Food and Nutrition Sciences
Vol.4 No.5(2013), Article ID:31346,6 pages DOI:10.4236/fns.2013.45073
β-Glucan Fiber from Spent Brewer’s Yeast Reduces Early Atherosclerosis Greater Than Psyllium in Hypercholesterolemic Syrian Golden Hamsters*
![]()
Department of Clinical Laboratory and Nutritional Sciences, Center for Health and Disease Research, University of Massachusetts Lowell, Lowell, USA.
Email: #Thomas_Wilson@uml.edu
Copyright © 2013 Thomas A. Wilson et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 12th, 2013; revised April 12th, 2013; accepted April 19th, 2013
Keywords: β-Glucan Fiber; Plasma Cholesterol; Aortic Fatty Streak; Psyllium; Hamsters
ABSTRACT
β-Glucans, mostly from oats, have been shown to reduce blood concentrations of total and LDL-cholesterol in animals and humans. After processing, spent brewer’s yeast, a by-product of the fermentation process, contains 85% - 90% β- glucans. The purpose of this study was to evaluate the effect of yeast-derived β-glucan fiber on plasma lipids and early atherosclerosis development in hamsters consuming a semi-purified hypercholesterolemic diet (HCD). Animals were fed either the HCD or the HCD containing psyllium or β-glucan fiber from yeast for 12 weeks. Both the psyllium and β-glucan fiber from yeast showed significant decreases in plasma total cholesterol, non-HDL-C, triacylglycerol, and aortic fatty streak area when compared to the HCD. Also, the β-glucan fiber from yeast had significantly less aortic fatty streak area compared to the psyllium diet. Findings from this study show that while both β-glucan fiber from yeast and psyllium produced similar reductions in plasma lipid and lipoprotein concentrations, the β-glucan fiber from yeast prevented the development early atherosclerosis better than psyllium in the hamsters.
1. Introduction
The link between elevated plasma LDL-cholesterol concentrations and the risk of developing coronary artery disease has been clearly established [1]. However, cholesterol-lowering medications are usually reserved for patients who have not reduced their cholesterol concentrations sufficiently with dietary management and exercise, have already had an infarction or stroke, or do not meet the American Heart Association guidelines for acceptable serum lipid concentrations [2]. The National Cholesterol Education Program (NCEP) recommends that most patients with abnormal serum lipid profiles follow the Step I or II diet plans, which include reductions in dietary total fat, saturated fat and cholesterol intake [2].
Not included in NCEP guidelines are specific recommendations about other dietary macronutrients, including fiber. Consumption of whole oats or oat bran has been shown to lower serum cholesterol concentrations in animal models of hypercholesterolemia [3-7]. This effect is largely attributed to the (1 ® 3) (1 ® 4)-β-D-glucan (i.e., β-glucan) component [8]. Spent brewers yeast (from breweries or bakeries) is also a rich source of β-glucans. In fact, after processing, the yeast contains 85% - 90% β-glucan, consisting of repeating units of β-D-glucose joined together in β-(1 ® 3) (1 ® 6) linkages [9]. Currently this material is sold as animal feeds, used as land fill or in biogas production [10]. Utilization of this residue as a dietary supplement would benefit the breweries, extract manufacturers and the environment [10,11].
While barley, oat bran [8] and yeast [10] have been shown to improve the blood lipid profile, the purpose of this study was to examine the effect on the development of early atherosclerosis, as measured by aortic fatty streak area, of yeast-derived β-glucan fiber in Syrian Golden F1B hamsters and to confirm that the yeast source of this fiber is as efficacious as a commonly used soluble fiber, psyllium, which is a gel-forming mucilage derived from the seed husk of Plantago Ovata, and has been shown to improve the lipid profile [12-14]. Hamsters were selected for this because they represent a useful model with respect to humans due to their similar lipoprotein profile when fed hypercholesterolemic diets and their ability to metabolize cholesterol in a similar manner [4,8,10,15,16].
2. Materials and Methods
2.1. Diets
All dietary ingredients and formulated diets were supplied by Research Diets, Inc., (New Brunswick, NJ). The semipurified, hypercholesterolemic control diet (HCD) contained 17 g/100g fat (coconut oil), 0.1 g/100g cholesterol and 10 g/100g cellulose. The experimental diets were formulated to contain either 10 g/100g psyllium or 10 g/100g Fibercel (yeast β-glucan fiber source) at the expense of cellulose (Table 1) and fed for 12 weeks.
2.2. Animals
Forty-eight male Syrian Golden hamsters (F1B strain, Charles River Laboratories International, Inc., Wilmington, MA) approximately 8 - 10 weeks of age were individually housed and acclimated to stainless steel caging and fed the HCD for 4 weeks. Hamsters were bled at both 2 and 4 weeks to determine plasma lipids and lipoprotein cholesterols. At the end of the fourth week, the
Table 1. Dietary composition (g/100g) of hamster feed1.
animals were divided into 3 groups (n = 16/group) and fed either the HCD, or the HCD containing psyllium or β-glucan fiber from yeast (β-glucan fiber) for 12 weeks. Animals were bled at 8 and 12 weeks to measure plasma lipids and lipoprotein cholesterols. At the end of the twelfth week, the animals were euthanized and aortic fatty streak analysis was performed. Experimental protocols were approved by the Institutional Animal Care and Use Committee. Hamsters were maintained in accordance with the guidelines of the Animal Care Committee at the University of Massachusetts-Lowell Research Foundation and the guidelines prepared by the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHEW publication No 85-23, revised 1985). Hamsters were housed in environmentally controlled conditions with an alternating 12-h light/dark cycle and given ad libitum access to food and water except when food was withheld for the experimental protocols described below. Food intake was measured every two or three days by measuring how much food was added versus how much food was removed and body weights were measured on a weekly basis.
2.3. Plasma Lipids and Lipoprotein Cholesterol Measurements
Blood was collected via the retro-orbital sinus into heparinized capillary tubes under ultrapure CO2/O2 (50/ 50) gas (Northeast Airgas, Salem, NH) anesthesia from hamsters fasted for 12 hours at weeks −2, 0, 8, and 12. Plasma was harvested after centrifugation at 1500× g at room temperature for 20 min and plasma lipid and lipoprotein cholesterol concentrations were measured using a Cobas Mira Plus Clinical Chemistry Autoanalyzer. Plasma total cholesterol (TC) [17] and triacylglycerol (TG) [18] concentrations were measured enzymatically using the Infinity Cholesterol Reagent (procedure #401) and Triglyceride (GPO-Trinder) Reagent (procedure 337) from Sigma Diagnostics (Sigma-Aldrich, St. Louis, MO). Plasma very lowand low-density lipoprotein cholesterol, which we combined and termed non-HDL-C, was precipitated with phosphotungstate reagent [19] and plasma high-density lipoprotein cholesterol (HDL-C) was measured in the supernatant using the plasma TC procedure. The concentration of non-HDL-C was calculated as the difference between plasma TC and HDL-C.
2.4. Aortic Fatty Streak Analysis
At the end of the twelfth week, hamsters were anesthetized with an IP injection of sodium pentobarbital (62.5 mg/mL at a dosage of 0.2 - 0.25 mL/200 gram body weight) (Henry Schein, Port Washington, NY) and aortic tissue was obtained as previously described [20,21]. Images from the entire mounted section were captured and analyzed using a model 3000 Image Analysis system (Image Technology Corp., Deer Park, NY). The analysis system was calibrated with a micrometer slide and the color threshold to identify oil red O (ORO) was set subsequent to analysis of the total ORO-stained area. Units of measurement of fatty streak area are expressed in μm2/mm2 of aortic tissue.
2.5. Statistics
Repeated-Measures (RM) One-Way Analysis of Variance (ANOVA) was used to examine the effect of treatment over time on plasma cholesterol concentrations and body weight using SigmaStat software (Jandel Scientific, San Rafael, CA) at p < 0.05 [22]. A One-Way ANOVA was used to examine the effect of treatment on food consumption and aortic fatty streak area. When differences were observed between experimental groups by RM ANOVA or ANOVA, a Student-Newman-Keuls post hoc test was performed using SigmaStat®5.
3. Results and Discussion
3.1. Animal Body Weights
The hamsters adapted well to all diets and all hamsters survived the dietary treatments. No differences were observed between the hamsters fed the HCD, β-glucan fiber, or psyllium diets for food consumption over the course of the 12-week study (14.7 + 2.20, 15.3 + 1.40, and 14.9 + 2.13, grams/day respectively). The body weights for the hamsters during the dietary treatment period also were not significantly different from each other for each time point. The body weights of the hamsters fed the HCD, β-glucan fiber, or psyllium diets at week 0 (93.5 + 1.78, 92.2 + 1.95, and 94.1 + 2.04, grams respectively) were significantly increased by week 12 (112.4 + 2.89, 110.6 + 3.45, and 109.3 + 2.13, grams respectively) (p < 0.05).
3.2. Plasma Lipid Concentrations
Plasma lipid and lipoprotein cholesterol concentrations in hamsters consuming the HCD were approximately the same in all groups following a 4-week lead-in. Following the lead-in period, hamsters were placed on the indicated diets. Plasma lipid and lipoprotein cholesterol concentrations measured at weeks 8 and 12 of the treatment period were not significantly different from each other for diet treatment, thus the mean of the two data points is stated. Compared to hamsters fed the HCD, a decrease in plasma TC, non-HDL-C, TG concentrations were observed in the hamsters fed the psyllium (−28%, −44%, and −46%, respectively) (p < 0.05) and β-glucan fiber (−30%, −46%, and −51%, respectively) (p < 0.05) diets (Table 2). No differences in plasma HDL-C concentrations were observed among any of the dietary treatments at the end of 12 weeks. Also, the hamsters fed the psyllium and β-glucan fiber diets were not different from each other for any plasma variable.
Elevated plasma cholesterol concentrations are associated with increased risk of coronary artery disease, whereas an elevation in plasma HDL-C concentration is inversely correlated with the incidence of cardiovascular disease [2]. A reduction in dietary total and saturated fat as well as cholesterol, are the main dietary treatments for patients with hypercholesterolemia [1]. However there is substantial evidence for the favorable lipid profile effects of certain dietary fibers [11,23-26]. The cholesterollowering activity of oat and barley is believed to be attributable to the β-glucan in soluble fiber fraction of these cereal grains. β-glucan is composed of large molecular weight water-soluble cell-wall polysaccharides consisting of (1 ® 3, 1 ® 4)-β-D-linked glucopyranosyl-monomers [27]. Concentrated β-glucan from both oats and barley lower plasma cholesterol concentrations in clinical studies [28,29] and in animal models of hypercholesterolemia [3-5,8,30-33].
In the current study, both the β-glucan fiber and psyllium diets lowered plasma TC, non-HDL-C, and TG concentrations while having no effect on plasma HDL-C concentrations compared to HCD diet. Recently, our laboratory showed that hamsters fed β-glucan fiber concentrate from oats or barley at both 4% and 8% by weight in the diet decreased plasma TC and non-HDL-C concentrations in a dose-dependent manner [8]. The magnitude of the decrease in TC concentrations in the
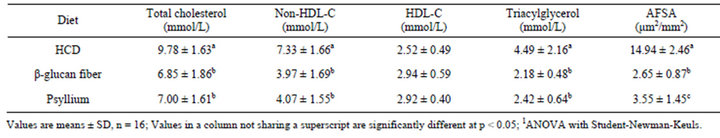
Table 2. Plasma lipids and lipoprotein cholesterol concentrations (average of weeks 8 and 12 bleeds) and aortic fatty streak area (AFSA) in hamsters fed the control diet and either β-glucan fiber or psyllium diets for 12 weeks1.
current study is consistent with previous studies evaluating the effect of β-glucan concentrates from barley [4,8,31] and similar to [5,8] or slightly greater in magnitude than the effect of oat bran and oat β-glucan concentrates in hamsters [3,4,30,34]. This effect was primarily attributable to decreased plasma non-HDL-C concentrations. The current study, however, did not show any changes in plasma HDL-C concentrations in hamsters fed the yeast-derived β-glucan fiber while previous work has reported decreases in plasma HDL-C concentrations in other studies evaluating the effect of β-glucan from barley or oat preparations in hamsters [4,5,8,30,31]. It is possible that the decreases in plasma HDL-C observed previously may be due to some other active ingredient that is present in the β-glucan preparations from oat and barley bran rather than due to the β-glucan itself.
It has been hypothesized that upon digestion, β-glucan increases small intestine viscosity due to its high molecular weight and its tendency to form viscous gummy solutions, resulting in reduced bile acid and cholesterol or triacylglycerol absorption, consequently lowering plasma cholesterol and triacylglycerols [27]. Wood et al. [35] reported a significant linear inverse relationship between glycemic response to a 50 g oral glucose load and the viscosity of oat b-glucan solutions. Wood and Beer [36] showed that processing such as extrusion reduced the MW of oat b-glucan but had little effect on its cholesterol-reducing properties. In rats, consumption of an ethanol-water and wet-milled oat b-glucan concentrate reduced serum TC by 30%, whereas a cold-water and wet-milled b-glucan concentrate reduced cholesterol by 10% as compared to rats fed a cellulose control diet, although the latter had higher MW and higher hydrodynamic volume [37]. Additionally, a recent study using hamsters demonstrated a similar cholesterol-lowering effect of oat b-glucan with molecular weight varying from 136 kDa to 1650 kDa [38]. Previously we evaluated the cholesterol-lowering activity of a reduced MW β- glucan concentrate prepared from barley in hamsters consuming a HCD [39]. Barley β-glucan concentrate was prepared from waxy hulless barley cultivars that have previously been shown to lower plasma cholesterol concentrations in hamsters consuming a HCD [8]. The β- glucan in this preparation had an average MW of 1000 kDa. A reduced MW β-glucan concentrate was prepared by limited enzymatic hydrolysis of the high MW barley b-glucan to an average MW of 175 kDa. Decreased plasma cholesterol and triacylglycerol concentrations were observed in hamsters consuming a HCD supplemented with concentrated barley β-glucan, regardless of the molecular weight [39]. Studies [24,34,40] have also shown that β-glucan inhibits the absorption of cholesterol from the gut, as demonstrated by significant increases in the excretion of fecal cholesterol and neutral sterols [8,39], however, fecal excretion of cholesterol and bile acids were not performed in the current study.
3.3. Early Atherosclerosis Development
At the end of the 12-wk treatment, compared to hamsters fed the HCD, aortic fatty streak area was significant less in the hamsters fed the psyllium (−76%, p < 0.05) and the β-glucan fiber (−82%, p < 0.05) diets (Table 2). Also, the hamsters fed the β-glucan fiber diet had significantly less aortic fatty streak area (−25%, p < 0.05) compared to the hamsters fed the psyllium diet.
Our main objective was to examine whether yeast-derived β-glucan fiber added to the diet of hypercholesterolemic hamsters would slowdown the development of early atherosclerosis similar to or better than another soluble fiber, psyllium, which has previously been shown to reduce blood lipids and lipoprotein cholesterol concentrations in humans [12-14] and in hamsters [41].
While the hamsters fed both the β-glucan fiber and psyllium diets had significantly less aortic fatty streak area compared to the hamsters fed the control diet, hamsters fed the yeast-derived β-glucan fiber diet had significantly less aortic fatty streak area compared to hamsters fed the psyllium diet, although similar decreases in plasma non-HDL-C were observed. This observation is particularly important because aortic fatty streak area is viewed as the hallmark in early atherosclerosis development. This is the first time that dietary β-glucan fiber derived from yeast has demonstrated such an effect on the aorta, although our previous study [8] did show a significant decrease in aortic cholesterol accumulation in hamsters fed β-glucan concentrates from oat and barley bran. Since we observed a greater decrease in aortic fatty streak and in hamsters fed the β-glucan diet compared to hamsters fed the psyllium diet, without differences in cholesterol-lowering activity, this suggests that additional anti-atherogenic mechanism(s) of action of yeastderived β-glucan fiber may be present other than a lowering of plasma non-HDL-C concentrations. It is unclear at this time what these potential other mechanisms maybe that are involved in reducing the aortic fatty streak area in hamsters fed the yeast-derived β-glucan fiber and future studies are needed to study this mechanism.
In conclusion, the results of the current study demonstrated that β-glucan derived from yeast can reduce plasma cholesterol concentrations, similar to psyllium, and the associated early atherogenic process, greater than psyllium, in hamsters. These results support the cholesterol-lowering and anti-atherogenic activity of yeastderived β-glucan fiber and also suggest a new market for spent brewers yeast.
4. Acknowledgements
The authors would like to thank Subbiah Yoganathan, Donato Vespa, Lorraine Misner, and Leena Laitinen for their technical assistance and Maureen Faul for her administrative assistance. This work was supported, in part, by a research grant to Robert J. Nicolosi from AlphaBeta Technology, Inc., Worcester, MA.
REFERENCES
- E. J. Schaefer, S. Lamon-Fava, L. M. Ausman, J. M. Ordovas, B. A. Clevidence, J. T. Judd, B. R. Goldin, M. Woods, S. Gorbach and A. H. Lichtenstein, “Individual Variability in Lipoprotein Cholesterol Response to National Cholesterol Education Program Step 2 Diets,” American Journal of Clinical Nutrition, Vol. 65, No. 3, 1997, pp. 823-830.
- Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults, “Summary of the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II),” Journal of the American Medical Association, Vol. 269, No. 23, 1993, pp. 3015-3023. doi:10.1001/jama.1993.03500230097036
- S. S. Jonnalagadda, F. W. Thye and J. L. Robertson, “Plasma Total and Lipoprotein Cholesterol, Liver Cholesterol and Fecal Cholesterol Excretion in Hamsters Fed Fiber Diets,” Journal of Nutrition, Vol. 123, No. 8, 1993, pp. 1377-1382.
- T. S. Kahlon, F. I. Chow, B. E. Knuckles and M. M. Chiu, “Cholesterol-Lowering Effects in Hamsters of β-GlucanEnriched Barley Fraction, Dehulled Whole Barley, Rice Bran, and Oat Bran and Their Combinations,” Cereal Chemistry, Vol. 70, No. 4, 1993, pp. 435-440.
- J. X. Zhang, E. Lundin, C. O. Reuterving, G. Hallmans, R. Stenling, E. Westerlund and P. Aman, “Effects of Rye Bran, Oat Bran and Soya-Bran Fibre on Bile Composition, Gallstone Formation, Gall-Bladder Morphology and Serum Cholesterol in Syrian Golden Hamsters (Mesocricetus auratus),” British Journal of Nutrition, Vol. 71, No. 6, 1994, pp. 861-870. doi:10.1079/BJN19940192
- W. J. L. Chen, J. W. Anderson and M. R. Gould, “Effects of Oat Bran, Oat Gum and Pectin on Lipid Metabolism of Cholesterol-Fed Rats,” Nutrition Reports International, Vol. 24, No. 6, 1981, pp. 1093-1098.
- A. P. De Groot, R. Luyken and N. A. Pikaar, “Cholesterol Lowering Effect of Rolled Oats,” Lancet, Vol. 282, No. 7302, 1963, pp. 303-304. doi:10.1016/S0140-6736(63)90210-1
- B. Delaney, R. J. Nicolosi, T. A. Wilson, T. Carlson, F. Frazer, G. H. Zheng, R. Hess, K. Ostergen, J. Haworth and G. Ostroff, “ß-Glucan Fractions from Barley and Oats Are Similarly Antiatherogenic in Hypercholesterolemic Syrian Golden Hamsters,” Journal of Nutrition, Vol. 133, No. 2, 2003, pp. 468-495.
- S. Bell, V. M. Goldman, B. R. Bistrian, A. H. Arnold and G. Ostroff, “Effect of Beta-Glucan from Oats and Yeast on Serum Lipids,” Critical Reviews of Food Science and Nutrition, Vol. 39, No. 2, 1999, pp. 189-202. doi:10.1080/10408399908500493
- R. J. Nicolosi, S. Bell, B. Bistrian, I. Greenberg, R. Forse and G. Blackburn, “Plasma Lipid Changes after Supple-Mentation with β-Glucan Fiber from Yeast,” American Journal of Clinical Nutrition, Vol. 70, No. 2, 1999, pp. 208-212.
- M. Suphantharika, P. Khunrae, P. Thanardkit and C. Verduyn, “Preparation of Spent Brewer’s Yeast β-Glucans with a Potential Application as an Immunostimulant for Black Tiger Shrimp,” Penaeus monodon. Bioresource Technology, Vol. 88, No. 1, 2003, pp. 55-60. doi:10.1016/S0960-8524(02)00257-2
- L. Moreno, B. Tresasaco, G. Bueno, J. Fleta, G. Rodriguez, J. Garagorri and M. Bueno, “Psyllium Fibre and the Metabolic Control of Obese Children and Adolescents,” Journal of Physiological Biochemistry, Vol. 59, No. 3, 2003, pp. 235-242. doi:10.1007/BF03179920
- J. Sandhu, G. Hudson and J. Kennedy, “The Gel Nature and Structure of the Carbohydrate of Ispaghula Husk ex Plantago Ovata Forsk,” Carbohydrate Research, Vol. 93, No. 2, 1981, pp. 247-259. doi:10.1016/S0008-6215(00)80854-X
- B. Olson, S. Anderson, M. Becker, J. Anderson, D. Hunninghake, D. Jenkins, J. Larose, J. Rippe, D. Roberts, D. Stoy, C. Summerbell, A. Truswell, T. Wolever, D. Morris and V. Fulgoni III, “Psyllium-Enriched Cereals Lower Blood Total Cholesterol and LDL Cholesterol, but Not HDL Cholesterol, in Hypercholesterolemic Adults: Results of a Meta-Analysis,” Journal of Nutrition, Vol. 127, No. 10, 1997, pp. 1973-1980.
- P. M. Kris-Etherton and J. Dietschy, “Design Criteria for Studies Examining Individual Fatty Acid Effects on Cardio-Vascular Disease Risk Factors: Human and Animal Studies,” American Journal of Clinical Nutrition, Vol. 65, Suppl. 5, 1997, pp. 1590S-1596S.
- D. K. Spady and J. M. Dietschy, “Rates of Cholesterol Synthesis and Low-Density Lipoprotein Uptake in the Adrenal Glands of the Rat, Hamster and Rabbit in Vivo,” Biochimical Biophysical Acta, Vol. 836, No. 2, 1985, pp. 167-175. doi:10.1016/0005-2760(85)90063-3
- C. C. Allain, L. S. Poon, C. S. G. Chan, W. Richmond and P. C. Fu, “Enzymatic Determination of Total Serum Choles-Terol,” Clinical Chemistry, Vol. 20, No. 4, 1974, pp. 470-475.
- G. Bucolo and H. David, “Quantitative Determination of Serum Triglycerides by the Use of Enzymes,” Clinical Chemistry, Vol. 19, No. 5, 1973, pp. 476-482.
- K. W. Weingand and B. P. Daggy, “Quantitation of High-Density Lipoprotein Cholesterol in Plasma from Ham-Sters by Differential Precipitation,” Clinical Chemistry, Vol. 36, No. 3, 1990, p. 575.
- T. L. Foxall, G. T. Shwaery, A. F. Stucchi, S. S. Wang and R. J. Nicolosi, “Dose-Response Effects of Doxazosin on Plasma Lipids, Lipoprotein Cholesterol and Aortic Fatty Streak Formation in Hypercholesterolemic Hamsters,” American Journal Pathology, Vol. 140, No. 6, 1992, pp. 1357-1363.
- M. C. Kowala, J. J. Nunnari, S. K. Durham and R. J. Nicolosi, “Doxazosin and Cholestyramine Similarly Decrease Fatty Streak Formation in the Aortic Arch of Hyper-Lipidemic Hamsters,” Atherosclerosis, Vol. 91, No. 1, 1991, pp. 35-49. doi:10.1016/0021-9150(91)90185-6
- G. W. Snedecor and W. G. Cochran, “Statistical Methods,” 6th Edition, Iowa State University Press, Ames, 1980.
- S. R. Glore, D. Van Treeck, A. W. Knehans and M. Guild, “Soluble Fiber and Serum Lipids: A Literature Review,” Journal of the American Dietetic Association, Vol. 94, No. 4, 1994, pp. 425-436. doi:10.1016/0002-8223(94)90099-X
- R. J. Illman and D. L. Topping, “Effects of Dietary Oat Bran on Faecal Steroid Excretion, Plasma Volatile Fatty Acids and Lipid Synthesis in Rats,” Nutrition Research, Vol. 5, No. 8, 1985, pp. 839-846. doi:10.1016/S0271-5317(85)80171-8
- S. Ink and R. Mathews, “Oatmeal and Oat Bran: Heart Health Benefits and More,” In: M. Yalpani, Ed., New Technologies for Healthy Foods and Nutraceuticals, ALT Press, Shrewsbury, 1997, pp. 195-233.
- S. Kalra and S. Jood, “Effects of Dietary Barley β-Glucan on Cholesterol and Lipoprotein Fractions in Rats,” Journal of Cereal Science, Vol. 31, No. 2, 2000, pp. 141-145.
- P. J. Wood, “Physicochemical Characteristics and Physiological Properties of Oat (1⋄3) (1⋄4)-β-D-Glucan,” In: P. J. Wood, Ed., Oat Bran, American Association of Cereal Chemists, St. Paul, 1993, pp. 83-112.
- J. T. Braaten, P. J. Wood, F. W. Scott, M. S. Wolynetz, M. K. Lowe, P. Bradley-White and M. W. Collins, “Oat β- Glucan Reduces Blood Cholesterol Concentration in Hypercholesterolemic Subjects,” European Journal Clinical Nutrition, Vol. 48, 1994, pp. 465-474.
- G. Onning, A. Wallmark, M. Persson, B. Akesson, S. Elmstahl and R. Oste, “Consumption of Oat Milk for 5 Weeks Lowers Serum Cholesterol and LDL Cholesterol in Free-Living Men with Moderate Hypercholesterolemia,” Annals of Nutrition and Metabolism, Vol. 43, 1999, pp. 301-209. doi:10.1159/000012798
- W. H. Yokoyama, B. E. Knuckles, A. Stafford and G. Inglett, “Raw and Processed Oat Ingredients Lower Plasma Cholesterol in the Hamster,” Journal of Food Science, Vol. 63, No. 4, 1998, pp. 713-715. doi:10.1111/j.1365-2621.1998.tb15820.x
- B. German, R. Xu, R. Walzem, J. E. Kinsella, B. Knuckles, M. Nakamura and W. Yokoyama, “Effects of Dietary Fats and Barley Fiber on Total Cholesterol and Lipoprotein Cholesterol Distribution in Plasma of Hamsters,” Nutrition Research, Vol. 16, No. 7, 1996, pp. 1239-1249. doi:10.1016/0271-5317(96)00127-3
- D. G. Oakenfull, R. L. Hood, G. S. Sidhu and H. S. Saini, “Effects of Barley and Isolated Barley β-Glucans on Plasma Cholesterol in the Rat,” In: D. J. Martin and C. W. Wrigley, Eds., Proceedings of Cereals International, Brisbane, 1991, pp. 344-349.
- M. L. Maqueda De Guevara, P. C. H. Morel, G. D. Coles and J. R. Pluske, “A Novel Barley β-Glucan Extract (Glucageltm) in Combination with Flax or Coconut Oil Influences Cholesterol and Triglyceride Levels in Growing Rats,” Proceedings of Nutrition and Society of Australia, Vol. 24, 2000, pp. 209-212.
- D. Rieckhoff, E. A. Trautwein, Y. Malkki and H. F. Ebersdobler, “Effects of Different Cereal Fibers on Cholesterol and Bile Acid Metabolism in the Syrian Golden Hamster,” Cereal Chemistry, Vol. 76, No. 5, 1999, pp. 788-795. doi:10.1094/CCHEM.1999.76.5.788
- P. J. Wood, J. T. Braaten, F. W. Scott, K. D. Riedel, M. S. Wolynetz and M. W. Collins, “Effect of Dose and Modification of Viscous Properties of Oat Gum on Plasma Glucose and Insulin Following an Oral Glucose Load,” British Journal of Nutrition, Vol. 72, No. 5, 1994, pp. 731-743. doi:10.1079/BJN19940075
- P. J. Wood, “Beer, Functional Oat Products,” In: G. Mazza, Ed., Functional Foods-Biochemical and Processing Aspects, Technomic Publishing Company, Inc., Lancaster, 1998, pp. 1-37.
- Y. Malkki, K. Autio, O. Hanninen, O. Myllymaki, K. Pelkonene, T. Suortti and R. Torronen, “Oat Bran Concentrates: Physical Properties of Beta-Glucan and Hypocholesterolemic Effects in Rats,” Cereal Chemistry, Vol. 69, No. 6, 1992, pp. 647-653.
- W. H. Yokoyama, B. E. Knuckles, D. Wood and G. E. Inglett, “Food Processing Reduces Size of Soluble Cereal β-Glucan Polymers without Loss of Cholesterol-Reducing Properties,” In: T.-C. Lee, Ed., Bioactive Compounds in Foods-Effects of Processing and Storage, ACS Symposium Series 816, American Chemical Society, Washington DC, 2002, pp. 105-116. doi:10.1021/bk-2002-0816.ch008
- T. A. Wilson, R. J. Nicolosi, B. Delaney, K. Chadwell, V. Moolchandani, T.K. Kotyla, G.H. Zheng, R. Hess, N. Knutson, L. Curry, L. Kolberg, M. Goulson and K. Ostergren, “Comparative Effects of Reduced and High Molecular Weight Barley β-Glucans on Early Atherosclerosis Risk Factors and Aortic Cholesterol Ester Accumulation in Hypercholesterolemic Syrian Golden Hamsters,” Journal of Nutrition, Vol. 134, No. 10, 2004, pp. 2617- 2622.
- P. A. Judd and A. S. Truswell, “The Effects of Rolled Oats on Blood Lipids and Fecal Steroid Excretion in Man,” American Journal of Clinical Nutrition, Vol. 34, No. 10, 1981, pp. 2061-2067.
- B. P. Daggy, N. C. O’Connell, G. R. Jerdack, B. A. Stinson and K. D. R. Setchell, “Additive Hypocholesterolemic Effect of Psyllium and Cholestyramine in the Hamster: Influence on Fecal Sterol and Bile Acid Profiles,” Journal of Lipid Research, Vol. 38, No. 3, 1997, pp. 491-502.
NOTES
*There is no conflict of interest to declare. All authors confirm that there is no professional affiliation, financial agreement, or other involvement with any company whose product figures prominently in the submitted manuscript.
#Corresponding author.

