Food and Nutrition Sciences
Vol. 2 No. 2 (2011) , Article ID: 4535 , 4 pages DOI:10.4236/fns.2011.22018
Induction of Apoptosis and Acetylation of Histone H3 and H4 by Arctigenin in the Human Melanoma Cell Line SK-MEL-28
![]()
Bioresource Science, Andong National University, Andong, Republic of Korea
E-mail: jhj@andong.ac.kr
Received February 5th, 2011; revised February 20th, 2011; accepted March 1st, 2011.
Keywords: Arctigenin, Apoptosis, Histone Acetylation, Caspases, Melanoma
ABSTRACT
Cutaneous melanoma is one of the most aggressive forms of skin cancer. Arctigenin, one of the major bioactive components of Arctii Fructus, has been reported to exhibit antio-xidant, antitumor and anti-inflammatory activities. In the present study, we investigated the effect of arctigenin on induction of apoptosis in highly metastatic SK-MEL-28 human melanoma cells. Arctigenin inhibited growth of SK-MEL-28 cells in a dose-dependent manner. Treatment of SK-MEL-28cells with arctigenin caused cleavage of caspases 3, 7 and 9, and poly (ADP-ribose) polymerase in a dose-dependent manner. Furthermore, acetylation of histone H3 and H4 in the SK-MEL-28 cells was dramatically increased by arctigenin treatment. Collectively, these findings indicate that arctigenin-induces apoptosis of SK-MEL-28 melanoma cells via activation of caspases and histone acetylation.
1. Introduction
Cancer presents constellation of diseases. For the primary prevention, therapeutic management and treatment, nutrition is a key factor. Decades of epidemiological studies have established a direct association between consumption of plant-based diets and a reduction in risk of cancer [1-4]. In recent years, efforts directed at the identification of bioactive ingredients in those diets and delineation of their mechanism(s) have demonstrated that even ubiquitous, non-nutritional secondary plant metabolites, such as flavonoids and polyphenolics widely present in foods consumed in the US, have significant health consequences [5-10].
Arctigenin, one of the major bioactive components from the fruits of the burdock, has been reported to exhibit antioxidant, antitumor and anti-inflammatory activities as a phenylpropanoid dibenzylbutyrolactone lignan [11-13]. Arctigenin occurs in a variety of plants including Bardanae fructus (Goboshi), Saussurea medusa (Compositae), T. nucifera and tropical climbing shrub Ipomea cairica [14-17].
Cutaneous melanoma is one of the most aggressive forms of skin cancer with high metastatic potential and strong resistance to radiation, immunotherapy and chemotherapy [18]. The role of diet and nutrition in prevention of cutaneous melanoma has recently become a very popular subject. An increasing number of studies have examined the effect of dietary manipulation and vitamin intake on cancer prevention, especially the use of dietary supplements [19]. Melanoma represents an enormous medical challenge. The incidence of melanoma in the USA is at least 50 000 new cases per year, or about 3% of all newly diagnosed cancers. Approximately 1 in 87 Americans alive today will develop melanoma in their lifetime, and the incidence of melanoma continues to increase faster than any other cancer [20]. The incidence of melanoma is rising at an alarming rate and has become a major public health concern in many countries. Despite extensive research the precise molecular determinants responsible for melanoma progression are yet to be delineated. Recent studies, however, have indicated that melanoma cells show dysfunction in the apoptotic program [21] which provided exciting new targets for rationally designed anti-melanoma therapeutic strategies. Apoptosis is a programmed cell death. It is executed by the activation of caspases, a family of cytoplasmic cysteine proteases. Caspase-3, caspase-7 and caspase-9, key components of the apoptotic machinery, cleave several cellular proteins including poly (ADP-ribose) polymerase (PARP) [22]. Also, the hyper-acetylation of histone plays a role in apoptosis.
In this study, we investigated whether arctigenin affected histone acetylation and the activation of caspases as part of a study for anti-cancer effect and its molecular mechanisms of arctigenin. This is the first report describing the effect of arctigenin on apoptosis of melanoma cells by inducing histone acetylation and activating caspases.
2. Materials and Methods
2.1. Chemical Regents
Arctigenin (C21H24O6) was purchased from Fluka Chemical Co. (USA). Anti-acetylated H3 lysine 9, anti-acetylated H3 lysine 14, anti-acetylated H4 lysine 8, anti-acetylated H4 lysine 12, anti-cleaved caspase 3, anti-cleaved caspase 7, anti-cleaved caspase 9, and anti-cleaved PARP were purchased from Cell Signaling Technology (USA). The secondary antibodies, HRP-Goat-anti rabbit IgG were purchased from Cell Signaling Technology (USA). Caspase-3 activity assay kit was purchased from Biovision (USA). All electrophoresis chemicals were purchased from Bio-Rad Labs (USA).
2.2. Cell Culture
The human melanoma cell line SK-MEL-28 was purchased from the American Type Culture Collection (ATCC). SK-MEL-28 cells were cultured in Dulbeco’s modified Eagle’s medium (DMEM) supplemented with 100 U/ml of penicillin, 100 µg/ml of streptomycin and 10% fetal bovine serum. The cells were incubated in an atmosphere of 5% CO2 at 37˚C and subcultured every 2 days. In all experiments, cells were grown to 80% - 90% confluence and subjected to no more than 20 cell passages.
2.3. MTT Assay for Cell Viability
The SK-MEL-28 cells (1 × 105) were seeded in each well of 96-well plates. After incubation with 0.5 μM, 1 μM, 5 μM and 10 μM arctigenin for 48 hours, the MTT solution (1 mg/ml in PBS) was added (50 μl/well). The plate was incubated for an additional 4 h at 37˚C, and the formazan crystals produced were dissolved in 100 μl of DMSO. Absorbance was measured by an enzyme-linked immunosorbent assay reader at 570 nm. The given values were calculated from the mean of three different experiments. Growth inhibition was estimated as the reduction in values from a DMSO control.
2.4. Western Blot
The SK-MEL-28 cells (5 × 105) were treated with arctigenin for 48 hours at 37˚C. After 48 hours, cell lysates were prepared in 100 μl lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 5 mM phenylmethylsulfonyl fluoride [PMSF], and 1 mM DTT) containing 1% Triton X-100. Insoluble material was removed by centrifugation at 15,000 g for 15 min at 4°C. The protein content in the supernatant was measured using a Bradford method. The protein extracts (50 μg/well) were separated by 15% SDS-PAGE and then transferred onto PVDF membranes. The membranes were blocked with 5% (w/v) non-fat dry milk in TBS-T (Tris-buffered saline containing 0.1% (v/v) Tween-20) at room temperature for 1 hour, and incubated overnight at 4°C with the primary antibody such as anti-cleaved-caspases (3, 7, 9), anti-cleaved-PARP, anti-acetylated H3 (lys 9, 14) and anti-acetylated H4 (lys 8, 12) (1 : 1000). Subsequently, the membranes were washed with TBS-T buffer, and blotted with secondary HRP-conjugated antibodies at room temperature for 1 hour and then washed again with TBS-T buffer. After washing, the membranes were treated with the detection agent (Amersham Biosciences) and immediately developed in Polaroid film.
2.5. Caspase-3 Activity Assay
After treatment with different concentration of arctigenin (0.5 µM, 1 µM, 5 µM and 10 µM), cells were washed with ice-cold PBS and lysed in lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 5 mM phenylmethylsulfonyl fluoride [PMSF], and 1 mM DTT) containing 1% Triton X-100 for 30 min on ice. Caspase-3 activity was determined using Caspase-3 colorimetric Assay kit according to the manufacturer protocol.
2.6. Statistical Analysis
For statistical analysis, data were analyzed by One-way ANOVA followed by Dunnett’s test. In western blot analysis, % relative density was calculated by the density using the software Un-SCAN-IT gel Version 5.1 (Silk Scientific, Inc.). Experiments were repeated at least three times.
3. Results
3.1. Effect of Arctigenin on Cell Viability
To determine the effect of arctigenin on cell viability, SK-MEL-28 cells were treated with the varying concentrations of arctigenin for 48 hours, and the cell viability was evaluated by the MTT assay. As shown in Figure 1, a significant decrease in cell viability was detected in a dose-dependent manner at 48 hours after the treatment.
3.2. Effect of Arctigenin on Activation of Caspases and PARP in SK-MEL-28 Cells
In a sequential manner, cytochrome c is released into the cytosol, where it recruits and activates procaspase-9 in the apoptosome [22]. Active caspase-9 cleaves and activates executioner caspases, including caspase-3 and 7, which cleave a broad spectrum of cellular target proteins, including PARP, thereby leading to cell death [22]. We evaluated whether induction of apoptosis by arctigenin through the activation of caspases 3, 7 and 9, and PARP by Western blot analysis. The treatment of arctigenin for 48 hours dose-dependently induced the cleavage of caspases 3, 7 and 9, and PARP in SK-MEL-28 cells (Figure 2). The activation of caspase-3 during arctigenin-induced apoptosis of SK-MEL-28 cells was further confirmed using a colorimetric caspase-3 activity assay. Arctigenin activated the caspase-3 activity in a dose-dependent manner (Figure 3).
3.3. Effect of Arctigenin on Acetylation of Histone H3 and H4 in SK-MEL-28 Cells
As core histones are mainly acetylated on specific lysine residues [23], directly the specific lysine acetylation of core histones H3 and H4 were examined. Histone H3 can be acetylated at lysines 9, 14, 23 and 28 at the N-terminal region [24]. Acetylation of each lysine within a histone tail is independently regulated [25], supporting the idea that each lysine has unique function. Acetylations of histone H3 at Lys9 and Lys14 play important roles in transcriptional activation and chromatin assembly [26]. As shown in Figure 4a, arctigenin strikingly increased the amount of acetylated histone H3 at lysine 9 and lysine 14 in a dose-dependent manner.
The acetylation of histone at lysine 8 and lysine 12 is involved in the regulation of transcription activation of several antiproliferative genes [27]. As shown in Figure 4b, arctigenin also induced hyper-acetylation of histone H4 at lysine 8 and lysine 12 in a dose-dependent manner.
4. Discussion
Apoptosis, also called the programmed cell death, is a specific form of cell death characterized by several morphological and biochemical events. Aberrant cell survival resulting from inhibition of apoptosis is expected to contribute to tumor progression and oncogenesis, and cancer cells often gain a selective growth advantage by blocking the apoptosis . The recent findings strongly suggest that resistance to apoptosis is also closely associated with metastatic potential of tumor cells . Therefore, induction of apoptosis of metastatic tumor cells is considered as one of the reasonable and promising chemopreventive or chemotherapeutic strategies. In the present study, we observ-ed that arctigenin induced SK-MEL-28 cell death.
Apoptosis may be triggered via either the death receptor or the mitochondrial pathway. In the mitochon
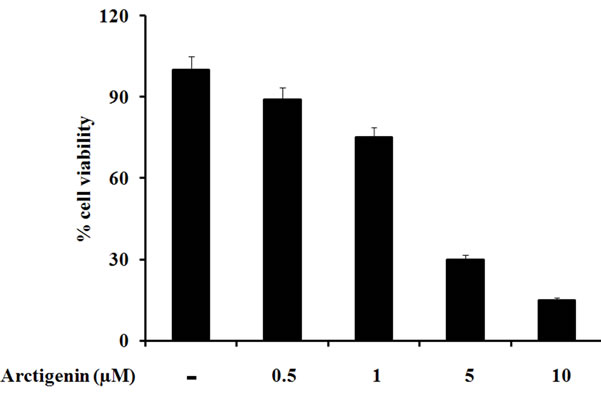
Figure 1. Effect of arctigenin on the SK-MEL-28 melanoma cell growth. The viability of cells was determined by the MTT assay as described in Materials and methods. The values of absorbance were calculated as % cell viability. The cells without arctigenin were treated with DMSO.
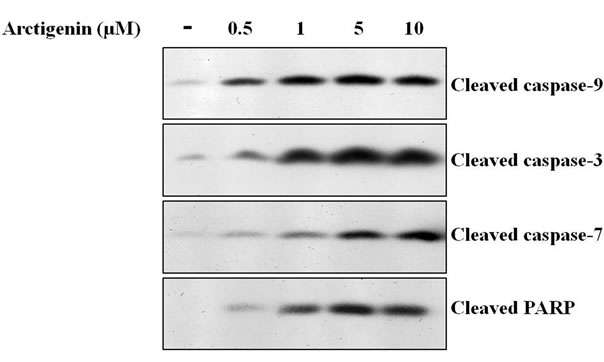
Figure 2. Effect of arctigenin on the cleavage of caspases 3, 7 and 9, and PARP. The cells were treated with arctigenin for 48 hours and 50 µg protein of cell lysates was subjected to SDS-PAGE as described in Materials and methods. The cells without arctigenin were treated with DMSO.
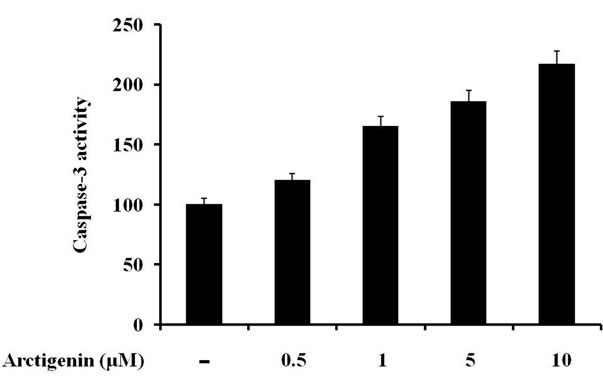
Figure 3. Effect of arctigenin on caspase-3 activation, values represented as percentage. The cells without arctigenin were treated with DMSO. Caspase-3 activity was determined using caspase-3 colorimetric assay kit according to the manufacturer protocol.
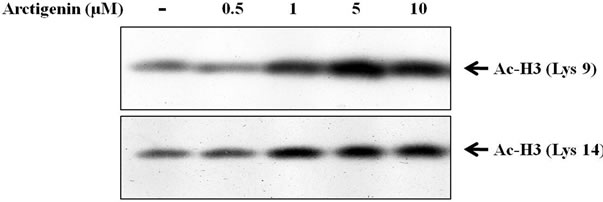 (a)
(a)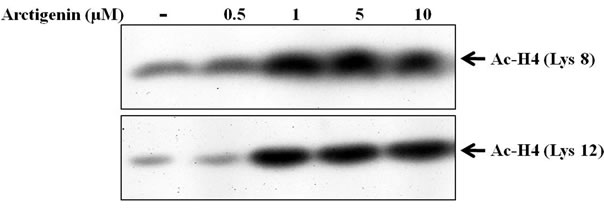 (b)
(b)
Figure 4. Effect of arctigenin on the acetylation of histone H3 and H4. The cells were treated with arctigenin for 48 hours and 50 µg protein of cell lysates was subjected to SDS-PAGE as described in materials and methods. The cells without arctigenin were treated with DMSO.
drial pathway, release of cytochrome c activates caspase-9 and subsequently caspases 3 and 7, leading to induction of apoptosis . We found that arctigenin induces cleavage of caspases 3, 9 and 7, and PARP. Thus, we conclude that arctigenin may induce apoptosis via the mitochondrial pathway.
In order to further elucidate the molecular mechanism responsible for the arctigenin-induced apoptosis in SK-MEL-28 cells, the effect of arctigenin on histone acetylation was analyzed. There is much work to suggest that the extent of histone acetylation can influence growth of cancer cells . A number of studies indicated that reduced proliferation of cancer cells was favored by agents that increase histone acetylation. Such compounds may have a role in cancer prevention or therapy .
In response to arctigenin, histone acetylation status increased after 48 hours of treatment to SK-MEL-28 cells. According to previous studies, acetylation of histone H3 at lysine 9 and H4 at lysine 8 was also involved in the regulation of transcription activation of several anti-proliferative genes . Arctigenin-induced histone hyper-acetylation could result either from a stimulation of HAT activity or an inhibition of HDAC activity. Several lines of evidence suggest that histone acetylation plays a role in the transcriptional regulation, probably by altering chromatin structure . The alteration of chromatin structure by histone acetylation has not been clarified but it may be important to explain the mechanism of regulation of gene expression by HDAC inhibitors. Thus, the molecular and cellular responses induced by arctigenin could be linked to its effect on histone acetylation and play a protective role on melanoma.
In summary, arctigenin suppresses the growth of the highly metastatic, SK-MEL-28 human melano-ma cells at least in part through induction of apoptosis mediated by activation of caspases and histone hyper-acetylation.
5. Acknowledgements
This work was supported by the second stage of BK21 grants from Ministry of Education and Human Resources Development, Korea.
REFERENCES
- R. Doll and R. Peto, “The Causes of Cancer: Quantitative Estimates of Avoidable Risks of Cancer in the United States Today,” Journal of National Cancer Institute, Vol. 66, No. 6, 1981, pp. 1191-1308.
- C. R. Wolf, “Chemoprevention: Increased Potential to Bear Fruit,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 98, No. 6, 2001, pp. 2941-2943. doi:10.1073/pnas.071042698
- M. B. Sporn and N. Suh, “Chemoprevention: An Essential Approach to Controlling Cancer,” Nature Reviews Cancer, Vol. 2, No. 7, 2002, pp. 537-543. doi:10.1038/nrc844
- V. L. Go, R. R. Butrum and D. A. Wong, “Diet, Nutrition, and Cancer Prevention: The Postgenomic Era,” Journal of Nutrition, Vol. 133, No. 11, 2003, pp. 3830S-3836S.
- C. S. Yang, J. M. Landau, M. T. Huang and H. L. Newmark, “Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds,” Annual Review of Nutrition, Vol. 21, 2001, pp. 381-406. doi:10.1146/annurev.nutr.21.1.381
- Y. Kuroda and Y. Hara, “Antimutagenic and Anticarcinogenic Activity of Tea Polyphenols,” Mutation Research, Vol. 436. No. 1, 1999, pp. 69-97.
- M. Nihal, N. Ahmad, H. Mukhtar and G. S. Wood, “Anti-proliferative and Proapoptotic Effects of epigallocatechin-3-gallate on Human Melanoma: Possible Implications for the Chemoprevention of Melanoma,” International Journal of Cancer, Vol. 114, No. 4, 2005, pp. 513-521. doi:10.1002/ijc.20785
- S. Caltagirone, C. Rossi, A. Poggi, F. O. Ranelletti, P. G. Natali, M. Brunetti, F. B. Aiello and M. Piantelli, “Flavonoids Apigenin and Quercetin Inhibit Melanoma Growth and Metastatic Potential,” International Journal of Cancer, Vol. 87, No. 4, 2000, pp. 595-600. doi:10.1002/1097-0215(20000815)87:4<595::AID-IJC21>3.0.CO;2-5
- J. A. Milner, S. S. McDonald, D. E. Anderson and P. Greenwald, “Molecular Targets for Nutrients Involved with Cancer Prevention,” Nutrition and Cancer, Vol. 41, No. 1-2, 2001, pp. 1-16. doi:10.1207/S15327914NC41-1&2_1
- G. J. Soleas, E. P. Diamandis and D. M. Goldberg, “Resveratrol: A Molecule Whose Time has Come? And gone?” Clinical Biochemistry, Vol. 30, No. 2, 1997, pp. 91-113. doi:10.1016/S0009-9120(96)00155-5
- S. Awale, J. Lu, S. K. Kalauni, Y. Kurashima, Y. Tezuka, S. Kadota and H. Esumi, “Identification of Arctigenin as an Antitumor Agent having the Ability to Eliminate the Tolerance of Cancer Cells to Nutrient Starvation,” Cancer Research, Vol. 66, No. 3, 2006, pp. 1751-1757. doi:10.1158/0008-5472.CAN-05-3143
- M. K. Cho, Y. P. Jang, Y. C. Kim and S. G. Kim, “Arctigenin, a Phenylpropanoid Dibenzylbutyrolactone Lignan, Inhibits MAP Kinases and AP-1 Activation via Potent MKK Inhibition: The Role in TNF-α Inhibition,” International Immunopharmacology, Vol. 4, No. 10-11, 2004, pp. 1419-1429. doi:10.1016/j.intimp.2004.06.011
- T. Matsumoto, K. Hosono-Nishiyama and H. Yamada, “Anti-proliferative and Apoptotic Effects of Butyrolactone Lignans from Arctium Lappa on Leukemic Cells,” Planta Medica, Vol. 72, No. 3, 2006, pp. 276-278. doi:10.1055/s-2005-916174
- Y. P. Jang, S. R. Kim, Y. H. Choi, J. Kim, S. G. Kim and G. J. Markelonis, T. H. Oh and Y. C. Kim, “Arctigenin Protects Cultured Cortical Neurons from Glutamate-Induced Neurodegeneration by Binding to Kainate Receptor,” Journal of Neuroscience Research, Vol. 68, No. 2, 2002, pp. 233-240. doi:10.1002/jnr.10204
- Y. P. Jang, S. R. Kim and Y. C. Kim, “Neuroprotective Dibenzylbutyrolactone Lignans of Torreya Nucifera,” Planta Medica, Vol. 67, No. 5, 2001, pp. 470-472. doi:10.1055/s-2001-15804
- S. Moritani, M. Nomura, Y. Takeda and K. Miyamoto, “Cytotoxic Components of Bardanae Fructus (Goboshi),” Biological & Pharmaceutical Bulletin, Vol. 19, No. 11, 1996, pp. 1515-1517.
- K. Umehara, M. Nakamura, T. Miyase, M. Kuroyanagi and A. Ueno, “Studies on Differentiation Inducers: VI. Lignan Derivatives from Arctium Fructus (2),” Chemical & Pharmaceutical Bulletin, Vol. 44, No. 12, 1996, pp. 2300-2304.
- J. F. Thompson, R. A. Scoley and R. F. Kefford, “Cutaneous Melanoma,” Lancet, Vol. 265, No. 9460, 2005, pp. 687-701.
- J. D. Jensen, G. J. Wing and R. P. Dellavalle, “Nutrition and Melanoma Prevention,” Clinics in Dermatology, Vol. 28, No. 6, 2010, pp. 644-649. doi:10.1016/j.clindermatol.2010.03.026
- T. M. Pawlik and V. K. Sondak, “Malignant Melanoma: Current State of Primary and Adjuvant Treatment,” Critical Reviews in Oncology/Hematology, Vol. 45, No. 3, 2003, pp. 245-264. doi:10.1016/S1040-8428(02)00080-X
- P. Hersey, “Apoptosis and Melanoma: How New Insights are Effecting the Development of New Therapies for Melanoma,” Current Opinion in Oncology, Vol. 18, No. 2, 2006, pp. 189-196. doi:10.1097/01.cco.0000208794.24228.9f
- Y. Shi, “Mechanisms of Caspase Activation and Inhibition during Apoptosis,” Molecular Cell, Vol. 9, No. 3, 2002, pp. 459-470. doi:10.1016/S1097-2765(02)00482-3
- V. A. Spencer and J. R. Davie, “Role of Covalent Modifications of Histones in Regulating Gene Expression,” Gene, Vol. 240, No. 1, 1999, pp. 1-12. doi:10.1016/S0378-1119(99)00405-9
- A. W. Throne, D. Kmiciek, P. Sautiere and C. Crane-Robinson, “Patterns of Histone Acetylation,” European Journal of Biochemistry, Vol. 193, No. 3, 1990, pp. 701-713. doi:10.1111/j.1432-1033.1990.tb19390.x
- D. G. Edmondson, J. K. Davie, J. Zhou, B. Mirnikjoo, K. Tatchell and S. Y. R. Dent, “Site-specific Loss of Acetylation upon Phosphorylation of Histone H3,” Journal of Biological Chemistry, Vol. 277, No. 33, 2002, pp. 29496-29502. doi:10.1074/jbc.M200651200
- B. D. Strahl and C. D. Allis, “The Language of Covalent Histone Modifications,” Nature, Vol. 403, No. 6765, 2000, pp. 41-45.
- N. Druesne, A. Pagniez, C. Mayeur, M. Thomas, C. Cherbuy, P. H. Duee, P. Martel and C. Chaumontet, “Diallyl Disulfide (DADS) Increases Histone Acetylation and p21(waf1/cip1) Expression in Human Colon Tumor Cell Lines,” Carcinogenesis, Vol. 25, No. 7, 2004, pp. 1227-1236.
- M. O. Hengartner, “The Biochemistry of Apoptosis,” Nature, Vol. 407, 2000, pp. 770-776. doi:10.1038/35037710
- D. Hanahan and R. A. Weinberg, “The Hallmarks of Cancer,” Cell, Vol. 100, No. 1, 2000, pp. 57-70. doi:10.1016/S0092-8674(00)81683-9
- R. J. Bold, S. Virudachalam and D. J. McConkey, “BCL2 Expression Correlates with Metastatic Potential in Pancreatic Cancer Cell Lines,” Cancer, Vol. 92, No. 5, 2001, pp. 1122-1129. doi:10.1002/1097-0142(20010901)92:5< 1122::AID-CNCR1429>3.0.CO;2-H
- H. S. Jun, T. Park, C. K. Lee, M. K. Kang, M. S. Park, H. I. Kang, Y. J. Surh and O. H. Kim, “Capsaicin Induced Apoptosis of B16-F10 Melanoma Cells through Downregulation of Bcl-2,” Food and Chemical Toxicology, Vol. 45, No. 5, 2007, pp. 708-715. doi:10.1016/j.fct.2006.10.011
- M. Mouria, A. S. Gukovskay, Y. Jung, P. Buechler, O. J. Hines, H. A. Reber and S. J. Pandol, “Food-derived Polyphenols Inhibit Pancreatic Cancer Growth through Mitochondrial Cytochrome c Release and Apoptosis,” International Journal of Cancer, Vol. 98, No. 5, 2002, pp. 761-769.
- U. Mahiknecht and D. Hoelzer, “Histone Acetylation Modifiers in the Pathogenesis of Malignant Disease,” Molecular Medicine, Vol. 6, No. 8, 2000, pp. 623-644.
- M. Grunstein, “Histone Acetylation in Chromatin Structure and Transcription,” Nature, Vol. 389, No. 6649, 1997, pp. 641-643.

