Food and Nutrition Sciences
Vol.05 No.14(2014), Article ID:49032,8 pages
10.4236/fns.2014.514152
Effects of drying on the biochemical composition of Atherina boyeri from the Tunisian coast
Mohamed Ali Ben Smida1, Alěs Bolje2, Anissa Ouerhani1, Manel Barhoumi1, Hassen Mejri1, M’hamed El Cafsi1, Rafika Fehri-Bedoui1
1Faculté des Sciences de Tunis, Université de Tunis El Manar, Tunis, Tunisie
2Fisheries Research Institute of Slovenia, Ljubljana, Slovenia
Email: ali.bensmida@Laposte.net
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 May 2014; revised 1 July 2014; accepted 13 July 2014
ABSTRACT
The effects of solar and experimental drying on the protein concentration and total fatty acid (TFA) content of the muscles of Atherina boyeri (sand smelt) were studied. The percentage of protein was 18.3% for fresh fish and 14.2% for sun-dried fish, while TFA content was 4.9 g/100g and 0.5 g/100g. After the drying experiment, the percentage of protein was 23% and the fatty acid (FA) content was 2.8 g/100g. The results show that natural or experimental drying favors saturated fatty acids. The n-3 polyunsaturated fatty acids (PUFAs) are less sensitive to heat than the n-6 PUFAs. From a nutritional point of view, it seems that the drying conditions, where parameters are T = 50˚C, moisture = 30% and air speed = 2 m/s, would be the most beneficial for the preservation of sand smelt.
Keywords:
Biochemical composition, solar drying, drying experimental, Atherina boyeri

1. Introduction
Fish is important for humans for its nutritional qualities and also for the choice it offers in taste [1] , texture, and the form in which it is marketed: whole, fillets, fresh, frozen, salted, smoked, dried, or processed [2] . Fish meat is the part that is most used by consumers. The fish meat is the most used in part for human consumption. The lipids of the flesh have a beneficial effect on human health due to the presence of n-3 polyunsaturated fatty acids (PUFAs) [3] .
The nutritional qualities of fish are generally greater than or equal to those of meat, and the protein content of fish flesh is, regardless of the species, equivalent to that of meat [4] . In addition, fish protein is more digestible than meat protein and the levels of essential amino acids are generally a little higher than those of meat [5] .
Proteins are able to soak up water and hold it against the force of gravity in a protein matrix [6] . Some species of fish have the ability to accumulate large amounts of fat to be used for the purposes of basic metabolism during migration and reproduction. The lipid content of a marketed species is essential information for consumers [7] .
Fish is a perishable product, and its consumption is spread throughout the year [8] . To keep the fish for eating long after it was caught, it is necessary to preserve or transform the fish [9] . This process also requires drying, which aims to reduce the water content of the fish to promote its preservation.
Water is a vector of bacterial infections, and chemical, and biochemical decomposition, and is involved in the degradation reactions of the product. It is therefore necessary to partially dehydrate the product to stabilize, by removing a portion of the so-called “free” water. Proper drying can only be achieved all year round only if the temperature, humidity, and ventilation are controlled. Without control of these three parameters, the drying time can be very long, depending on the climatic conditions [10] .
In Tunisia, sand smelt is known as the “Cherkaou” and is consumed in the areas of Monastir, Djerba, and Gabes. It is preserved by conventional drying and is consumed in this form [11] . Given the importance of fatty acids and proteins for the proper functioning of the human body, it is of interest to quantify the fatty acid and protein contents of the flesh of A. boyeri. In this study, we compared the effects of traditional and artificial drying on the protein and fatty acid contents of A. boyeri.
2. Materials and Methods
2.1. Sampling and biological parameters
Fresh samples of A. boyeri came from the region of Monastir. These samples were collected in winter 2010. The average total length (TL) and the average total weights (TW) of the individuals are shown in Table 1.
2.2. Drying conditions
The fish were divided into several groups, each consisting of six samples (n = 6). One group was subjected to solar drying (SN). Another group was analyzed fresh (F). The other fish was subjected to different experimental conditions. It was dehydrated by convective drying at variable air speeds (1 m/s and 2 m/s) at different temperatures (45˚C, 50˚C, 60˚C, and 70˚C) and relative humidity (20%, 30%, and 40%). The experimental conditions are summarized in Table 2.
The drying tests were conducted in a closed-loop drying system using forced hot air convection. The average diameter of the fish was 14 mm, and the initial water content varied between 75% and 80%. The conditions of the drying air were kept constant during each test. A high precision balance is equipped with a data output enables via a software acquisition weight during the drying process. The temperature inside and on the surface of A. boyeri was measured using thermocouples connected to a data acquisition channel. The air flow in the test section was perpendicular to the surface of the product to be dried in order to obtain optimum air-fish contact.
2.3. Determination of moisture content
This measurement was made on fresh fish and dried fish. The fish was weighed prior to being placed in an oven to determine the wet weight (Ph). It was then placed in an oven at a temperature of 105˚C for 24 hours until a constant weight was reached (Ps). Moisture was calculated using the following formula:
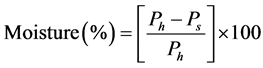
2.4. Extraction of total fatty acids
The extraction of total fatty acids (TFAs) was performed in the presence of chloroform-methanol (2:1 v/v) [12] . The total lipids obtained were stored in chloroform-methanol-butylatedhydroxytoluene (BHT) at −28˚C. For
Table 1. Biometric features A. boyeri.
Table 2. Experimental drying conditions.
further analysis, the fatty acids were transformed into methyl esters, according to Cecchi et al. [13] . The quanti- fication of the fatty acids is based on an internal standard not present in our samples, methyl nonadecanoate or C19:0 (Sigma Aldrich, Corporate Headquarters, St. Louis, MO).
2.5. Identification and quantification of fatty acids
Methyl esters of TFAs were separated, identified, and quantitated by gas chromatography using a HP 6890 gas chromatograph with a split/splitless injector with electronic pressure control and a flame ionisation detector was used for the analysis. Separation was performed with a 30 m HP Innowax capillary column with an internal diameter of 250 μm and a 0.25 μm film thickness, the stationary polar phase of the column being polyethylene glycol.
2.6. Protein determination
The determination of protein content was based on a solution of serum albumin [14] . The sample was incubated for 30 minutes away from light and then the optical density at a 540 nm was measured.
2.7. Statistical analysis
Mean comparison was performed by analysis of variance (ANOVA) followed by Duncan test at the significance level of p < 0.05. All statistical analyses were carried out using the software program SPSS version 13 (SPSS Inc., Chicago, Illinois).
3. Results
The percentages of moisture protein and total fatty acids (TFA) of fresh and sun-dried A. boyeri are shown in Table 3.
3.1. Effects of experimental drying on protein content
After subjecting A. boyeri to different experimental drying conditions, the protein content of the flesh varied significantly. The results are shown in Table 4.
A maximum of 23% protein was obtained with E2. The effects of different temperatures were observed with E2 (23%), E6 (18%), and E8 (18%); the difference between the three conditions was significant. Protein content varied significantly with moisture, with a significant difference between E2 (23%) and E3 (19.6%). The drying rate appeared to have a destructive effect on the protein content; the destructive effect of protein content was greater with low drying speed of 1 m/s. the percentages were 23% and 17.6% for E2 and E9, respectively.
3.2. Effects of drying on TFA content
TFA content after extraction was expressed in g per 100 g of fish. The TFA contents in fresh and sun-dried A. boyeri were 4.9 ± 1.9 g/100g and 0.5 ± 0.0 g/100g respectively (Table 3). Drying may be a necessary step in food preservation. We subjected A. boyeri to different experimental drying conditions. The TFAs obtained are shown in Table 4. Drying appeared to affect the TFA content of the flesh of A. boyeri. The values varied from 4.9 g/100g
Table 3. Water (%), protein (%), and total fatty acid content (g/100g) of fresh and sun-dried A. boyeri (mean ± SE, n = 6).
Table 4. Comparative table of protein content (%) and total fatty acid content (g/100g) of fish dried at different experi- mental conditions (mean ± SE, n = 6; superscript letters indicate inter group statistical differences, p < 0.05).
in fresh fish, depending on the experimental conditions. Conditions E2, E3, E4 and E9 seem to be the best for the preservation of TFAs in A. boyeri; TFA content varied from 2.6 to 2.8 g/100g.
Variations in temperature and moisture content led to significant variations in TFA content between the experimental conditions. At 60˚C, the moisture content did not cause significant variation between E2 (2.8 g/100g) and E3 (2.6 g/100g). By contrast, at 70˚C, a significant difference was found between E4 and E1 where the TFA content was 2.6 g/100g and 1.8 g/100g, respectively. The drying rate did not seem to affect the TFA content. No significant difference was found between E3 and E9.
3.3. A. boyeri lipid profile
Under experimental drying condition (E1 - E9), 22 fatty acids were identified in the tissues of A. boyeri (Table 5). Five fatty acids were particularly prominent in the lipid composition of fresh fish. The palmitic fatty acid (C16:0) (20.2% - 26.9%) and stearic acid (C18:0) (7.8% - 10.4%) predominated among the saturated fatty acids (SFAs). Oleic acid (C18:1n-9) was the main component of the monounsaturated fatty acids (MUFAs), varying between 8.9% and 14.4% of the TFAs. It was followed by vaccenic acid (C18:1n-7) and palmitoleic acid (C16:1n-7). Among the PUFAs, the majority were docosahexaenoic fatty acids (DHA, C22:6n-3) and eicosapentaenoic acid EPA (C20:5n-3). Their cumulative percentages varied between 6.8% and 25.5% of the TFAs. Natural drying (SN) promoted the formation of saturated fatty acids (SFA) with palmitic acid C16:0 as the predominant fatty acid; oleic acid was the predominant MUFA; and DHA was the predominant PUFA.
3.4. Composition of fatty acid groups
3.4.1. In fresh fish and sun-dried fish
Natural drying (SN) promotes the formation of saturated fatty acids that reach a percentage of 67.2%, while in the fresh fish, the percentage of saturated fatty acids is 42.6% of the TFAs (Table 5).
3.4.2. Under experimental drying
For fresh fish, natural drying seemed to affect MUFA content; while PUFA content remained stable (Figure 1). There seemed to be an inverse relationship between the percentage of SFAs and MUFAs. The most favorable conditions for the preservation of A. boyeri were those that showed a minimum percentage of SFA and a maximum percentage of unsaturated fatty acids (MUFA + PUFA). The results of E5, E6, and E7 met these conditions.
According to Figure 2, solar drying (SN) affected the percentage of unsaturated fatty acids. The PUFAs that were most sensitive to experimental drying were those of the n-6 family. Fatty acids of the n-3 family did not seem to be affected by experimental drying; the content was higher than in fresh fish. Two points stood out, E1 and E5, with respective percentages of 30.1 and 30.4% of the TFAs. Among the MUFAs, n-9 fatty acids were more resistant to experimental drying than n-7 fatty acids.
We can also see from Table 5 that the n-3/n-6 ratio varied between 2.9 and 4.8. The highest ratios of 4.2 and 4.8 were observed in E5 and E8, respectively, which had the lowest percentages of n-6 fatty acids (7.5% and 6.8% of the TFAs, respectively). Of interest from a nutritional point of view, the levels of EPA + DHA were expressed
Figure 1. Percentages of saturated fatty acids (SFA), monoun- saturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) under different experimental conditions.
Table 5. Fatty acid profile under different experimental conditions (%TFA, values with superscript letters indicate inter group statistical differences, p < 0.05).
PI: peroxidisability index = (0.025 × monoenes) + (1 × dienes) + (2 × trienes) + (4 × tetraenes) + (6 × pentaenes) + (8 × hexaenes). HH: hypocholesterolaemic/hypercholesterolaemic ratio = (C18:1n-9 + C18:2n-6 + C20:4n-6 + C18:3n-3 + C20:5n-3 + C22:5n-3 + C22:6n-3)/(C14:0 + C16:0). EPA + DHA*: mg/100g de poisson.
Figure 2. Percentages of n-3, n-6, n-7, and n-9 fatty acids under different experimental conditions.
in mg/100g of fish. These levels varied depending on the experimental conditions. The levels were higher than 300 mg/100g except with natural drying (SN), E6, and E8 where the values were lower than 300 mg/100g.
The peroxidisability index (PI) represents the relationship between the fatty acid composition of tissue and its susceptibility to oxidation. This index showed high values of 246 and 238 in E1, E7, and E6, respectively, and a value of 187 for E4. For the hypocholesterolemic/hypercholesterolemic ratio, the values were 2 and 1.96 in E6 and E7, respectively.
4. Discussion
The drying of fish is performed to reduce some of the so-called “free” water that is a vector of various infections and is involved in the degradation reactions. Drying helps promote preservation. During natural drying, the fish is exposed to air and natural light. Drying in the sun can be performed for 3 to 10 days, but drying times of 1 to 3 days are more common [15] .
The protein content of fresh A. boyeri was 18.3%. With natural drying, the surface of the fish dries faster and hardens, locking moisture inside, which slows down the drying process and promotes the degradation of proteins [16] . In this study, we obtained a protein content of 14.2 g/100g. Protein destruction is accelerated when the products are subjected to high temperatures for an extended period of time [16] . The total fatty acid (TFA) content of fish affects the biochemical characteristics postmortem. In this study, the TFA content of fresh fish was 4.9 g/100g; it was 0.5 g/100g with sun-dried fish. When drying conditions change (E1 to E9), the contents of total fatty acids vary from 1.1 g/100g to 2.8 g/100g.
The exposure of A. boyeri for extended periods to solar drying led to the degradation of lipids. The formation of lipid oxidation by-products reduces the nutritional quality and increases health risks [17] because they are associated with aging, altered membranes, heart disease, and cancer [18] . Photo-oxidation explains the low trans fat content of fish exposed to the air [19] .
Taking into account productivity, one might think that drying with warm air would take less time than natural drying. In E2, E3, E4, and E9, the TFA content increased in parallel with the temperature. However, a high temperature of 70˚C (E1) can alter the final TFA content, which was 1.8 g/100g. It seems that the E2 conditions would be ideal for the preservation of AGT in A. boyeri.
In fresh A. boyeri, palmitic acid was the major component of the SFAs, with a relative percentage of 57.5% (of SFAs), followed by stearic acid with a relative percentage of 29.3% (of SFAs). The profile of fatty acids in A. boyeri was distinguished by the presence of myristic acid (C14:0), with a relative percentage of 9% (of SFA). During natural drying, the respective percentages of these fatty acids were 51.4%, 42.2%, and 2.9% (of SFA). These three fatty acids cannot be considered as a whole because they differ in their structure, metabolism, cellular functions, and even their deleterious effects in case of excess [20] . Myristic acid represents a small proportion of the TFAs in the animal body (between 0.5% and 2% of TFA) [21] .
The three main MUFAs in fresh A. boyeri were palmitoleic acid (C16:1n-7), oleic acid (C18:1n-9), and vaccenic acid (C18:1n-7), with relative percentages of 24.6%, 44%, and 28.2% (of MUFA). These three fatty acids were identified as major fatty acids in natural drying, with relative percentages of 13%, 55.6%, and 31.2% (of MUFA), respectively. Oleic acid is characteristic of fish tissue [22] and is actively synthesized by the cells [23] . Under the action of ACAT (acylCoA-cholesterol acyltransferase), oleic acid binds to cholesterol [23] . Cholesterol esters thus formed represent the form of the transport of cholesterol in lipoproteins [22] . Under experimental drying, the highest percentage of oleic acid was observed with E6 and E8, with 57.7% and 55.4% (MUFA), respectively.
A. boyeri, in addition to being a source of protein, contains large amounts of PUFAs. In fresh fish, the percentage was 34.7% (of TFA), and between 33.2% and 41.4% (TFA) with experimental drying. The n-6 PUFA content was 11% (of TFA) in fresh fish. This category is represented by linoleic acid (LA, C18:2n-6), acid which has a percentage of 5.1% and by arachidonic acid (ARA, C20:4n-6) with a respective percentage of 5% (TFA). As shown in Table 5, under different experimental conditions, ARA was the main n-6 PUFA except with E5. The percentages of n-3 PUFAs were higher than those of n-6 PUFAs, as shown by the n-3/n-6 ratios in Table 5. The highest ratios of 4.8 and 4.2 were observed with E5 and E8, respectively. This ratio is useful for comparing the nutritional value of fish oils [24] . Fish and fishery products rich in n-3 fatty acids and low in n-6 fatty acids are considered good for human health [25] . An increase in the n-3/n-6 ratio is essential to help the body use n-3 fatty acids. A low ratio indicates that the enzymes that convert fatty acids to their active forms are likely to be used by n-6 PUFAs [26] . The percentage of EPA + DHA is responsible for variations in the n-3/n-6 ratios [26] .
Research has shown that there are significant health benefits of a diet rich in EPA and DHA. DHA and ARA are important in neonatal health [27] [28] , in particular in eye and brain development. A number of countries including Canada and the United Kingdom, and organizations such as the World Health Organization (WHO) and North Atlantic Treaty Organization have advocated dietary recommendations for n-3 PUFAs. These recommendations are 0.300 to 0.500 mg/day EPA + DHA [29] . Based on the results in Table 5, these recommendations can easily be met by consuming 100 g fresh A. boyeri (i.e., 680 mg EPA + DHA), or 200 g of dried fish with E6 (540 mg EPA + DHA), E7 (632 mg EPA + DHA) or E8 (492 mg EPA + DHA).
The PI index [30] shows the relationship between the unsaturated fatty acid composition of tissue and its susceptibility to oxidation. It provides information on the quality of the product that when the value of PI is high, polyunsaturated fatty acids are susceptible to be oxidized. The higher the PI value, the higher fat fish is likely to be oxidized. Factors that influence lipid oxidation are either intrinsic, such as the fatty acid composition of lipids (number and position of unsaturation), the presence of pro-oxidants (heme, metal ions, and enzymes) or natural antioxidants (tocopherols and carotenoids), and external factors such as temperature, light, oxygen, water activity, and the conditions of storage and processing [31] . The PI of fresh A. boyeri tissue was 176; the PI of sun- dried A. boyeri was 83. Although the conditions E1, E5, E6, and E7 had the highest values on the PI index, they also showed a greater sensitivity to oxidation than other conditions. The values that were closest to that of fresh fish were 187 (E2) and 195 (E4). The values that are closest to fresh fish are 187 and 195 and they correspond to drying conditions E4 and E2.
The relationship between hypocholesterolaemic and hypercholesterolaemic fatty acids (HH) [32] provides information on the effects of specific fatty acids on cholesterol metabolism. The highest ratios that are most beneficial were found with E6 (2), E7 (1.96), E1 (1.82), and E9 (1.82).
Acknowledgements
The authors thank Mrs. Hedia Chaabane who contributed to the achievement of this work.
References
- Robb, D.H.F., Kestin, S.C., Warriss, P.D. and Nute, G.R. (2002) Muscle Lipid Content Determines the Eating Quality of Smoked and Cooked Atlantic Salmon (Salmo Salar). Aquaculture, 205, 345-358. http://dx.doi.org/10.1016/S0044-8486(01)00710-4
- Dumay, J. (2006) Extraction de lipides en voie aqueuse par bio-réacteur enzymatique combiné à l’ultracentrifugation: Application à la valorisation de co-produits de poisson (Sardina pilchardus). Ph.D. Dissertation, Ecole Polytechnique de l’Université de Nantes, 283. http://archimer.ifremer.fr/doc/2006/these-1556.pdf
- Harper, C.R. and Jacobson, T.A. (1987) The Role of Omega-3 Fatty Acids in the Prevention of Coronary Heart Disease. Archives of Internal Medicine, 161, 2185-2192. http://dx.doi.org/10.1001/archinte.161.18.2185
- Piclet, G. (1987) Le poisson aliment. Composition—Intérêt nutritionnel. Cahiers de la Nutrition et de la Diététique, 4, 317-336.
- Médale, F., Lefèvre, F. and Corraze, G. (2003) Qualité nutritionnelle et diététique des poissons. Cahiers de Nutrition et de Diététique, 38, 37-44.
- Okland, H.M.W., Stoknes, I.S., Remme, J.F., Kjerstad, M. and Synnes, M. (2005) Proximate Composition, Fatty Acid and Lipid Class Composition of the Muscle from Deep Sea Teleosts and Elasmobranchs. Comparative Biochemistry and Physiology—Part B, 140, 437-443. http://dx.doi.org/10.1016/j.cbpc.2004.11.008
- Grégoire, F., Dionne, H. and Lévesque, C. (1994) Contenu en gras chez le maquereau bleu (Scomber scombrus L.) en 1991 et 1992. Rapport canadien à l’Industrie sur les sciences halieutiques et aquatiques, 220, 82.
- FAO (2008) Assurer l’approvisionnement régional des produits aquatiques: Perspectives suite aux études de cas de la FAO dans l’évaluation des pertes post-capture—Comité des pêches continentales et de l’aquaculture pour Afrique, Quinzième session. FAO, Lusaka.
- Choubert, G. (2010) Procédés de conservation/transformation et qualité sensorielle du poisson. 13ème Journées Sciences des Muscles et Technologies des Viandes, Clermont-Ferrand, 19-20 Octobre 2010, 91-98.
- Knockaert, C. (1990) Le fumage du poisson. http://archimer.ifremer.fr/doc/00004/11490/
- Bouriga, N., Ben Alaya, H., Selmi, S., Azouz, S., Faure, E. and Trabelsi, M. (2009) Effets de deux procédés de séchage sur la qualité lipidique des athérines de l’île de Djerba (Tunisie). Société des Sciences Naturelles de Tunisie, 36, 44-51.
- Folch, J., Lees, M. and Sloane-Stanley, G.A. (1957) A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. Journal of Biological Chemistry, 226, 497-509.
- Cecchi, G., Basini, S. and Castano, C. (1985) Méthanolyse rapide des huiles en solvant. Revue française des corps gras, 4, 163-164.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent. Journal of Biological Chemistry, 193, 265-275.
- FAO/UN (1992) Fish and Fuel. Food and Forests. Perspectives on Post-Harves Losses in Uganda. Fishin Project: P O Box 521. FAO/UN, Kampala.
- Ariyawansa, S. (2000) The Evaluation of Functional Properties of Fish Meal. United Nations University, Fisheries Training Programme, Project Final, Sri Lanka, 1-25.
- Frankel, E.N. (1996) Antioxidants in Lipid Foods and Their Impact on Food Quality. Food Chemistry, 57, 51-55. http://dx.doi.org/10.1016/0308-8146(96)00067-2
- Suja, K.P., Abraham, J.T., Thamizh, S.N., Jayalekshmy, A. and Arumughan, C. (2004) Antioxidant Efficacy of Sesame Cake Extract in Vegetable Oil Protection. Food Chemistry, 84, 393-400. http://dx.doi.org/10.1016/S0308-8146(03)00248-6
- Ashton, I.P. (2002) Understanding Lipid Oxidation in Fish. In: Bremmer, H.A., Ed., Safety and Quality Issues in Fish Processing, Woodhead Publishing Limited, Cambridge, 254-285. http://dx.doi.org/10.1533/9781855736788.2.254
- Agence Française de Sécurité Sanitaire des Aliments (2010) Avis de l’Agence Française de Sécurité Sanitaire des Aliments. Actualisation des apports nutritionnels conseillés pour les acides gras, Saisie 2006-SA-0359, 1-10.
- Rioux, V. and Legrand, P. (2001) Métabolisme et fonctions de l'acide myristique. Oléagineux, Corps Gras Lipides, 8, 161-166.
- Steffens, W. (1997) Effects of Variation in Essential Fatty Acids in Fish Feeds on Nutritive Value of Freshwater Fish for Humans. Aquaculture, 151, 97-119. http://dx.doi.org/10.1016/S0044-8486(96)01493-7
- Legrand, P. (2007) Les acides gras: Structures, fonctions Apports nutritionnels conseillés. Cahiers de Nutrition Diététique, 42, 7-12.
- Pigott, G.M. and Tucker, B.W. (1990) Seafood: Effects of Technology on Nutrition. Marcel Dekker, Inc., New York.
- Sargent, J.R. (1997) Fish Oils and Human Diet. British Journal of Nutrtion, 78, 5-13. http://dx.doi.org/10.1079/BJN19970131
- Hossain, M.A. (2011) Fish as Source of n-3 Polyunsaturated Fatty Acids (PUFAs), Which One Is Better-Farmed or Wild? Advance Journal of Food Science and Technology, 3, 455-466.
- Horrocks, L.A. and Yeo, Y.K. (1999) Health Benefits of Docosahexaenoic Acid (DHA). Pharmacological Research, 40, 211-225. http://dx.doi.org/10.1006/phrs.1999.0495
- Innis, M.S. (2000) The Role Dietary n-6 and n-3 Fatty Acids in the Developing Brain. Developmental Neuroscience, 22, 474-480. http://dx.doi.org/10.1159/000017478
- Kris-Etherton, P.M., Harris, W.S. and Appel, L.J. (2002) Fish Consumption, Fish Oil, Omega-3 Fatty Acid and Cardiovascular Disease. Circulation, 106, 2747-2757. http://dx.doi.org/10.1161/01.CIR.0000038493.65177.94
- Erickson, M.C. (1992) Variation of Lipid and Tocopherol Composition in Three Strains of Channel Catfish (Ictalurus punctatus). Journal of the Science of Food and Agriculture, 59, 529-536. http://dx.doi.org/10.1002/jsfa.2740590416
- Hsieh, R.J. and Kinsella, J.E. (1989) Lipoxygenase Generation of Specific Volatile Flavor Carbonyl Compounds in Fish Tissues. Journal of Agricultural and Food Chemistry, 37, 279-286. http://dx.doi.org/10.1021/jf00086a001
- Santos-Silva, J., Bessa, R.J.B. and Santos-Silva, F. (2002) Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs. II. Fatty Acid Composition of Mea. Livestock Production Science, 77, 187-194. http://dx.doi.org/10.1016/S0301-6226(02)00059-3




