Food and Nutrition Sciences
Vol. 3 No. 11 (2012) , Article ID: 24495 , 6 pages DOI:10.4236/fns.2012.311202
Soluble Fiber Improves Management of Diarrhea in Elderly Patients Receiving Enteral Nutrition
![]()
1Department of Neuropsychopharmacology and Hospital Pharmacy, Nagoya University Graduate School of Medicine, Nagoya, Japan; 2School of Pharmacy, Kinjo Gakuin University, Nagoya, Japan; 3Department of Gastroenterology and Hepatology, Mie University Graduate School of Medicine, Tsu, Japan; 4Kameyama Kaisei Hospital, Kameyama, Japan.
Email: *kato-y@med.nagoya-u.ac.jp
Received September 24th, 2012; revised October 24th, 2012; accepted November 3rd, 2012
Keywords: Enteral Nutrition; Soluble Dietary Fiber; Diarrhea; Plasma Short-Chain Fatty Acids; Plasma Diamine Oxidase; Elderly Patients
ABSTRACT
Dietary fiber is a non-digestible carbohydrate providing beneficial effects for bowel health. The aim of this study was to evaluate the clinical effects of fiber supplementation in enteral feeding on elderly patients suffering from diarrhea. This study was conducted in 15 patients (7 men and 8 women, 79.0 ± 7.5 years) who had loose stools or diarrhea during enteral nutrition. The enteral formula was supplemented with soluble dietary fiber (5.2 g/day) for 3 weeks, which was then discontinued for 1 week to confirm its effects. The effects of soluble dietary fiber on stool frequency, the Bristol Stool Form Scale (which is designed to measure stool consistency), plasma diamine oxidase (DAO) activity, and concentrations of plasma short-chain fatty acids (SCFA) were evaluated. After supplementation with soluble dietary fiber, there were no significant differences in stool frequency but there was a significant improvement in stool consistency (P < 0.05). Furthermore, ingestion of soluble dietary fiber resulted in increased plasma DAO activity and significantly increased levels of plasma SCFA (P < 0.05). Supplementation with soluble dietary fiber may be beneficial for improving stool consistency in patients suffering from diarrhea during enteral nutrition. A further controlled trial is warranted to examine the preventive effects of soluble dietary fiber in patients suffering from diarrhea.
1. Introduction
Enteral nutrition support is required when oral food intake is insufficient or is likely to be absent for a period of more than 5 - 7 days. Furthermore, support may be required in patients with insufficient oral food intake over longer periods [1]. Patients are suitable for longer term enteral nutrition if they have a functioning and accessible gastrointestinal tract. Thus, enteral nutrition is used for in-patients and out-patients in a wide range of disease states [2].
Diarrhea during enteral nutrition is a common complication. Whelan et al. reported that the incidence of diarrhea was 2% - 95% in enteral feeding cases [3]. Gastrointestinal symptoms during enteral nutrition can be influenced by various factors, such as administration method of the nutrient (composition, temperature, and rate of application), antibiotic prescription, abnormal secretion of water, enteropathogenic colonization, and the disease state [4-7]. The administration of low-residue formulas in enteral nutrition has been reported to induce small intestinal mucosal atrophy or enhance intestinal tract permeability [8,9].
Dietary fiber is a non-digestible carbohydrate that selectively stimulates the activity and/or growth of intestinal flora. Dietary fiber can be fermented into methane, hydrogen, carbon dioxide, and short-chain fatty acids (SCFA) by intestinal flora [10]. Fermentability of soluble fiber by intestinal flora is generally much greater than that of insoluble fiber [11]. SCFA, the most important energy substance for the colonic mucosal epithelium, promotes the absorption of water and sodium, regulates bowel function, and reverses colonic fluid secretion induced by enteral nutrition [12]. Therefore, dietary fiber supplementation has been recommended to normalize bowel function, improve feeding tolerance, and reduce diarrhea in patients receiving enteral nutrition [13]. (Elia, 2008, Systematic review and meta-analysis: the clinical and physiological effects of fibre-containing enteral formulae)However, the efficacy of dietary fiber supplementation has not been examined in detail.
In this study, we determined whether supplementation with soluble dietary fiber in an enteral formula improves diarrhea or loose stools in tube-fed patients. The clinical availability of soluble dietary fiber was evaluated by measuring the frequency of bowel movements, fecal features, plasma diamine oxidase (DAO) activity, which reflects the integrity and maturity of the small intestinal mucosa [14], and concentration of plasma SCFA.
2. Materials and Methods
2.1. Subjects
This study was conducted in 15 elderly in-patients of the Kameyama Kaisei hospital. Patients in medical wards necessitating enteral nutrition through a gastrostomy tube with loose stools or diarrhea were eligible. Patients with gastrointestinal diseases, or those using antibiotics, antidiarrheal agents, or probiotics were excluded from the study. All patients, or their legal representative, received an explanation of the study and gave their informed consent. The study was approved by the Human Research Ethics Committee of Kinjo Gakuin University.
2.2. Enteral Formula
Patients received fiber-free formula for 1 week (before fiber administration), then fiber-enriched formula for 3 weeks (fiber administration period), and then fiber-free formula for 1 week (discontinue fiber). During the fiberfree formula, patients received an enteral formula (K-2S, Kewpie Co.) that contained, per 100 mL feed; 100 kcal, 3.5 g protein, 93 mg sodium, 74 mg potassium, 60 mg calcium, 41 mg phosphorus, and was fiber free (Table 1). During the fiber-enriched formula, patients received fiber-free formula supplemented with soluble dietary fiber 5.2 g per day. A soluble dietary fiber, EDF (Fibro Co., Ltd.), was used. EDF is a powder containing 2.6 g of psyllium per package. This product contains 1.0 - 5.0 mg of sodium, has 2.9 kcal, and can be easily dissolved in water. Both fiber-free and fiber-enriched formula were administered twice a day through a gastrostomy tube with a continuous injector.
2.3. Blood Sample
To determine plasma DAO activity and SCFA concentration, a 5 mL blood sample was collected in the morning after an overnight fast the day before administration of fiber and then at weekly intervals after fiber administration, including 1 week after discontinuation. Blood samples were centrifuged (1000 × g, for 10 min) and plasma aliquots were stored at −20˚C until determination.
2.4. Fecal Analysis
Patients were asked to fill out a daily questionnaire reporting the frequency of their bowel movements and stool consistency. Stool consistency was assessed using the Bristol Stool Form Scale [15]. The Bristol Stool Form Scale was devised on the basis of 7 categories in
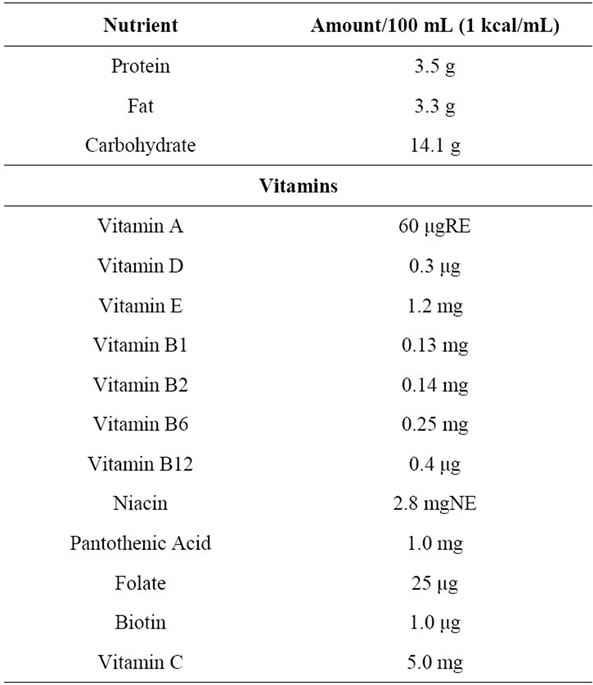
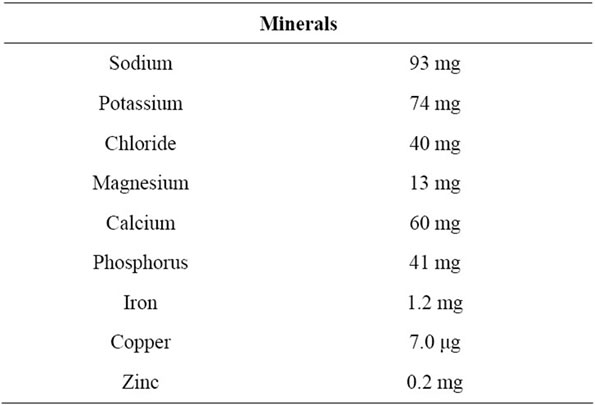
Table 1. Enteral nutrition constituents administered.
which stools were scored according to cohesion and crack, as follows: 1) separate hard lumps, like nuts; 2) sausage-shaped, but lumpy; 3) like a sausage but with cracks on its surface; 4) like a sausage or snake, and smooth and soft; 5) soft blobs with clear-cut edges; 6) fluffy pieces with ragged edges, a mushy stool; and 7) watery, no solid pieces, entirely liquid.
2.5. Plasma DAO Activity
Plasma DAO activity was measured using a high sensitivity colorimetric method according to Takagi et al. [16]. Briefly, a 0.04 mL sample was incubated with 0.6 mL of 25 mmol/L piperazine-N,N 9-bis(2-ethanesulphonic acid) buffer (pH 7.2) at 37˚C for 30 min. Then, 0.6 mL of 25 mmol/L 2-(4-morpholino) ethanesulphonic acid with 0.1 mol/L DA-67, 2.4 units/L peroxidase and 5000 units/L ascorbate oxidase was added to the mixture and incubation was allowed to proceed for 60 min. The enzyme reaction was stopped by the addition of 0.04 mL of 30 mmol/L sodium diethyldithiocarbamate and absorbance was measured at 668 nm.
2.6. SCFA Analysis
The concentration of plasma SCFA was measured using high performance liquid chromatography (HPLC) [17]. The HPLC apparatus used was a Shimadzu LC-20A system (Shimadzu, Kyoto, Japan). The UV-VIS detector was set to 400 nm and a YMC-Pack FA column (6.0 × 250 mm; YMC, Kyoto, Japan) was used with a column oven (CTO-20AC; Shimadzu) heated to 50˚C. The mobile phase consisted of acetonitrile-methanol-water (30: 16:54 v/v, pH 4 - 5 adjusted by 0.01 N HCl) and the flow rate was 1.2 mL/min. Ethanol (400 μL) was added to 100 μL of each plasma sample and vortexed. After centrifugation at 14,000 rpm for 20 min, the supernatant was added to 200 μL of an internal standard (50 μM) and fatty acid was pre-labeled using a Shortand Long-Chain Fatty Acid Analysis Kit (YMC, Kyoto, Japan). SCFA derivatives were extracted with n-hexane and diethyl ether, and were then evaporated to dryness. The residue was reconstituted with methanol and injected into the HPLC system.
2.7. Data Analysis
All data are expressed as means ± standard error, and statistical analysis was performed using Statview version 5.0, or the statistical software R version 2.10.1. To compare continuous variables, a one-way analysis of variance followed by Dunnett’s test for post-hoc analysis was performed. Steel’s test was used to compare ordered variables. Differences between means were considered to be significant at a P-value of <0.05.
3. Results
3.1. Patients
Fifteen patients (7 men and 8 women) necessitating enteral nutrition through a gastrostomy tube due to cerebrovascular diseases (e.g. cerebral infarction, intracerebral hemorrhage, and subarachnoid hemorrhage) were recruited for this study; however, one patient was subsequently excluded due to the discontinuation of enteral feeding. The remaining 14 patients (7 men and 7 women) were aged 79.0 ± 7.8 years.
3.2. Frequency of Bowel Movements
Changes in bowel frequency are shown in Figure 1. There were no significant differences in bowel frequency between baseline and after soluble fiber supplementation.
3.3. Stool Consistency
Figure 2 shows the differences in stool consistency. The Bristol Stool Form Scale was sequentially lower during the 3-week fiber administration period than that before soluble fiber supplementation, and these changes were significant after 3 weeks of fiber supplementation (4.86 ± 0.66 on the 3rd week vs. 5.67 ± 0.52 at baseline; P < 0.05 versus the baseline). The score increased one week after the discontinuation of fiber administration.
3.4. Plasma DAO Activity
Differences in plasma DAO activity are shown in Figure 3. Plasma DAO activity increased sequentially during fiber administration, and then decreased after discontinuation. No significant difference was observed between baseline and after fiber administration.

Figure 1. Changes in stool frequency after supplementation with dietary fiber. Values are presented as mean ± standard error of the mean (n = 14).
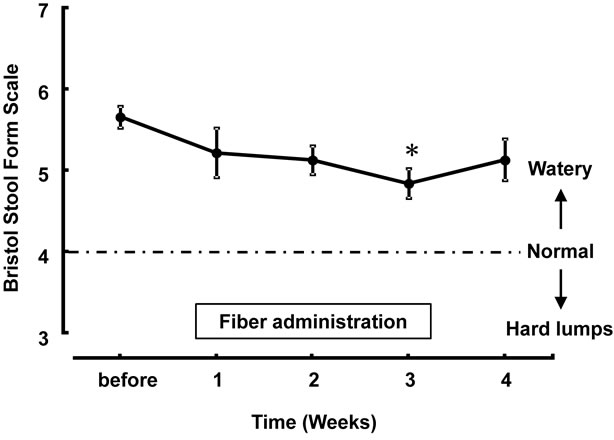
Figure 2. Changes in Bristol Stool Form Scale after supplementation of dietary fiber. Values are presented as mean ± standard error of the mean (n = 14). *P < 0.05, significantly different from the level before use.
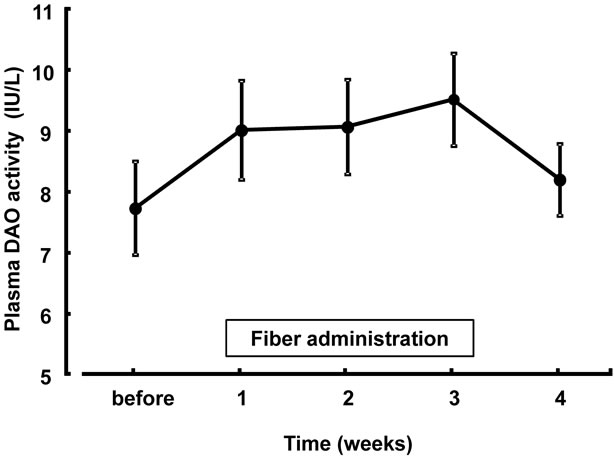
Figure 3. Changes in plasma DAO activity after supplementation with dietary fiber. Values are presented as mean ± standard error of the mean (n = 14). Normal plasma DAO levels of 50 volunteers were 10.0 ± 1.5 IU/L. DAO, diamine oxidase.
3.5. Concentration of Plasma SCFA
Changes in the concentration of plasma SCFA in patients during and after the cessation of fiber administration are illustrated in Figure 4. No significant amounts of propionate or butyrate were detected at any time point, whereas acetate was detectable in the plasma of patients. The concentration of plasma SCFA (acetate) was sequentially higher during fiber administration than that before fiber supplementation. The concentration of plasma SCFA was significantly higher after 3 weeks of fiber supplementation (27.35 ± 3.16 on the 3rd week vs. 33.50 ± 2.83 on the baseline; F(4.13) = 2.80, P < 0.05 versus the baseline) and was lower after discontinuation than that after 3 weeks of fiber supplementation.
4. Discussion
Diarrhea is the most common problem associated with enteral tube feeding and can lead to dehydration with a subsequent negative impact on healthcare costs, includeing nursing time and prolonged length of hospital stay. Although the causes of diarrhea during enteral nutrition are multiple and often poorly understood, the absence of dietary fiber in enteral nutrition has been implicated as a cause for diarrhea [18].
Dietary fiber is a type of carbohydrate that cannot be digested by the human digestive process. However, upon reaching the colon, anaerobic bacteria are able to degrade some dietary fiber through fermentation [10]. A soluble dietary fiber product was used in this study because the fermentability of soluble fiber by intestinal flora is generally much greater than that of insoluble fiber [11]. In addition, EDF can be easily and evenly mixed with other enteral nutrients.
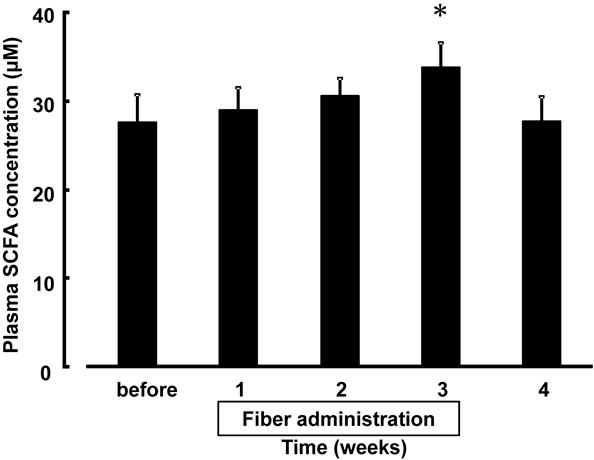
Figure 4. Changes in the concentration of plasma SCFA after supplementation with dietary fiber. Values are presented as mean ± standard error of the mean (n = 14). *F(4.13) = 2.80, P < 0.05, significantly different from the level before use. SCFA, short-chain fatty acids.
In the present study, bowel frequency was unaffected by fiber supplementation. Meta-analysis conducted mainly on intensive care unit and surgical patients revealed that fiber-containing feeds had no significant effect on bowel frequency, but meta-regression analysis demonstrated a significant moderating effect of fiber on bowel frequency [12]. In other words, fiber supplementation can decrease bowel frequency in those with high bowel frequency, and increase it in those with low bowel frequency. Thus, our study supports existing data that shows bowel movement is not altered by fiber supplementation.
Fibre administration significantly decreased the Bristol Stool Form Scale: we observed normal stools after 3 weeks of fiber supplementation, with gradual but significant improvements in fecal forms. This observation agrees with previous reports on the effects of psyllium [19,20]. It has been demonstrated that supplementation with psyllium is associated with an increase in the water-holding capacity of stool solids. We suggest that the patients in our study may have experienced an improvement in stool water-holding capacity due to dietary fiber intake.
DAO is an enzyme that deaminates histamine and polyamines. DAO activity is at its highest in the small intestinal mucosa and shows only very low activity in all other tissues [21]. It is also specifically localized in the tip of mature villus cells [22]. Thus, mucosal DAO activity reflects the integrity and maturity of the small intestinal mucosa [23]. In addition, it has been reported that plasma DAO levels correlate with mucosal DAO content and structural changes in the intestinal mucosa [13]. Thus, plasma DAO activity has been considered to reflect the integrity and maturity of the small intestinal mucosa. Hosoda et al. reported that total parenteral nutrition, several types of enteral feeding, and elemental diets cause atrophy of the digestive tract mucosa, whereas the addition of dietary fiber prevents atrophy of the mucosal epithelium in rats [9]. In this clinical study, changes in plasma DAO activity before and after administration of dietary fiber were not significantly different; however, plasma DAO activity tended to increase after administration of fiber. Therefore, dietary fiber may have some preventive effect on mucosal epithelial atrophy in the intestine.
SCFA are the main by-products of fermentation of non-starch polysaccharides by resident anaerobic microbiota in the colon. SCFA, such as acetate, propionate and butyrate, are produced mainly by intestinal bacteria in the process of digesting dietary fiber [24,25]. Acetate levels are quantitatively the highest, followed by propionate and butyrate. These fatty acids comprise approximately 90% of the overall SCFA produced in the digestive tract [26]. Increases in the level of SCFA have been shown to reduce stool water during enteral nutrition [27]. Additionally, most SCFA are absorbed by the large intestine and thus promote the proliferation of digestive tract mucosal epithelial cells, which are then used as an energy source in the digestive tract [11]. Some studies showed a significant increase in the concentrations of fecal SCFA and this may reflect supplementation with fiber [28,29]. However, these concentrations may be insufficient as indicators of SCFA production because most SCFA produced in the colon are absorbed. In the present study, we attempted to estimate the concentration of plasma SCFA. No significant amounts of propionate or butyrate were detected in the plasma, which is compatible with high colonic metabolism and/or hepatic clearance of these compounds [30]. Plasma acetate concentrations were significantly increased after fiber supplementation, suggesting that acetate concentrations in the plasma may reflect SCFA produced by fiber fermentation in the colon.
In summary, supplementation of soluble fiber may increase the production of SCFA by fiber fermentation, promote proliferation of intestinal mucosal epithelial cells, and prevent mucosal epithelial atrophy. This may result in an increase in plasma DAO activity, which reflects morphological changes in the small intestinal villous tissues, and fecal forms may be improved because supplementation with fiber increases the water-holding capacity.
5. Conclusion
Our results suggest that supplementation with soluble dietary fiber in patients who develop diarrhea or loose stools during enteral nutrition serve to improve the management of diarrhea. Based on the present findings in a small sequential self-controlled study, a further controlled clinical trial is warranted to examine the preventive effects of soluble dietary fiber.
REFERENCES
- M. Stroud, H. Duncan and J. Nightingale, “Guidelines for Enteral Feeding in Adult Hospital Patients,” Gut, Vol. 52, Suppl. 7, 2003, pp. vii1-vii12.
- C. B. Pearce and H. D. Duncan, “Enteral Feeding. Nasogastric, Nasojejunal, Percutaneous Endoscopic Gastrostomy, or Jejunostomy: Its Indications and Limitations,” Postgraduate Medical Journal, Vol. 78, 2002, pp. 198-204. doi:10.1136/pmj.78.918.198
- K. Whelan, “Enteral-Tube-Feeding Diarrhoea: Manipulating the Colonic Microbiota with Probiotics and Prebiotics,” The Proceedings of the Nutrition Society, Vol. 66, No. 3, 2007, pp. 299-306. doi:10.1017/S0029665107005551
- G. Bleichner, H. Bléhaut, H. Mentec and D. Moyse, “Saccharomyces Boulardii Prevents Diarrhea in Critically Ill Tube-Fed Patients. A Multicenter, Randomized, Double-Blind Placebo-Controlled Trial,” Intensive Care Medicine, Vol. 23, No. 5, 1997, pp. 517-523. doi:10.1007/s001340050367
- P. A. Guenter, R. G. Settle, S. Perlmutter, P. L. Marino, G. A. Desimone and R. H. Rolandelli, “Tube Feeding-Related Diarrhea in Acutely Ill Patients,” Journal of Parenteral and Enteral Nutrition, Vol. 15, No. 3, 1991, pp. 277-280. doi:10.1177/0148607191015003277
- T. E. Bowling, A. H. Raimundo, G. K. Grimble and D. B. Silk, “Colonic Secretory Effect in Response to Enteral Feeding in Humans,” Gut, Vol.35, No. 12, 1994, pp. 1734-1741. doi:10.1136/gut.35.12.1734
- D. Z. Bliss, S. Johnson, K. Savik, C. R. Clabots, K. Willard and D. N. Gerding, “Acquisition of Clostridium Difficile and Clostridium Difficile-Associated Diarrhea in Hospitalized Patients Receiving Tube Feeding,” Annals of Internal Medicine, Vol. 129, No. 12, 1998, pp. 1012- 1019.
- R. M. Goldstein, T. Hebiguchi, G. D. Luk, F. Taqi, T. R. Guilarte, F. A. Franklin et al., “The Effects of Total Parenteral Nutrition on Gastrointestinal Growth and Development,” Journal of Pediatric Surgery, Vol. 20, No. 6, 1985, pp. 785-791. doi:10.1016/S0022-3468(85)80044-0
- N. Hosoda, M. Nishi, M. Nakagawa, Y. Hiramatsu, K. Hioki and M. Yamamoto, “Structural and Functional Alterations in the Gut of Parenterally or Enterally Fed Rats,” The Journal of Surgical Research, Vol. 47, No. 2, 1989, pp. 129-133. doi:10.1016/0022-4804(89)90076-0
- J. H. Cummings, “Dietary Fiber,” British Medical Bulletin, Vol. 37, No.1, 1981, pp. 65-70.
- L. D. Bourquin, E. C. Titgemeyer and G. C. Fahey, “Fermentation of Various Dietary Fiber Sources by Human Fecal Bacteria,” Nutrition Research, Vol. 16, 1996, pp. 1119-1131. doi:10.1016/0271-5317(96)00116-9
- J. M. W. Wong, R. de Souza, C. W. C. Kendall, A. Emam and D. J. A. Jenkins, “Colonic Health: Fermentation and Short Chain Fatty Acids,” Journal of Clinical Gastroenterology, Vol. 40, No. 3, 2006, pp. 235-243. doi:10.1097/00004836-200603000-00015
- M. Elia, M. B. Engfer, C. J. Green and D. B. Silk, “Systematic Review and Meta-Analysis: The Clinical and Physiological Effects of Fibre-Containing Enteral Formulae,” Alimentary Pharmacology and Therapeutics, Vol. 27, No. 2, 2008, pp. 120-145. doi:10.1111/j.1365-2036.2007.03544.x
- G. D. Luk, T. M. Bayless and S. B. Baylin, “Diamine Oxidase (Histaminase). A Circulating Marker for Rat Intestinal Mucosal Maturation and Integrity,” Journal of Clinical Investigation, Vol. 66, No. 1, 1980, pp. 66-70. doi:10.1172/JCI109836
- S. J. Lewis and K. W. Heaton, “Stool form Scale as a Useful Guide to Intestinal Transit Time,” Scandinavian Journal of Gastroenterology, Vol. 32, No. 9, 1997, pp. 920-924. doi:10.3109/00365529709011203
- K. Takagi, M. Nakao, Y. Ogura, T. Nabeshima and A. Kunii, “Sensitive Colorimetric Assay of Serum Diamine Oxidase,” Clinica Chimica Acta, Vol. 226, No. 1, 1994, pp. 67-75. doi:10.1016/0009-8981(94)90103-1
- H. Miwa, C. Hiyama and M. Yamamoto, “High-Performance Liquid Chromatography of Short-and Long-Chain Fatty Acids as 2-Nitrophenylhydrazides,” Journal of Chromatography, Vol. 321, 1985, pp. 165-174. doi:10.1016/S0021-9673(01)90433-9
- K. Shankardass, S. Chuchmach, K. Chelswick, C. Stefanovich, S. Spurr, J. Brooks et al., “Bowel Function of Long-Term Tube-Fed Patients Consuming Formulae with and without Dietary Fiber,” Journal of Parenteral and Enteral Nutrition, Vol. 14, No. 5, 1990, pp. 508-512. doi:10.1177/0148607190014005508
- M. I. McBurney, “Potential Water-Holding Capacity and Short-Chain Fatty Acid Production from Purified Fiber Sources in a Fecal Incubation System,” Nutrition, Vol. 7, No. 6, 1991, pp. 421-424.
- H. H. Wenzl, K. D. Fine, L. R. Schiller and J. S. Fordtran, “Determinants of Decreased Fecal Consistency in Patients with Diarrhea,” Gastroenterology, Vol. 108, 1995, pp. 1729-1738. doi:10.1016/0016-5085(95)90134-5
- T. Biegański, “Biochemical, Physiological and Pathophysiological Aspects of Intestinal Diamine Oxidase,” Acta Physiologica Polonica, Vol. 34, No. 1, 1983, pp. 139-154.
- L. D’Agostino, G. D’Argenio, C. Ciacci, B. Daniele, V. Macchia and G. Mazzacca, “Diamine Oxidase in Rat Small Bowel: Distribution in Different Segments and Cellular Location,” Enzyme, Vol. 31, No. 4, 1984, pp. 217-220.
- M. C. Wolvekamp and R. W. de Bruin, “Diamine Oxidase: An Overview of Historical, Biochemical and Functional Aspects,” Digestive Diseases, Vol. 12, No. 1, 1994, pp. 2-14. doi:10.1159/000171432
- J. H. Cummings and G. T. Macfarlane, “Role of Intestinal Bacteria in Nutrient Metabolism,” Journal of Parenteral and Enteral Nutrition, Vol. 21, No. 6, 1997, pp. 357-365. doi:10.1177/0148607197021006357
- W. E. Roediger, “Role of Anaerobic Bacteria in the Metabolic Welfare of the Colonic Mucosa in Man,” Gut, Vol. 21, No. 9, 1980, pp. 793-798. doi:10.1136/gut.21.9.793
- D. L. Topping and P. M. Clifton, “Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides,” Physiological reviews, Vol. 81, No. 3, 2001, pp. 1031-1064.
- T. E. Bowling, A. H. Raimundo, G. K. Grimble and D. B. Silk, “Reversal by Short-Chain Fatty Acids of Colonic Fluid Secretion Induced by Enteral Feeding,” Lancet, Vol. 342, 1993, pp. 1266-1268. doi:10.1016/0140-6736(93)92360-6
- H. A. Majid, P. W. Emery and K. Whelan, “Faecal Microbiota and Short Chain Fatty Acids in Patients Receiving Enteral Nutrition with Standard or Fructo Oligosaccharides and Fibre Enriched Formulas,” Journal of Human Nutrition and Dietetics, Vol. 24, No. 3, 2011, pp. 260-268. doi:10.1111/j.1365-277X.2011.01154.x
- S. A. Kapadia, A. H. Raimundo, G. K. Grimble, P. Aimer and D. B. A. Silk, “Influence of Three Different FiberSupplemented Enteral Diets on Bowel Function and Short-Chain Fatty Acid Production,” Journal of Parenteral and Enteral Nutrition, Vol. 19, No. 1, 1995, pp. 63-68. doi:10.1177/014860719501900163
- J. H. Cummings, E. W. Pomare, W. J. Branch, C. P. Naylor and G. T. Macfarlane, “Short Chain Fatty Acids in Human Large Intestine, Portal, Hepatic and Venous Blood,” Gut, Vol. 28, No. 10, 1987, pp. 1221-1227. doi:10.1136/gut.28.10.1221
NOTES
*Corresponding author.

