American Journal of Plant Sciences
Vol.5 No.18(2014), Article ID:48832,7 pages
DOI:10.4236/ajps.2014.518285
Linuron Biologically Effective Dose for Glyphosate-Resistant Giant Ragweed (Ambrosia trifida L.) Control in Soybean (Glycine max L.)
Kimberly D. Walsh, Nader Soltani*, Lynette R. Brown, Peter H. Sikkema
University of Guelph Ridgetown Campus, Ridgetown, Canada
Email: *nsoltani@uoguelph.ca
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 May 2014; revised 15 July 2014; accepted 8 August 2014
ABSTRACT
Glyphosate-resistant (GR) giant ragweed (Ambrosia trifida L.) was first identified in Canada in 2008 and has since been found throughout southwestern Ontario. Six field trials were conducted over a two-year period (2012, 2013) on Ontario farms with GR giant ragweed to evaluate the efficacy of linuron applied pre-plant (PP) in soybean (Glycine max (L.) Merr.). The dose required for 50%, 80%, and 95% GR giant ragweed control was 1238, 2959, and 6018 g·ai·ha−1 four weeks after application (WAA), respectively. The linuron dose needed for 50%, 80%, and 95% reduction in density was 1554, 3181, and 5643 g·ai·ha−1 and 1204, 2496, and 4452 g·ai·ha−1 for dry weight, respectively. Application of 7874 g·ai·ha−1 linuron was needed to obtain soybean yields that were 90% of the weed-free control; approximately 3.5 times the maximum field recommended dose. To achieve 95% and 98% yields, greater than 8640 g·ai·ha−1 linuron was required. Application of linuron plus glyphosate PP in soybean will help to control GR giant ragweed as well as reduce GR selection pressure.
Keywords:Giant Ragweed, Glyphosate-Resistant, Linuron, Soybean

1. Introduction
The selection of resistance in weeds to glyphosate is a significant hurdle towards the sustainability and continued utility of glyphosate-resistant (GR) crops. Largely due to the widespread adoption of GR crops, including soybean (Glycine max (L.) Merr.), corn (Zea mays L.), and canola (Brassica napus L.), over reliance on a single herbicide mode of action has resulted in a strong selection intensity favoring resistant weed biotypes [1] -[3] . The repeated use of glyphosate does not specifically change a weed, but rather selects for biotypes with natural genetic resistance to 5-enolpyruvylshikimate-3-phosphate synthase inhibition [4] . Consequently, weed community shifts are inevitable and are an inherent consequence of growing GR crops recurrently with concomitant use of glyphosate [4] . Annual weed species that are widely distributed, occur in high population densities, are prolific seed producers, and have efficient gene dissemination by means of seed or pollen have often been associated with propensity for herbicide resistance [5] .
Giant ragweed (Ambrosia trifida L.) is native to North America and frequently found throughout the eastern United States and central Canada [6] -[8] . This annual weed is herbaceous, erect, and best characterized by its immense stature, reaching heights of up to 5 m [9] . Considered noxious according to the Ontario Weeds Act, giant ragweed can be found growing in river valleys, meadows, roadsides, wastelands, fencerows, and fertile agricultural soils [6] [10] . Giant ragweed exhibits early emergence and vigorous growth which enables it to compete readily for resources and depress crop yields [6] [11] . Reference [12] reported that densities of only two plants per 9 m row could reduce soybean yields by up to 50%. It is therefore preferable that populations be managed early to prevent both crop losses due to competition, as well as limit giant ragweed seed bank additions.
Giant ragweed flowers from August to October in Ontario and is wind-pollinated [7] [13] . Giant ragweed plants are monoecious, having both male and female flowers present on individual plants [14] . The female, seed bearing flowers are clustered at the base of elongated groupings of the male, pollen producing flowers [7] [9] . Giant ragweed pollen is a trigger for allergies and a known contributor of hay fever [15] . The male flowers shed pollen in excess, which allows giant ragweed plants to cross-pollinate and create genetic variation within populations [7] . Genetic diversity is frequently associated with proclivity for resistance, as demonstrated in weeds belonging to Amaranthus, Conyza, Lolium, as well as Ambrosia genera [5] [16] .
In 2008, a population of giant ragweed from a field near Windsor, Ontario failed to be controlled by a standard glyphosate application. Upon investigation, resistance to glyphosate was confirmed at two times the field recommended dose [17] . In 2009 and 2010, a survey of southwestern Ontario further confirmed GR giant ragweed at 47 additional locations across three counties, indicating that the distribution of resistant plants was much broader than originally anticipated [18] . Resistant populations continued to be identified, with 34 more sites confirmed in 2012, five of which exhibited multiple resistance to both glyphosate as well as acetolactate synthase inhibiting herbicides [19] . In response to the discovery of GR giant ragweed in Ontario, producers have been forced to diversify their weed management tactics including the incorporation of herbicides with additional modes of action.
Linuron is a substituted-urea herbicide registered for application in various crops including corn, soybean, wheat (Triticum aestivum L.), oats (Avena sativa L.), and barley (Hordeum vulgare L.) [20] . Linuron provides residual control of many newly established and germinating annual grass and broadleaf weeds and may be soilor foliar-applied at a dose of 1125 to 2250 g·ai·ha−1 in soybean, in Ontario [20] [21] . Linuron inhibits photosynthesis by binding to the QB-binding niche of the D1 protein of the photosystem II complex in chloroplast thylakoid membranes [1] . Sensitive weeds include green foxtail Setaria viridis (L.) Beauv, wild mustard (Sinapsis arvensis L.), common lambsquarters (Chenopodium album L.), common ragweed (Ambrosia artemisiifolia L.), and redroot pigweed (Amaranthus retroflexus L.) [22] . While the risk of herbicide resistance tolinuron is higher than that of glyphosate, its relative exposure supports implementation into GR cropping systems [16] [20] [23] .
As GR giant ragweed continues to be identified, routine application of only glyphosate will no longer provide effective control. In southwestern Ontario, giant ragweed seedlings are among the first weeds to emerge. Recent studies on GR giant ragweed have concluded that control prior to soybean planting is an effective management tactic [24] [25] . The objective of this study was to therefore determine the biologically effective dose of linuron for the control GR giant ragweed when applied pre-plant (PP) in soybean.
2. Materials and Methods
Six field experiments were established in 2012 and 2013 on Ontario farms with GR giant ragweed. Resistance to glyphosate was confirmed prior to the establishment of field trials at each location [18] . The experiments were conducted at locations near McGregor and Windsor (two sites) in 2012, and Tilbury, Harrow, and McGregor, Ontario, Canada in 2013. All of the experiments were established in no-till soybean fields. All sites were fertilized according to soil test results; soil characteristics for each location are listed in Table1
The experiments were arranged in a randomized complete block design with four replications. Dose response treatments included glyphosate (900 g·ae·ha−1) plus linuron applied at 135, 270, 540, 1080, 2160, 4320, and 8640 g·ai·ha−1. Experiments included a weed-free (900 g·ae·ha−1 glyphosate; 500 g·ai·ha−1 2,4-D ester) and a GR weedy control (900 g·ae·ha−1 glyphosate); plots measured 2 by 10 m. Throughout the growing season, weedfree plots were maintained by hoeing and hand weeding as needed. Herbicide treatments were applied with a CO2-pressurized backpack sprayer calibrated to deliver 200 L·ha−1 aqueous solution at 240 kPa. Boom length was 1.5 m with four ultra-low drift nozzle tips (ULD 120-04, Hypro, New Brighton, MN, USA), spaced 50 cm apart. Giant ragweed maximum height and density at the time of herbicide application are listed in Table2
Following herbicide application, GR soybean cultivars were seeded mid-May to early June at a depth of 3 to 4 cm using a no-till seeder (Table 2).
Weed control was evaluated relative to the GR weedy control at 2, 4, and 8 WAA on a scale of 0 (no control) to 100% (complete control). Weed density and dry weight per square meter were determined at 4 WAA by counting and cutting giant ragweed plants at the soil surface from two 0.5 m2 quadrats per plot. Plants were dried at 60˚C to constant moisture and then weighed. The soybean crop was harvested by taking two meters of plants out of one row in each plot, weight and moisture were recorded, and yields were adjusted to 13% moisture.
Statistical Analysis
Data were analyzed using non-linear regression (PROC NLIN) in SAS 9.2 [26] . The weed-free control was not
Table 2. Location, relative humidity (RH), and giant ragweed maximum height and density at the time of treatment as well as soybean agronomic information for experiments conducted during 2012 and 2013, in Ontario.
included in the regression analysis. Weed density and dry weight were converted to a percent of the GR weedy control and yield was converted to a percent of the weed-free control prior to analysis. All parameters were regressed against linuron dose, designated as DOSE in the equations.
The equation used for percent weed control (exponential to maximum), using a four parameter log-logistic model was:
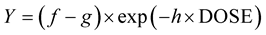 (1)
(1)
where f is the upper asymptote, g is the magnitude of the response and h is the slope of the response [27] .
For percent density and dry weight, an inverse exponential equation was used:
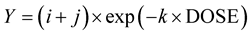 (2)
(2)
where i is the lower asymptote, j is the magnitude of the response and k is the slope of the response [27] .
For percent yield a dose response equation was used:
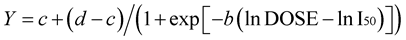 (3)
(3)
where c is the lower asymptote, d is the upper asymptote, b is the slope and I50 is the dose which gives a response halfway between c and d [27] .
Regression equations were used to calculate predicted linuron doses (g·ai·ha−1) required to give 50%, 80%, and 95% percent control of giant ragweed or a 50%, 80%, and 95% reduction in percent weed density or dry weight (ED50, ED80, and ED95), or the dose which gave 90%, 95%, and 98% yield of the weed-free control (ED90, ED95, ED98). If any linuron dose was predicted to be higher than 8640 g·ai·ha−1, it was simply expressed as “>8640” as it would be inappropriate to extrapolate outside the range of doses evaluated in these experiments.
3. Results and Discussion
No significant environment by dose interaction was present for the variables evaluated, thus data from all environments were pooled.
To establish 50% control of GR giant ragweed, 1371, 1238, and 1670 g·ai·ha−1 linuron was required at 2, 4, and 8 WAA, respectively (Table 3). However, the doses needed for 80% GR giant ragweed control were increased to 4326, 2959, and 3943 g·ai·ha−1 for the same time intervals, respectively (Table 3). These correspond to about 2, 1.3, and 1.8 times the recommended maximum linuron dose. In other PP linuron studies, Reference [24] demonstrated greater than 95% control of GR giant ragweed, while Reference [25] reported good, yet variable, control ranging from 23% to 99% at 8 WAA, in southwestern Ontario. This discrepancy in control may be due to application timing as Reference [24] applied PP linuron from May 20 to June 3, and Reference [25] during May 1 to June 2. In this study, linuron was applied May 1 to 17 (Table 2), therefore the comparative low GR giant ragweed control may be due, in part, to early application dates. Linuron has also been shown to be effective at controlling 86% and 99% of herbicide-resistant waterhemp (Amaranthus tuberculatus var. rudis) at 10 WAA when applied as early as May 11 and May 29, respectively [28] . Additionally, the primarily wide-spaced soybean rows in this study (Table 2) may have influenced GR giant ragweed control. Reference [29] examined linuron weed control in soybeans spaced 18 to 107 cm and found that weed control increased as the distance between rows decreased. Narrow row spacing has also been reported to delay the critical time for weed removal [30] . Miller et al. [31] determined the biologically effective rate of saflufenacil/dimethenamid-p in soybean and found that excessive moisture and below average temperatures resulted in higher doses of herbicide to maintain the same level of weed control. This experiment detected no significant environment by dose interaction and therefore dissimilar environmental conditions may produce altered linuron weed control values.
Based on the statistical analysis, the predicted dose of linuron required to reduce GR giant ragweed density and dry weight was similar to that required for visible control (Table 3). The dose needed to reduce density by 50%, 80%, and 95% was 1554, 3181, and 5643 g·ai·ha−1; this corresponds to a 1, 1.4, and 2.5 multiple of the maximum field recommended dose (Table 3). The amount of linuron required to reduce GR giant ragweed dry weight was slightly lower relative to density values (Table 3). To reduce dry weight by 50%, 80%, and 95%, 1204, 2496, and 4452 g·ai·ha−1 of linuron was required, respectively (Table 3). Based on this study, a grower who applies glyphosate (900 g·ae·ha−1) plus linuron (2250 g·ai·ha−1) PP in soybean can expect a reduction in GR giant ragweed density and dry weight greater than 50%, but less than 80% (Table 3). However, there may
Table 3 . Regression parameter estimates and predicted linuron doses from exponential to maximum models of percent weed control 2, 4 and 8 WAA, inverse exponential models of percent weed density and dry weight 4 WAA as a percent of the glyphosate-resistant weedy control and dose response models of soybean yield as a percent of the weed-free controla.
aAbbreviations: AMBTR, giant ragweed; WAA, weeks after herbicide application. bExponential to maximum parameters (Equation (1)): f, upper asymptote; g, magnitude of response; h, slope of response. Inverse exponential parameters (Equation (2)): i, lower asymptote; j, magnitude of response; k, slope of response. Dose response parameters (Equation (3)): b, slope; c, lower asymptote; d, upper asymptote; I50, dose required for 50% response. cR50, R80, and R95 are the doses required to give weed control of 50%, 80%, 95%, respectively, for a given weed species; R50, R80, and R95 are the doses required to give a 50%, 80%, and 95% reduction in percent density or dry weight, respectively, for a given weed species; R90, R95, and R98 are the doses required to give yields of 90%, 95%, and 98%, respectively, of the weed-free control.
have been mitigating factors in this study as a standard linuron PP application has been shown to reduce GR giant ragweed dry weight by up to 98% in similar experiments conducted in southwestern Ontario [24] [25] .
Higher doses of linuron were needed to obtain soybean yields comparable to the weed-free control (Table 3). To achieve a 90% soybean yield, 7874 g·ai·ha−1 linuron was required; approximately 3.5 times the maximum recommended dose (Table 3). For 95% and 98% yields, the linuron dose needed was greater than 8640 g·ai·ha−1, the highest dose evaluated in this study (Table 3). Interestingly, application of the maximum field dose was insufficient to establish a soybean yield even 50% of the weed-free control (Table 3) while other published reports have observed considerably higher yields. Linuron plus glyphosate applied PP has been previously reported to give soybean yields of up to 59% [25] and 90% [24] of the weed-free control, when targeting GR giant ragweed. A similar study examined control of herbicide-resistant common waterhemp and found that linuron applied pre-emergence in soybean was sufficient to establish 94% on the weed-free control [28] .
4. Conclusion
In this experiment, the PP application of linuron plus glyphosate at the maximum field dose provided fair to acceptable control of GR giant ragweed in soybean. To establish 95% yield of the weed-free control, more than 8640 g·ai·ha−1 linuron was required. If required, a subsequent soybean post-emergence herbicide application may be required as giant ragweed can greatly reduce yield, even at low densities [12] . While predicted weed control was less than ideal, application of more than one mode of action will decrease the selection pressure for resistant weed biotypes and broaden the spectrum of weeds controlled. Diversified herbicide programs benefit growers who have GR weeds as well as ones who do not. However, herbicide choice is only one aspect of integrated weed management [32] and auxiliary management tactics including timing of application and soybean row spacing may enhance PP linuron efficacy and thus require further investigation.
Acknowledgements
The authors would like to acknowledge Chris Kramer for his expertise and technical assistance. Funding for this study was provided by the Grain Farmers of Ontario and TKI Nova Source.
References
- Vencill, W.K., et al. (2012) Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Science, 60, 2-30. http://dx.doi.org/10.1614/WS-D-11-00206.1
- Duke, S.O. and Powles, S.B. (2009) Glyphosate-Resistant Crops and Weeds: Now and in the Future. AgBioForum, 12, 346-357.
- Powles, S.B. (2008) Evolved Glyphosate-Resistant Weeds around the World: Lessons to Be Learnt. Pest Management Science, 64, 360-365. http://dx.doi.org/10.1002/ps.1525
- Owen, M.D. (2008) Weed Species Shifts in Glyphosate-Resistant Crops. Pest Management Science, 64, 377-387. http://dx.doi.org/10.1002/ps.1539
- Thill, D.C. and Lemerle, D. (2001) World Wheat and Herbicide Resistance. In: Powles, S.B. and Shaner, D.L., Eds., Herbicide Resistance and World Grains, CRC Press, New York, 165-194.
- Abul-Fatih, H.A. and Bazzaz, F.A. (1979) The Biology of Ambrosia trifida L. II. Germination, Emergence, Growth, and Survival. The New Phytologist, 83, 817-827. http://dx.doi.org/10.1111/j.1469-8137.1979.tb02312.x
- Bassett, I.J. and Crompton, C.W. (1982) The Biology of Canadian Weeds: 55: Ambrosia trifida L. Canadian Journal of Plant Science, 62, 1003-1010. http://dx.doi.org/10.1139/b62-015
- United States Department of Agriculture (2012) Plants Profile Database. http://plants.usda.gov/java/
- Johnson, B., et al. (2007) Biology and Management of Giant Ragweed. https://www.extension.purdue.edu/extmedia/BP/GWC-12.pdf
- Cowbrough, M.J. (2006) Noxious Weed Profile—Ragweed spp. http://www.omafra.gov.on.ca/english/crops/facts/info_ragweed.htm
- Abul-Fatih, H.A. and Bazzaz, F.A. (1979) The Biology of Ambrosia trifida L. I. Influence of Species Removal on the Organization of the Plant Community. The New Phytologist, 83, 813-816. http://dx.doi.org/10.1111/j.1469-8137.1979.tb02312.x
- Baysinger, J.A. and Sims, B.D. (1991) Giant Ragweed (Ambrosia trifida) Interference in Soybeans (Glycine max). Weed Science, 39, 358-362.
- Ontario Ministry of Agriculture, Food, and Rural Affairs (2003) Ontario Weeds: Giant Ragweed. http://www.omafra.gov.on.ca/english/crops/facts/ontweeds/giant_ragweed.htm
- Payne, W.W. (1963) The Morphology of the Inflorescence of Ragweeds (Ambrosia-Franseria: Compositae). American Journal of Botany, 50, 872-880. http://dx.doi.org/10.2307/2439774
- Bassett, I.J. and Terasmae, J. (1962) Ragweeds, Ambrosia Species, in Canada and Their History in Postglacial Time. Canadian Journal of Botany, 40, 141-150. http://dx.doi.org/10.1139/b62-015
- Heap, I. (2014) The International Survey of Herbicide Resistant Weeds. http://www.weedscience.org
- Sikkema, P.H. and Tardi, F.J. (2009) Suspected Glyphosate-Resistant Giant Ragweed in Ontario. Proceedings of the 64th Annual Meeting of the NCWSS, Champaign, 7-10 December 2009, 167.
- Vink, J.P., Soltani, N., Robinson, D.E., Tardif, F.J., Lawton, M.B. and Sikkema, P.H. (2012) Occurrence and Distribution of Glyphosate-Resistant Giant Ragweed (Ambrosia trifida L.) in Southwestern Ontario. Canadian Journal of Plant Science, 92, 533-539. http://dx.doi.org/10.4141/cjps2011-249
- Follings, J., Soltani, N., Robinson, D.E., Tardif, F.J., Lawton, M.B. and Sikkema, P.H. (2013) Distribution of Glyphosate and Cloransulam-Methyl Resistant Giant Ragweed (Ambrosia trifida L.) Populations in Southern Ontario. Agricultural Sciences, 4, 570-576. http://dx.doi.org/10.4236/as.2013.410077
- Ontario Ministry of Agriculture, Food and Rural Affairs (2013) Guide to Weed Control. Publication 75. Toronto.
- Smith, A.E. and Emmond, G.S. (1975) Persistence of Linuron in Saskatchewan Soils. Canadian Journal of Soil Science, 55, 145-148. http://dx.doi.org/10.4141/cjss75-021
- Soltani, N., Nurse, R., Shropshire, C. and Sikkema, P. (2011) Weed Management in Cranberry Bean with Linuron. Canadian Journal of Plant Science, 91, 881-888. http://dx.doi.org/10.4141/cjps2011-018
- Beckie, H.J. (2006) Herbicide-Resistant Weeds: Management Tactics and Practices. Weed Technology, 20, 793-814. http://dx.doi.org/10.1614/WT-05-084R1.1
- Vink, J.P., Soltani, N., Robinson, D.E., Tardif, F.J., Lawton, M.B. and Sikkema, P.H. (2012) Glyphosate-Resistant Giant Ragweed (Ambrosia trifida L.) Control with Preplant Herbicides in Soybean [Glycine max (L.) Merr.]. Canadian Journal of Plant Science, 92, 913-922. http://dx.doi.org/10.4141/cjps2012-025
- Follings, J., Soltani, N., Robinson, D.E., Tardif, F.J., Lawton, M.B. and Sikkema, P.H. (2013) Control of Glyphosate Resistant Giant Ragweed in Soybean with Preplant Herbicides. Agricultural Sciences, 4, 195-205. http://dx.doi.org/10.4236/as.2013.44028
- SAS Institute Inc. (2008) The SAS System. Version 9.2., SAS Institute, Cary.
- Seefeldt, S.S., Jensen, J.E. and Fuerst, E.P. (1995) Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technology, 9, 218-227.
- Vyn, J.D., Swanton, C.J., Weaver, S.E. and Sikkema, P.H. (2007) Control of Herbicide-Resistant Common Waterhemp (Amaranthus tuberculatus var. rudis) with Preand Post-Emergence Herbicides in Soybean. Canadian Journal of Plant Science, 87, 175-182. http://dx.doi.org/10.4141/P06-016
- Kust, C.A. and Smith, R.R. (1969) Interaction of Linuron and Row Spacing for Control of Yellow Foxtail and Barnyardgrass in Soybeans. Weed Science, 17, 489-491.
- Knezevic, S.Z., Evans, S.P. and Mainz, M. (2003) Row Spacing Influences the Critical Timing for Weed Removal in Soybean (Glycine max). Weed Technology, 17, 666-673. http://dx.doi.org/10.1614/WT02-49
- Miller, R.T., Soltani, N., Robinson, D.E., Kraus, T.E. and Sikkema, P.H. (2012) Biologically Effective Rate of Saflufenacil/Dimethenamid-p in Soybean (Glycine max). Canadian Journal of Plant Science, 92, 517-531.http://dx.doi.org/10.4141/cjps2011-253
- Harker, K.N. and O’Donovan, J.T. (2013) Recent Weed Control, Weed Management, and Integrated Weed Management. Weed Technology, 27, 1-11. http://dx.doi.org/10.1614/WT-D-12-00109.1
Abbreviations
AMBTR, giant ragweed;
GR, glyphosate-resistant;
PP, pre-plant;
WAA, weeks after herbicide application.
NOTES

*Corresponding author.


