American Journal of Plant Sciences
Vol.3 No.7(2012), Article ID:20687,4 pages DOI:10.4236/ajps.2012.37111
Influence of Bavistin and Silver Thiosulphate on in Vitro Regeneration of Asclepias curassavica (L.) Using Nodal Explants
![]()
1Department of Plant Sciences, College of Agriculture and Rural Development, Wollega University, Nekemte, Ethiopia; 2Division of Crop Improvement, Sugarcane Breeding Institute, Coimbatore, India; 3Department of Plant Physiology & Biotechnology, UPASI Tea Research Foundation Tea Research Institute, Valparai, India; 4Department of Biotechnology, Dravidian University, Kuppam, India.
Email: *knchandu1@gmail.com
Received April 16th, 2012; revised May 9th, 2012; accepted May 20th, 2012
Keywords: Bavistin; Regeneration; Asclepiadoideae; Silver Thiosulphate
ABSTRACT
The effect of bavistin and the ethylene inhibitor (silver thiosulphate) on shoot regeneration using nodal explants of Asclepias curassavica (L.) has been investigated. Among the different concentrations studied, highest number of shoots was obtained on MS media with 200 mg/L bavistin. Among the varying concentrations (10 - 100 μM/L) of silver thiosulphate tested, highest number of shoots was obtained on MS medium amended with 60 μM/L silver thiosulphate without growth regulators. This study also establishes the stronger cytokinin like activity of bavistin. Effect of different growth additives like coconut milk, ascorbic acid and casein hydrolysate were tested on direct shoot regeneration. Among the different growth additives tested casein hydrolysate showed better and reproducible result at 0.025% in combination with 3 mg/L KN + 0.5 mg/L NAA. Antioxidants, activated charcoals and polyvinyl pyrrolidone were used to remove phenolics. Activated charcoal removed the phenolic exudates completely at 0.025% and prevented the browning of media and thus enhanced the frequency of regeneration (85%). The microshoots developed through in vitro regeneration were transferred to rooting media containing IBA alone and in combination with KN and the highest number of roots was observed on MS medium with IBA 1 mg/L + 0.2 mg/L KN.
1. Introduction
Asclepias curassavica (L.) (Tropical milkweed) is an erect, evergreen sub shrub belonging to the sub family Asclepiadoideae, in Apocynaeceae family [1]. Its root extracts are widely used as an emetic and laxative. A decoction of the plant is used as an abortifacient. Roots are of medicinal importance (termed “Pleurisy root”) and are used as an expectorant for pneumonia, lung problems, treat warts, fever, ringworm and bleeding. In general, Asclepiadoideae plants are source of cytotoxic and cardiac glycosides consisting of highly valuable products of medicinal importance. World wide shift towards herbal medicinal inclination over synthetic pharmaceuticals has resulted in overexploitation of number of plants with medicinal values. Several species, of known important drugs sources are being exploited a great deal as they are the only source of these drugs and in their effectiveness in production. Although India harbours rich plant diversity, with ever increasing population and deforestation have adversely affected their status; medicinal plants in particular [2].
Endemicity, restricted distribution, small population, inaccessible areas and anthropogenic pressure have caused decline in wild population of many species making their status as rare [3]. It is imperative that viable strategies ought to be implemented to conserve the surviving population at least, the critically important medicinal species from further loss. The conventional approaches to conservation and preservation include the in situ and ex situ conservation strategies. However, for many rare species, in situ preservation is not a feasible option owing to increasing human disturbance. Under such circumstances in vitro regeneration is undoubtedly an efficient means of ex situ conservation of plant diversity [4,5].
With the advent of biotechnological approaches, culturing plant cells and tissues have turned out to be easier and a boon for conserving and propagating valuable, rare and endangered medicinal plants. At present, tissue culture methods are used in almost 600 companies throughout the world to produce more than 500 million units annually from 50,000 varieties of plants [6]. The global biotechnology business is estimated to be round 150 billion U.S. dollars, of which 50% - 60% is in agribusiness and the annual demand of tissue culturally raised products constitutes about 15 billion US dollars with an annual growth rate of about 15 per cent [7].
Antimicrobial agents (antibiotics and fungicides) are generally used in plant tissue culture media to eliminate microorganisms that are present in explants or arise as laboratory contaminants. Several of such agents are reported to effect in vitro cell culture and plant regeneration. Certain agents like carbendazim, fenbendazole and imazalil were found to be least toxic to plant cells and had a broad spectrum fungicidal activity [8]. Ag+ ions of the ethylene inhibitor silver thiosulphate inhibit activity of ethylene in a various plant species [9]. The ethylene inhibiting effect of Ag+ is due to an interference believed with ethylene binding [10]. The positive effect of Ag+ ions suggests that ethylene produced by inoculated explants inhibits shoot organogenesis [11]. The beneficial effects of ethylene inhibitors on organogenesis for plant regeneration have been widely reported [12-14]. The influence of Bavistin and silver thiosulphate has been well documented in many medicinal plants such as Mentha piperita [15], Stevia rebaudiana [16] and effect of Bavistin with adenine sulphate has been studied in Picrorhiza scrophulariiflora [17].
The positive role of different growth additives coconut milk, ascorbic acid and casein hydrolysate has been reported extensively in the in vitro propagation of Gymnema sylvestre [18]. Antioxidants like polyvinyl pyrrolidone and activated charcoal have been used to reduce the phenolic exudates in a variety of plant tissue culture protocols. Hence the present study was aimed in understanding the influence of these growth additives as well as antioxidants in the in vitro shoot multiplication of A. curassavica. However no such attempts have been made in A. curassavica. Hence, the present study was aimed to comprehend the influence of bavistin and silver thiosulphate on in vitro shoot regeneration from nodal explants of A. curassavica.
2. Materials and Methods
In the present study, A. curassavica seeds were collected from Tirumala hills, Tirupati, Andhra Pradesh (A.P.) during March, 2005. The seeds were germinated in the garden, Department of Biotechnology, S.V. University, Tirupati, A.P. Actively growing shoots with five to six nodes were used as explants. The explants were washed under running tap water for 5 - 10 minutes, presoaked in liquid detergent (1% tween 20) for 1 - 5 minutes and surface sterilized in 70% ethanol for 60 seconds and Mercuric chloride (0.1%) for 1 - 5 minutes. Then they were rinsed with sterile double distilled water for 4 - 5 times. After trimming the cut ends, the explants were blotted on sterile filter paper discs. Nodal explants of A. curassavica were cultured on MS media supplemented with Bavistin (fungicide) at different concentrations ranging from 10 - 400 mg/L containing 3% (w/v) sucrose. The media was solidified with 0.8% (w/v) agar. The pH of the medium was adjusted to 5.8. In addition, to trace the effect of silver thiosulphate on direct shoot regeneration, the nodal explants of A. curassavica were cultured on MS media supplemented with silver thiosulphate at different concentrations (10 - 100 μM) along with growth additives and antioxidants. The effect of different growth additives and antioxidants were tested on shoot regeneration from nodal explants of A. curassavica. Effect of different concentrations of growth additives such as coconut milk (CM) and casein hydrolysate (CH) and antioxidants like activated charcoal (AC), poly vinyl pyrrolidone (PVP) and Ascorbic acid (AA) were studied. Different adjuvants were tested in combination with various cytokinins at their optimum concentrations either individually or in combination with different auxins. Experiments were set up in a completely randomized design and each treatment had 4 replicates of 5 explants each. All data are statistically analyzed by Analyses of Variance (ANOVA) and Duncan’s Multiple Range Test (DMRT).
3. Results and Discussion
Bavistin in direct shoot regeneration appeared to have much stronger cytokinin-like activity. Bavistin is a systemic fungicide that belongs to benzimidazole family. Benzimidazoles are a group of organic fungicides with systemic action that are extensively used in agriculture [19]. It has been reported that the molecular structure of methyl benzimidazole carbamate (or) carbendiazim has some resemblance to kinetin, adenine and to many other cytokinins based on adenine [20]. In addition, it has also been demonstrated that these compounds can have beneficial effects on the physiology of the plant [21] for example, benzomyl (carbamate of methyl-N-butyl-carbamyl-benzimidazole), one of the most effective and extensively used benzimidazoles, has a cytokinin activity in soy and radish [22,23].
In the present investigation Bavistin (fungicide) was used to study the effect on axillary bud proliferation in addition to its antifungal activity. Among the varying concentrations used for direct shoot regeneration, highest frequency of shoot regeneration of 70% was obtained at 250 mg/L Bavistin containing MS media. The results of the study indicated the differences in frequency of shoot regeneration, number of shoots/explants and length of shoot, the results are tabulated in Table 1 with respect to Bavistin concentrations. Our study showed that at 250 mg/L concentration, Bavistin showed maximum shoot regeneration frequency and it was significant at p < 0.01. Similarly number of shoots/explants and length of shoots showed statistically signification results at concentrations 150, 200 and 250 mg/L compared to other concentrations of Bavistin (Figure 1). Earlier studies conducted in other plant systems have also shown that bavistin/fungicides [20] promote shoot regeneration. In present study, the influence of Bavistin in shoot regeneration of A. curassavica cultures is possibly due to its “cytokinin-like” activity, results are tabulated in Table 1. The breakdown products of Bavistin trigger shoot regeneration resulting in enhanced biosynthesis of endogenous cytokinins within the cultures.
The beneficial effects of the ethylene inhibitor silver thiosulphate (STS) on organogenesis have been widely

Figure 1. Axillary bud explants of A. curassavica cultured on MS medium supplemented with various concentration of Bavistin and Silver thiosulphate individually. (A) Shoot bud initiation from nodal explants on MS medium + Bavistin (100 mg/L); (B) Multiple shoot formation from nodal explants on MS medium + Bavistin (200 mg/L); (C) Shoot bud initiation from nodal explants on MS medium + STS (30 µM/L); (D) Multiple shoot formation from nodal explants on MS medium + STS (50 µM/L); (E) Rhizogenesis from in vitro cultured plants on MS media; (F) Well rooted plants ready for hardening.
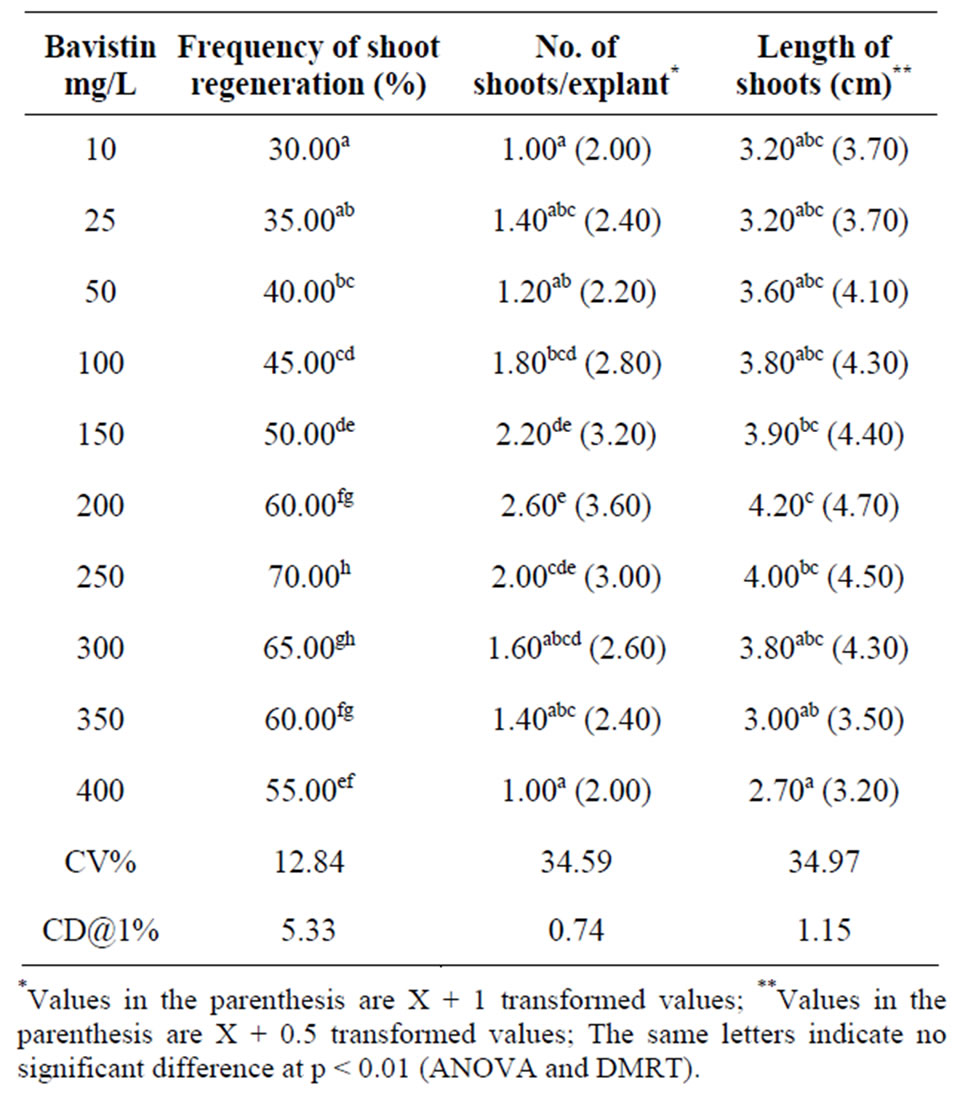
Table 1. Effect of different concentrations of Bavistin on direct shoot regeneration from seedling nodal explants of A. curassavica in MS media. Observations: After 4 weeks.
reported [11-14]. The Ag+ ions inhibit ethylene action in a wide variety of ethylene induced responses in plants. The ethylene inhibiting effect of Ag+ is believed to be due to an interference with ethylene binding [10]. The positive effect of Ag+ ions in shoot organogenesis suggests that ethylene produced by cultured explants inhibits shoot organogenesis of those explants [11].
Experiments were conducted to scrutinize the effect of varying concentrations of ethylene inhibitor STS on shoot regeneration of A. curassavica. STS tested on direct shoot regeneration from nodal explants of A. curassavica was 10 - 100 μM/L in MS medium. Increasing the concentration of STS enhanced regeneration capacity of MS medium. The results of the study indicated the differences in frequency of shoot regeneration, number of shoots/explants and length of shoot, the results are tabulated in Table 2 with respect to varying concentration of STS. Our study showed significant higher percentage of at 40, 70 - 100 μM/L concentrations of STS, maximum shoot regeneration frequency was obtained at 80 μM and it was significant at p < 0.01. Number of shoots/explants from 30 - 80 μM/L concentrations of STS produced significant results and length of shoots showed statistically signification results at concentrations of 30 - 80 μM/L compared to other concentrations of STS. Maximum shoot length 4.70 (5.20) at 50 μM/L concentrations was observed, results are tabulated in Table 2. Hence optimal
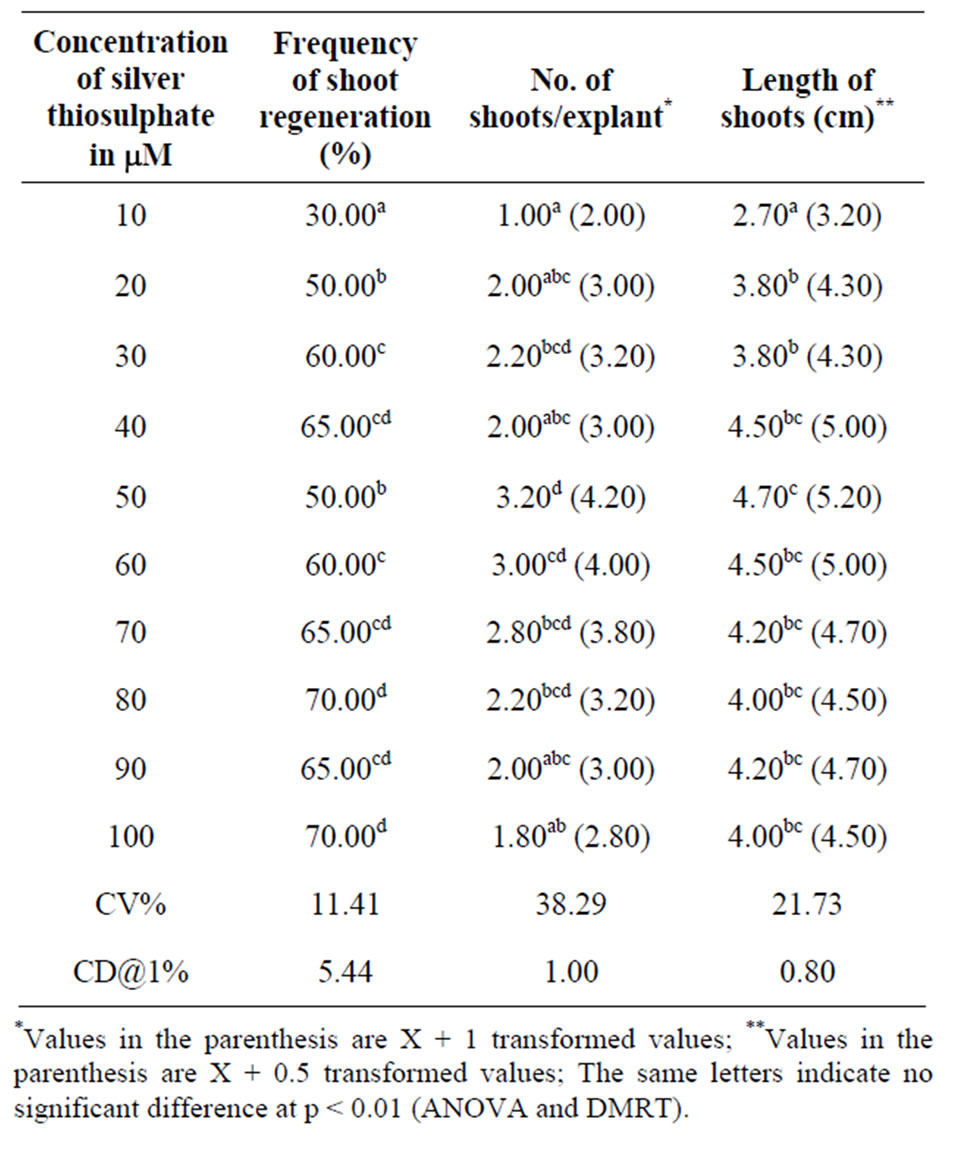
Table 2. Effect of silver thiosulphate on direct shoot regeneration from nodal explants of A. curassavica in MS media. Observations: After 4 weeks.
concentrations of STS may prove to be a useful media supplement in plant tissue culture.
The presence of casein hydrolysate (0.025%) in the growth medium produces appreciable amount of callus from the basal cut end of the explant. A mean shoot number of 3.2 was observed at 3 mg/L KN + 0.5 mg/L NAA medium when 0.025% of casein hydrolysate was added. Casein hydrolysate did not showed any effect regarding shoot length in both KN and BAP containing media. Addition of ascorbic acid enhanced shoot number. The highest shoot number of 3.0 was achieved with 3 mg/L KN + 0.5 mg/L NAA when 0.01% of ascorbic acid was added to the medium. But a significant decrease in shoot length was observed on this medium compared to the medium without ascorbic acid. Addition of coconut milk had no significant effect on shoot morphogenesis. There was no enhancement of shoot number with addition of Coconut Milk to the media containing 2 mg/L BAP + 0.5 mg/L NAA and 3.0 mg/L KN + 0.5 mg/L NAA. A slight decrease in shoot number (2.5) was observed in 2 mg/L BAP + 0.5 mg/L NAA containing medium with addition of coconut water and slight increase in shoot number (3.2) was noted in 3 mg/L KN + 0.5 mg/L NAA medium and the results are tabulated in Table 3.
Similar result was reported on other Asclepiadacean, Hemidesmus indicus by [24] where addition of 10% coconut water has resulted in highest number of shoots. It has been stated that ascorbic acid will increase the metabolic activity and accelerate the release of sugars [25], thereby enhancing the organogenic ability. Similar response with ascorbic acid was observed by [3,26-28]. More over ascorbic acid is a strong reducing agent that scavenges the free oxygen radicals present if any in the medium. Comparatively, the other growth adjuvants coconut water (10%) and casein hydrolysate (0.025%) were found ineffective in improving either shoot number (or) quality in the present investigation. This may support the view that for most tissue culture purposes, addition of undefined supplements containing amino acids may be unnecessary when correct balance of inorganic salts were present in the medium [29]. Callus formed at the cut end of the nodal explants readily turned brown and retarded the vigorous growth of shoot when they were cultured on medium with casein hydrolysate, possibly due to maladjustment of cells to the excessive organic nitrogen [18].
The antioxidants activated charcoal (0.025%) and polyvinyl pyrrolidone (0.025%) were used to remove phenolics. Activated charcoal subdued the phenolic exudates completely at 0.025% and prevented the browning of media and thus enhanced the frequency of regeneration (85%) data not shown. In absence of activated charcoal it was observed that media turned to brown in colour, prolonged cultures get contaminated. Comparatively other antioxidant (PVP) checked did not show much effect and frequency of regeneration was also less i.e., 75%. The addition of activated charcoal having beneficial effects were reported in Gymnema sylvestrae [30], Wattakata volubilis [31].
In vitro derived shoots with a length of (3 - 5 cm) were excised and transferred to MS medium supplemented with different concentrations of auxins such as IBA, IAA and NAA (0.1 - 2.0 mg/l) alone and in combination with KIN. In all the concentrations tried, highest number of roots was observed on MS medium with IBA 1 mg/L + 0.2 mg/L KN. Highest rooting frequency (85%), with a maximum number of roots (12.2 ± 0.53) was observed in this combination, results are tabulated in Table 3.
4. Conclusion
From the results it is noticeable that fungicide bavistin, ethylene inhibitor silver thiosulphate does not shows any harmful effect on shoot regeneration of Asclepias curassavica. Thus these promissory compounds may be useful as a media supplement to develop efficient protocols for in vitro propagation as it favors the shoot formation. Casein hydrolysate can be used as an important growth factor that promotes shoot regeneration frequency of Asclepias curassavica in the present study. Activated charcoal
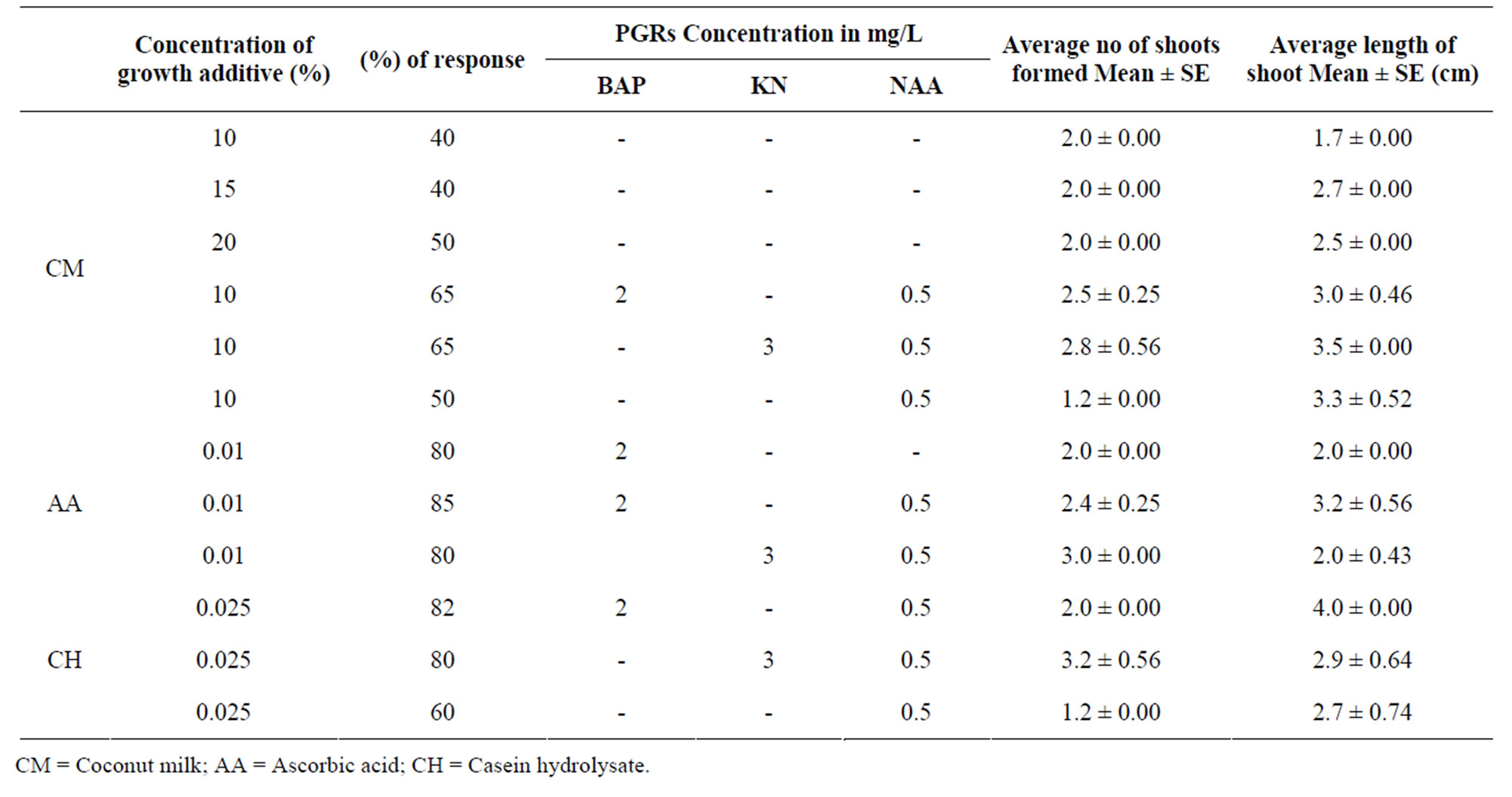
Table 3. Effect of different growth adjuvants on multiple shoot regeneration from nodal explants of A. curassavica in MS media supplemented with different growth regulators. Observations: After 4 weeks (The results are mean (SE±) of 20 independent determinations).
removed the phenolic exudates completely and prevented the browning of media and thus enhanced the frequency of regeneration.
5. Acknowledgements
S Hemadri Reddy would like to thank Department of Biotechnology, S.V. University, Tirupati, Andhra Pradesh for providing Doctoral fellowship to complete this work.
REFERENCES
- M. E. Endress and P. V. Bruyns, “A Revised Classification of the Apocynaceae s.l.,” Botanical Review, Vol. 66, No. 1, 2000, pp. 1-56. doi:10.1007/BF02857781
- S. S. Samant, U. Dhar and L. M. S. Palni, “Medicinal Plants of Indian Himalaya: Diversity, Distribution Potential Values,” Himavikas Publication, No. 13, Gyan Prakash, Nainital, 1998.
- N. Komalavalli and M. V. Rao, “In Vitro Micropropagation of Gymnema elegans—A Rare Medicinal Plant,” Indian Journal of Experimental Biology, Vol. 35, 1997, pp. 1088-1092.
- P. Knogstrup, S. Baldursson and J. V. Norgard, “Ex Situ Genetic Conservation by Use of Tissue Culture,” Operational Botany, Vol. 113, 1992, pp. 49-53.
- M. F. Fay, “In What Is in Vitro Culture Appropriate to Plant Conservation?” Biodiversity and Conservation, Vol. 3, No. 2, 1994, pp. 176-183. doi:10.1007/BF02291887
- I. K. Vasil and T. A. Thorpe, “Plant Cell and Tissue Culture,” Kluwer Academic Publishers, Dordrecht, 1994.
- S. Govil and S. C. Gupta, “Commercialisation of Plant Tissue Culture in India,” Plant Cell, Tissue and Organ Culture, Vol. 51, No. 1, 1997, pp. 65-73. doi:10.1023/A:1005873221559
- R. Shields, S. J. Robinson and P. A. Anslow, “Use of Fungicides in Plant Tissue Culture,” Plant Cell Reports, Vol. 3, No. 1, 1984, pp. 33-36. doi:10.1007/BF00270226
- L. Burgos and N. Alburquerque, “Ethylene Inhibitors and Low Kanamycin Concentrations Improve Adventitious Regeneration from Apricot Leaves,” Plant Cell Reports, Vol. 21, No. 12, 2003, pp. 1167-1174. doi:10.1007/s00299-003-0625-6
- E. M. Beyer, “Effect of Silver Ion, Carbon Dioxide, and Oxygen on Ethylene Action and Metabolism,” Plant Physiology, Vol. 63, No. 1, 1979, pp. 169-173. doi:10.1104/pp.63.1.169
- G. L. Chi, D. G. Barfield, G. E. Sim and E. C. Pua, “Effect of AgNO3 and Aminoethoxyvinylglycine on in Vitro Shoot and Root Organogenesis from Seedling Explants of Recalcitrant Brassica genotypes,” Plant Cell Reports, Vol. 9, No. 4, 1990, pp. 195-198. doi:10.1007/BF00232178
- D. D. Songstad, D. R. Duncan and J. M. Widholm, “Effect of 1-Aminocyclopropane-1-carboxylic Acid, Silver Nitrate, and Noborn-Adiene on Plant Regeneration from Maize Callus Cultures,” Plant Cell Reports, Vol. 7, No. 4, 1988, pp. 262-265. doi:10.1007/BF00272538
- K. M. Charaibi, A. Latche, J. P. Roustan and J. Fallot, “Stimulation of Shoot Regeneration from Cotyledons of Helianthus annuus by the Ethylene Inhibitors, Silver and Cobalt,” Plant Cell Reports, Vol. 10, 1991, pp. 204-207.
- H. P. Bais, G. Sudha and G. A. Ravishankar, “Influence of Putrescine, Silver Nitrate and Polyamine Inhibitors on the Morphogenetic Response in Untransformed and Transformed Tissues of Cichorium intybus and Their Regenerants,” Plant Cell Reports, Vol. 20, No. 6, 2001, pp. 547-555. doi:10.1007/s002990100367
- P. Sujana and C. V. Naidu, “Influence of Bavistin, Cefotaxime, Kanamycin and Silver Thiosulphate on Plant Regeneration of Mentha piperita (L.)—An Important Multipurpose Medicinal Plant,” Journal of Phytology, Vol. 3, 2011, pp. 36-40.
- D. Preethi, T. M. Sridhar and C. V. Naidu, “Effect of Bavistin and Silver Thiosulphate on in Vitro Plant Regeneration of Stevia rebaudiana,” Journal of Phytology, Vol. 3, 2011, pp. 74-77.
- P. Bantawa, O. S. Roy, P. Ghosh and T. K. Mondal, “Effect of Bavistin and Adenine Sulphate on in Vitro Shoot Multiplication of Picrorhiza scrophulariiflora Pennell.: An Endangered Medicinal Plant of Indo-China Himalayan Regions,” Plant Tissue Culture and Biotechnology, Vol. 19, No. 2, 2009, pp. 237-245.
- N. Komalavalli and M. V. Rao, “In Vitro Micropropagation of Gymnema sylvestre—A Multipurpose Medicinal Plant,” Plant Cell, Tissue and Organ Culture, Vol. 61, No. 2, 2000, pp. 97-105. doi:10.1023/A:1006421228598
- C. J. Delp, “Benzimidazole and Related Fungicides,” In: H. Lyr, Ed., Modern Selective Fungicides, 2nd Edition, Gustav Fisher Verlag, Jena, 1987, pp. 291-303.
- R. K. Tripathi and S. Ram, “Induction of Growth and Differentiation of Carrot Callus Cultures by Carbendazim and Benzimidazole,” Indian Journal of Experimental Biology, Vol. 20, 1982, pp. 674-677.
- P. C. Garcia, R. M. Rivero, J. M. Ruiz and L. Romero, “The Role of Fungicides in the Physiology of Higher Plants: Implications for Defense Responses,” Botanical Review, Vol. 69, No. 2, 2003, pp. 162-172. doi:10.1663/0006-8101(2003)069[0162:TROFIT]2.0.CO;2
- K. G. M. Skene, “Cytokinin-Like Properties of the Systemic Fungicide Benomyl,” Journal of Horticultural Science, Vol. 47, 1972, pp. 179-182.
- T. H. Thomas, “Investigations into the Cytokinin-Like Properties of Benzimidazole Derived Fungicides,” Annals of Applied Biology, Vol. 76, No. 2, 1974, pp. 237-241. doi:10.1111/j.1744-7348.1974.tb07977.x
- D. Raghuramulu, “In Vitro Morphogenetic Studies of Hemidesmus indicus and Cynanchum callialatum. (Asclepiadaceae),” Ph.D. Thesis, Sri Krishnadevaraya University, Ananthapur, 2001.
- R. W. Joy-Iv, K. R. Patel and T. A. Thorpe, “Ascorbic Acid Enhancement of Organogenesis in Tobacco Callus,” Plant Cell, Tissue and Organ Culture, Vol. 13, No. 3, 1988, pp. 219-228. doi:10.1007/BF00043670
- A. Ahuja, M. Verma and S. Grewal, “Clonal Propagation of Ocomum Species by Tissue Culture,” Indian Journal of Experimental Biology, Vol. 20, 1982, pp. 455-458.
- N. Sharma and K. P. S. Chandel, “Effect of Ascorbic Acid on Axillary Shoot Induction in Tylophoro indica (Burm. f.) Merril,” Plant Cell, Tissue and Organ Culture, Vol. 29, No. 2, 1992, pp. 109-113. doi:10.1007/BF00033615
- V. Sarasan, E. Y. Sonia and G. M. Nair, “Regeneration of Indian Sarasapilla, Hemidesmus indicus R. Br., through Organogenesis and Somatic Embryogenesis,” Indian Journal of Experimental Biology, Vol. 32, 1994, pp. 284- 287.
- E. F. George and P. D. Sherrington, “Handbook and Directory of Commercial Laboratories,” In: Plant Propagation by Tissue Culture, Exegetics Ltd., Eversley, 1984, p. 709.
- C. G. Sudha, “Tissue Culture Studies on Three Rare Medicinal Plants of India, Holostemma anulare, Janakia arayalpathra and Rauwolfia micrantha,” Ph.D. Thesis, University of Kerala, India, 1996.
- T. Chakradhar, “In Vitro Culture, Physiological, Phytochemical and Antimicrobial Studies of a Medicinal Plant. Wattakaka volubilis (L.f.) stapf (Asclepiadaceae),” Ph.D. Thesis Submitted to S.K. University, Ananthapur, 2004.
NOTES
*Corresponding author.

