American Journal of Plant Sciences
Vol.3 No.6(2012), Article ID:20030,8 pages DOI:10.4236/ajps.2012.36095
Sterilization of Hibiscus rosa-sinensis L. Vegetative Explants Sourced from Plants Grown in Open Environment and Influences of Organic Ingredients on In Vitro Direct Regeneration
![]()
1Department of Microbiology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Malaysia; 2Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Malaysia; 3PT Lengkuk Technology, Felda Biotechnology Centre, Nilai, Malaysia.
Email: janna@biotech.upm.edu.my
Received March 26th, 2012; revised April 20th, 2012; accepted April 30th, 2012
Keywords: Hibiscus rosa-sinensis L.; Murashige and Skoog Medium; Axillary Bud; Sucrose; Organic Ingredient; Activated Charcoal
ABSTRACT
This paper reports on the effects of organic ingredients in facilitating direct shoot regeneration from nodal explants of Hibiscus rosa-sinensis L. This paper also compares the sterilization conditions for 3 types of explants (node, internode, and shoot tip) harvested from an open field. The optimized sterilization conditions for the explants were 40% Clorox- 20 min exposure, 10% Clorox-15 min exposure, and 5% Clorox-40 min exposure for the node, internode and shoot tip, respectively. In the direct shoot regeneration using the nodal explants, we found MS medium containing 40 g/L sucrose, 0.3% (w/v) activated charcoal, and supplementations with myo-inositol, thiamine and nicotinic acid were suitable. The in vitro shoot survival rate was 30% with a mean leaf numbers of 2.68 produced, and a mean leaf length of 1.71 cm achieved after 5 weeks of culture on the modified medium.
1. Introduction
Hibiscus rosa-sinensis L., commonly known as “Chinese Hibiscus”, is an evergreen perennial plant that grows in tropical and sub-tropical regions. It has a charismatic bright red, five-petal, and single layer flower, and the leaves are alternate along the branch.
Pharmacological investigations of the genus Hibiscus indicated the existence of some species with interesting biological activities such as anti-hypertensive [1], anti-inflammatory [2], hepato-protective [3], anti-tumoric [4], anti-diabetic [5], anti-convulsive [6], anti-oxidative [7,8], and anti-mutagenic [9,10].
Traditional propagation of Hibiscus spp. is dependent on vegetative means such as cuttings or grafting. Vegetative propagation can shorten the length of the juvenile period and also allows genotypes combination [11]. However, such traditional asexual propagation cannot circumvent the very fundamental problems of long growing period, large growing space, small plant quantity, and inefficiency. After approximately 100 years of developmentplant tissue culture technique provides an alternative to solving those problems. In this propagation method, only small pieces of plant tissue are required to regenerate on plant tissue culture medium under sterile conditions. The plant tissues can be the internodes, nodes, shoot tips (stems), root tips, calli, leaves, seed embryos, or anthers.
Due to the strikingly encouraging effects of plant growth regulator (PGR) in accelerating, inducing, and maintaining in vitro shoots, direct in vitro shoots establishment and multiplication from explants often involve the application of PGRs such as 6-benzylaminopurine (BAP) [12, 13], zeatin [14], BAP plus kinetin (KIN) [15], BAP plus indole-3-acetic acid (IAA) [16], or BAP plus naphthalene-3-acetic acid (NAA) [17]. Christensen et al. [13]) reported that the most suitable multiplication medium for H. rosa-sinensis L. was demonstrated to be a modified Murashige and Skoog (MS) medium containing 2.2 μM BAP and increased concentrations of calcium at 9 mM and iron at 295 μM provided as ethylenediamine di-2-hydroxyphenyl acetate ferric (Fe-EDDHA). Bhalla et al. [18] modified the MS medium strength coupled with BAP in inducing direct shooting from H. rosa-sinensis but concluded that BAP was not suitable in their study. While in other hibiscus species, H. cannabinus (kenaf), Herath et al. [12] reported that 8.8 μM of BAP was the best treatment, but was contradicting Zapata et al. [19] who revealed BAP at 4.4 μM completely suppressed shoot growth of kenaf. Both reports again contradicted Ayadi et al. [20] who found that the additions of PGRs [BAP, NAA, and indoleacetic acid (IBA)] to the MS medium were detrimental to the cultures as they reduced buds formation, inhibited shoots growth and roots induction. Hence, it seems crucial that focus be targeted at the medium ingredients rather than exogenous supplementation of PGRs in inducing shoots formation or elongation, as an alternative to optimize direct in vitro shoot establishment and multiplication of hibiscus explants.
In this paper, we present the results on the effectiveness of shoot induction through modification of the MS medium ingredients, without involving any PGRs. We also report the effectiveness of sodium hypochlorite (NaOCl) in controlling the balance between contamination and survival levels for three types of H. rosa-sinensis L. vegetative explants, and highlight the optimized conditions for explants sterilization of this plant species, which were harvested from open field. Since most of the reported studies on hibiscus species were focused on samples obtained from plants grown in greenhouses, this paper provides alternative reference for plants grown in the open fields.
2. Materials and Methods
2.1. Culture Medium
All the media used were based on MS basal salts and vitamins [21]. No PGR was added. For media used in the sterilization process, 20 g/L of sucrose was added to half strength MS (1/2 MS) basal medium while for subsequent direct shoot induction, different sucrose concentrations (10, 20, 30, 40, 50 g/L), activated charcoal [0.1, 0.2, 0.3, 0.4 and 0.5% (w/v)] and different combinations of organic ingredients [myoinositol + thiamine.HCl, myoinositol + thiamine.HCl + nicotinic acid, myoinositol + thiamine.HCl + pyridoxine.HCl, myoinositol + thiamine. HCl + nicotinic acid + pyridoxine.HCl, and myoinositol + thiamine.HCl + nicotinic acid + pyridoxine.HCl + glycine] were tested. The pH of the medium was adjusted to 5.7 - 5.8 using 1 M NaOH before adding 2.7 g/L gelrite agar (for sterilization study) or 1.6 g/L gelrite agar (for organogenesis). The medium was autoclaved at 121˚C for 15 min. In the sterilization study, the explants were cultured on solid medium in Petri dishes while for shoot induction, the explants were placed on solid medium in culture tubes (diameter: 2.5 cm; height: 10 cm). Each treatment consisted of 50 replicates and the whole experiment was carried out twice.
2.2. Explant Surface Sterilization
Three types of explants—shoot tip, node, and internodewere harvested from young shoots of H. rosa-sinensis L. plants (the shoot harvested contained a maximum of three nodes counting from the shoot tip) that grew beside the Sultan Abdul Samad Library, Universiti Putra Malaysia, Selangor Darul Ehsan, Malaysia, and was authenticated with the Voucher Specimen Number: SK1560/08.
The nodes, internodes and shoot tips were cut into 2 cm long before washing under running tap water for 30 min. One drop of detergent (Dynamo) was added into the water and the washing was continued for an additional min. The detergent was removed under the running tap water and the explants were rinsed with distilled water three times. Commercial Clorox solution [5.25% (w/v) NaOCl] diluted to concentrations ranging from 5 to 50% (v/v), were tested on decontaminating all explants except the shoot tips, which were only subjected to 5% to 20% (v/v) of the Clorox solution. All treatments (except for shoot tips) were subjected to a Clorox exposure time ranging from 5 to 30 min at 5-min intervals; while for shoot tip, it was 10 to 60 min exposure time at 10-min intervals (Table 1). One drop of Tween-20 was added to the Clorox solution prior to sterilization. After each treatment, the explants were thoroughly washed with sterile deionized water 3 times [22], each comprising 10 min. After the sterilization steps above and prior to culturing on the nutrient medium, each node, internode and shoot tip explants were trimmed into 1 cm length. Each treatment for the node and internode explants, respectively, consisted of 45 replicates, while shoot tip comprised of 25 replicates due to limited samples. All cultures were maintained at 27˚C ± 1˚C under a 16 h-photoperiod provided by cool-white fluorescent tubes at 25 mMol photon/m2/s.
For sterilization, observation was taken daily for 35 days to record the percentages of contamination and survival of the explants. All experiments were carried out twice.
2.3. Direct Organogenesis
Sterile nodal explants were cultured in tubes containing the relevant MS medium formulation (as described previously in culture medium). The nodal explants were first subjected to treatment of different concentrations of sucrose. Following which, the optimized sucrose concentration was chosen for subsequent assessment of the organic ingredients and activated charcoal. The explants were maintained at 27˚C ± 1˚C under a 16 h-photoperiod provided by cool-white fluorescent tubes at 25 mMol photon/m2/s. Observation was taken weekly for 6 weeks to
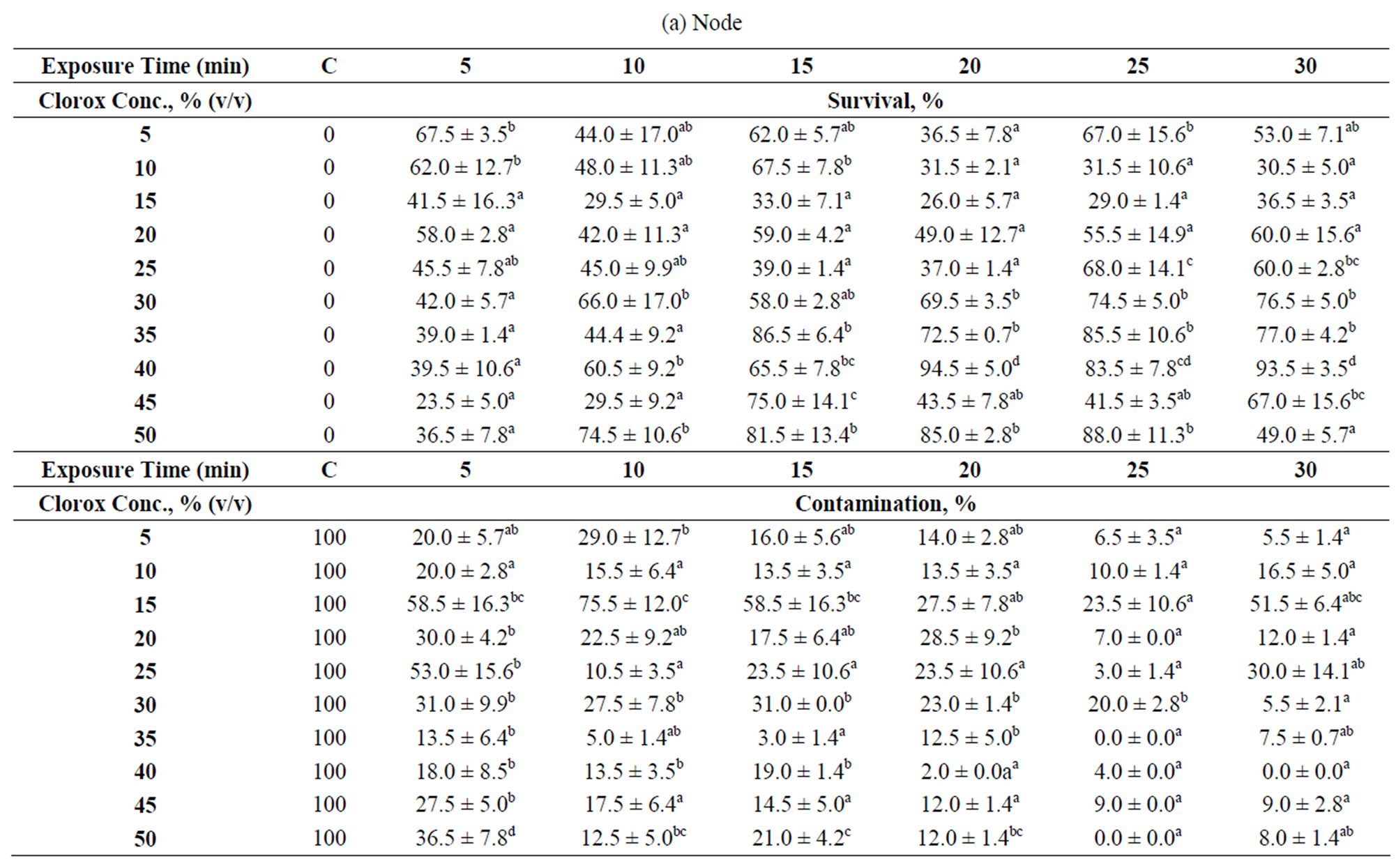
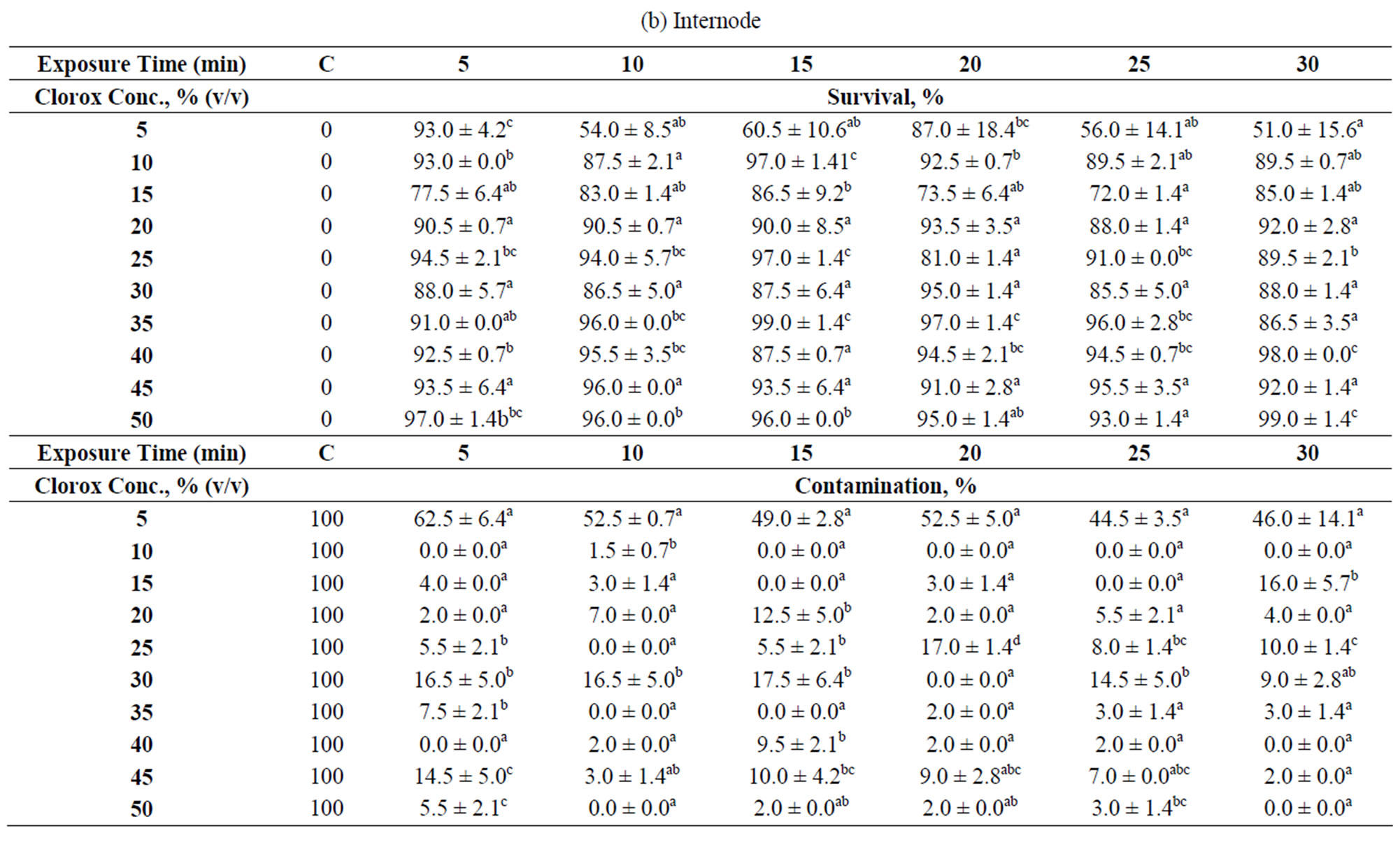

Table 1. Explants treatments at different Clorox concentrations and exposure times. Data were taken after 35 days of culture which were displayed as survival and contamination percentages: (a) Node; (b) Internode; (c) Shoot tip. “C” denotes control. The numbers followed by different superscript letters (a, b, c, d, e) within a column are significantly different at p < 0.05 by Duncan’s multiple range test (DMRT). Data is the mean ± standard deviation (SD). Means are the results of 2 experiments.
record the percentages of survival and regeneration of explants, mean leaf length (cm), and mean number of leaf. All experiments were carried out twice.
2.4. Statistical Analysis
The experiments were performed in a completely randomized design (CRD). Data were subjected to one-way analysis of variance (ANOVA) using SPSS version 16 software (SPSS Inc., Chicago, USA). Multiple comparisons among means were performed using Duncan’s multiple range test with the level of significance at p = 0.05.
3. Results and Discussion
3.1. Explant Surface Sterilization
Three types of explants (node, internode and shoot tip) were obtained from young hibiscus stems and subjected to different surface sterilization conditions as summarized in Table 1. The best sterilization condition for each explant type was scored based on the highest survival rate with minimal contamination.
The results show that nodal explants is the most difficult to sterilize, followed by shoot tip and internode. The nodal explant (node) bears an axillary bud located in a “V” shape trough, formed between the main stem and leaf petiole. Such shape easily traps contaminants and poses a barrier to water current during sterilization. The internodal explant has a smooth and small surface area compared to the other two explants (node and shoot tip) used in this study. A smooth surface is a poor “landing site” for microbial attachment because they are often easily ousted under strong water current, and hence, a lower chance for the explants to get contaminated. Both of these characteristics seem to contribute to a low contamination percentage (<10%) and high survival rate (>80%) as observed for 90% of the treatments (Table 1(b)).
For the shoot tip and nodal explants, the results show that increasing Clorox concentration with short exposure time was not effective in removing contaminants. For a 5-min exposure time, the contamination percentage using 5% (v/v) Clorox is comparable to almost all the Clorox levels tested. However, increasing the exposure time seems to be more favorable. Indeed, most researchers used longer exposure time (≥10 min) for nodal explant sterilization from varying plant species [23-25]. If a shorter exposure time is required, usually HgCl2 is used [26,27]. In this study, 75% of the treatments were able to confine the contamination percentages within 20%, and the explants survival percentages within the range of 30% - 70%. The best treatment for the node is 40%—20 min [2.1% (w/v) NaOCl)], a concentration comparable to that of the seeds from other plants [28,29]. It is interesting to find that Christensen et al. [13] applied 0.01% (w/v) NaOCl for H. rosa-sinensis nodal explants sterilization, a dose 210 times lower than the best treatment for the nodal explants found in this study. Such discrepancies could be attributed to the location of the sampling site—greenhouse versus open environment. This indicates that by controlling the plants’ exposure to outside environment can limit the level of contamination on the plant itself.
By taking into account of both the contamination and survival percentages, 10% - 15% min is the best treatment for the internode. Internodal explants subjected to this treatment did not show any contamination and almost all survived (Table 1(b)). However, under a situation where both the nodal and internodal explants were to be subjected to the disinfectant treatment simultaneously, 40% - 20% min is applicable because both the contamination and survival percentages are not affected significantly (Tables 1(a) and (b)).
The shoot tip was found to be susceptible to dehydration after being excised from the mother plant. We found that the shoot tips did not survive with 30% (v/v) Clorox or higher and also when the sterilization time was 10 min or more. The best treatment for shoot tip is 5%—40 min [0.2625% (w/v) NaOCl], a treatment comparable to Jo et al. [30] who used about 0.15% (w/v) of NaOCl with 30 min exposure for shoot tip of Alocasia amazonica. In contrast, Misra and Chakrabarty [22] used higher concentration4% (w/v) NaOCl, and shorter exposure time, 5 min, to sterilize Rosa clinophylla shoot tip.
3.2. Direct Organogenesis
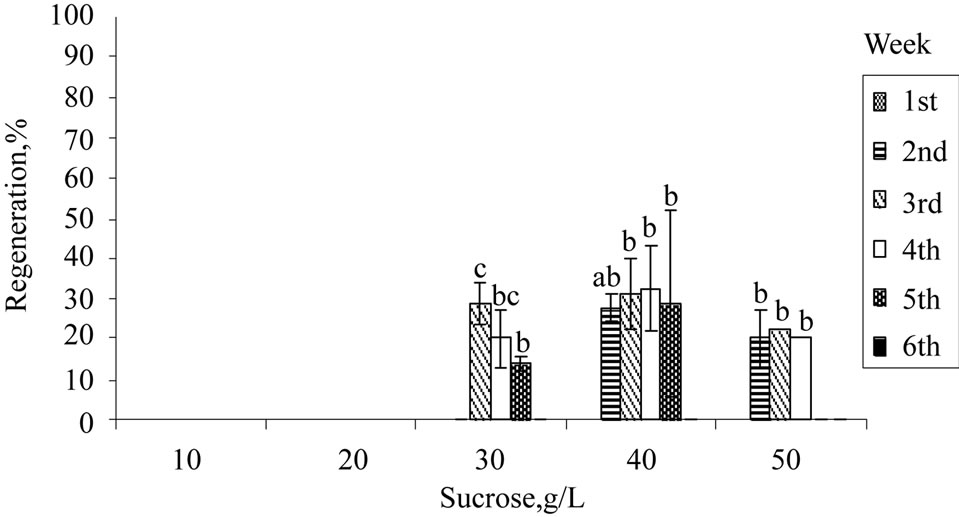
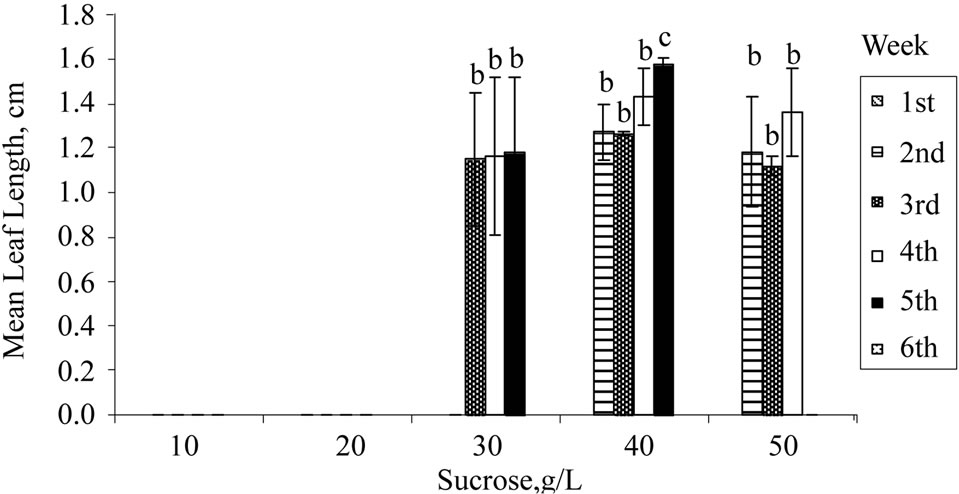
Apart from exogenous supply of PGRs, growth and induction of in vitro shoots are influenced by exogenous carbon source. The in vitro plants are either heterotrophic or semi-autotrophic, and hence an external supply of carbon is necessary. Sucrose is readily hydrolysed into glucose and fructose during autoclave, with the former being used up first and then fructose. Sucrose at 30 g/L (standard MS medium) was used for H. rosasinensis [13,18], H. cannabinus [12,20], H. sabdariffa [31,32] and other in vitro cultures. From the results obtained (Figure 1(a)), 10 and 20 g/L of sucrose were unsuitable for shoot induction. Growth of the shoots improved significantly at 30 g/L or higher. Further increment at 40 g/L showed that 30% of the explants regenerated, and the mean number of leaves produced and mean leaf length achieved were the highest. However, sucrose of more than 40 g/L did not improve the regeneration or the growth rates of the explants.
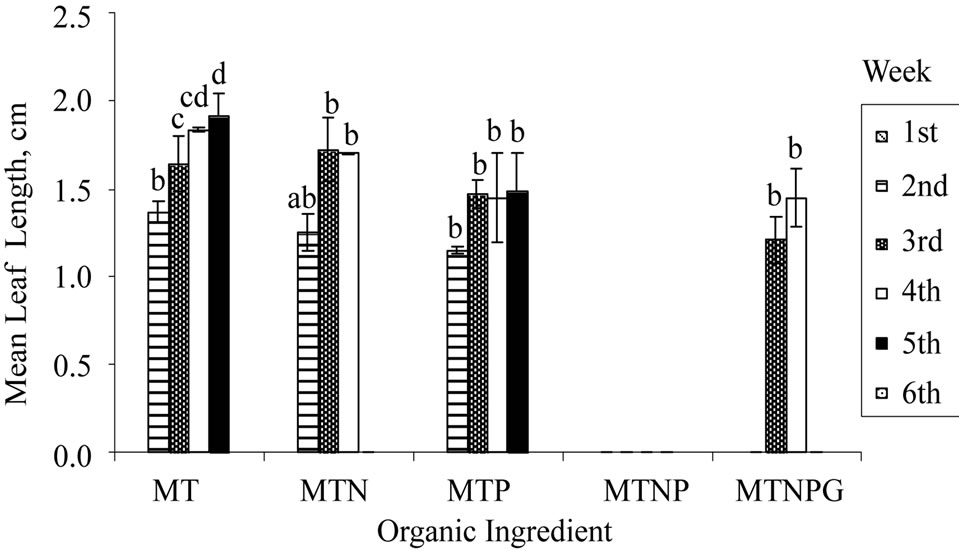
Four types of vitamins (myo-inositol, thiamine, pyridoxine, and nicotinic acid) and one amino acid, glycine were present in the standard MS medium formulation. Of the five supplements, myo-inositol and thiamine are essential for the culture of plant cells in vitro. Thiamine is an essential co-factor in carbohydrate metabolism and is involved in the biosynthesis of certain amino acids in its active form, thiamin diphosphate [33]. Plant cells and tissues could produce some essential vitamins in vitro but at a sub-optimal level, hence an exogenous supply of vitamin is always crucial. Similar to other plant species, all vegetative propagations of H. rosa-sinensis as reported involved the complete MS organic supplementations [13, 18,34]. In contrast to previous reports, the results obtained in this study (Figure 1(b)) revealed that shoots induction with myoinositol and thiamine (MT) supplementations only were achieved at a percentage higher than that with myoinositol, thiamine, and nicotinic acid (MTN). However, the shoots grown with organic supplements consisted of MTN gave better qualitative results of greenier and healthier-looking shoots (result not shown).
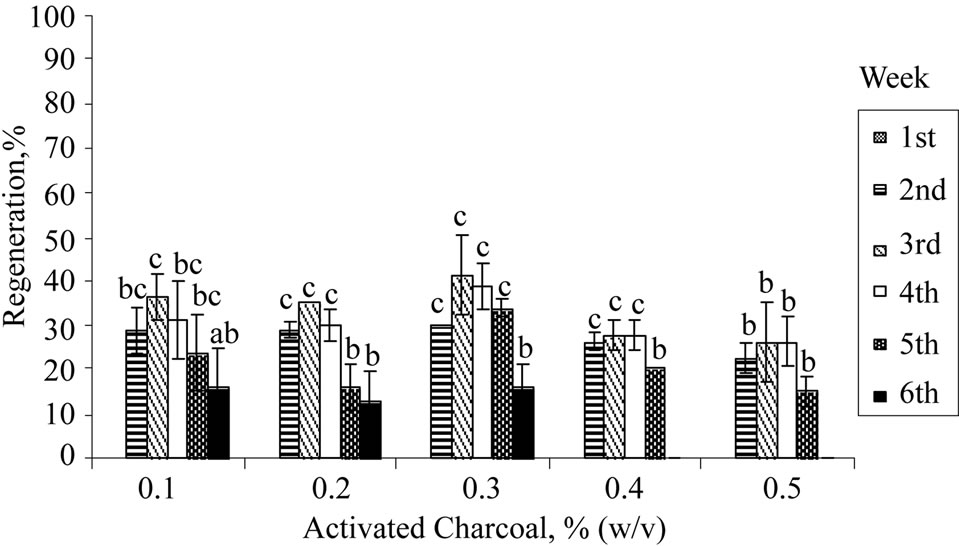
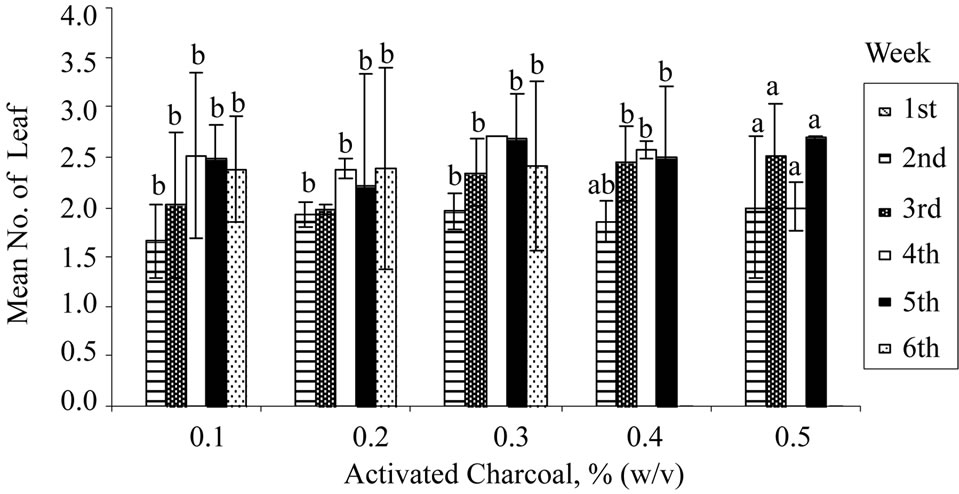
Activated charcoal or carbon (AC) had been reported to be a useful supplement in the plant tissue culture medium for several purposes such as improving rooting [35], shoot elongation [36], preventing necrosis [37], and preventing browning [38]. The fine network of pores and large surface area of AC serves as a useful scavenger to adsorb inhibitory phenolic compounds. As shown in Figure 1(c), nodal explant cultured on medium augmented with 0.3% (w/v) of activated charcoal achieved the highest shoot regeneration rate (40%) at the 3rd week. Leaf abscission and shoot necrosis were delayed until the 6th week,

 (a) Sucrose
(a) Sucrose


 (b) Organic Ingredient
(b) Organic Ingredient
 (c) Activated Charcoal
(c) Activated Charcoal
Figure 1. Regeneration of nodal explants cultured on MS medium, mean number of leaf shooted, and mean leaf length obtained: (a) Sucrose; (b) Organic ingredient; (c) Activated charcoal. Data were taken after 6 weeks of culture. Error bars indicate standard deviations (n = 2). Different letters (a, b, c, d) indicate values that are significantly different at p < 0.05 by Duncan’s multiple range test (DMRT). G: Glycine; M: Myoinositol; N: Nicotinic acid; P: Pyridoxine; T: Thiamine.
Figure 1(c). However, further increment of AC concentration did not improve the observable growth performance of the shoots as shown in Figure 1(c). This may be due to adsorption of the nutrient ions (Cu2+ or Zn2+) and other organic supplements (vitamins) in the medium by the excess amount of AC resulting in lower amounts of nutrients and supplements being made available to the growing cultures [39].
4. Conclusion
The morphology of an explant does affect the effectiveness of the sterilization process. The source of the explants may also play a role in this aspect. Comparison of the three explants used in this study revealed that the most difficult explant type to sterilize is the node while the easiest are the internode and shoot tip. However, shoot tip is prone to desiccation at high Clorox concentration. The recommended MS medium formulation for shoot induction of H. rosa-sinensis L. nodal explant is at least 40 g/L of sucrose supplemented with myoinositol, thiamine.HCl, nicotinic acid and 0.3% (w/v) activated charcoal.
5. Acknowledgements
Chew Tiong Dar is a recipient of a Graduate Research Fellowship from the Universiti Putra Malaysia. Authors would like to thank Associate Professor Dr. Rusea Go, Department of Biology, Faculty of Science, Universiti Putra Malaysia, for her invaluable assistance in identifying the plant species.
REFERENCES
- C. C Chen, J. D. Hsu, S. F. Wang, H. C. Chiang, M. Y. Yang, E. S. Kao, Y. C. Ho and C. J. Wang, “Hibiscus sabdariffa Extract Inhibits the Development of Atherosclerosis in Cholesterol-Fed Rabbits,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 18, 2003, pp. 5472-5477. doi:10.1021/jf030065w
- A. A. Dafallah and Z. Al-Mustafa, “Investigation of the Anti-Inflammatory Activity of Acacia nilotica and Hibiscus sabdariffa,” American Journal of Chinese Medicine, Vol. 24, No. 3-4, 1996, pp. 263-269. doi:10.1142/S0192415X96000323
- C. J. Wang, J. M. Wang, W. L. Lin, C. Y. Chu, F. P. Chou and T. H. Tseng, “Protective Effect of Hibiscus Anthocyanins against Tert-Butyl Hydroperoxide-Induced Hepatic Toxicity in Rats,” Food and Chemical Toxicology, Vol. 38, No. 5, 2000, pp. 411-416. doi:10.1016/S0278-6915(00)00011-9
- H. H. Lin, J. H. Chen, W. H. Kuo and C. J. Wang, “Chemopreventive Properties of Hibiscus sabdariffa L. on Human Gastric Carcinoma Cells through Apoptosis Induction and JNK/p38 MAPK Signaling Activation,” Chemico-Biological Interactions, Vol. 165, No. 1, 2007, pp. 59-75. doi:10.1016/j.cbi.2006.10.011
- S. Venkatesh, J. Thilagavathi and D. Shyamsundar, “AntiDiabetic Activity of Flowers of Hibiscus rosa-sinensis,” Fitoterapia, Vol. 79, No. 2, 2008, pp. 79-81. doi:10.1016/j.fitote.2007.06.015
- V. S. Kasture, C. T. Chopde and V. K. Deshmukh, “Anticonvulsive Activity of Albizzia lebbeck, Hibiscus rosa sinensis and Butea monosperma in Experimental Animals,” Journal of Ethnopharmacology, Vol. 71, No. 1-2, 2000, pp. 65-75. doi:10.1016/S0378-8741(99)00192-0
- V. Hirunpanich, A. Utaipat, N. P. Morales, N. Bunyapraphatsara, H. Sato, A. Herunsalee and C. Suthisisang, “Antioxidant Effects of Aqueous Extracts from Dried Calyx of Hibiscus sabdariffa Linn. (Roselle) in Vitro Using Rat Low-Density Lipoprotein (LDL),” Biological and Pharmaceutical Bulletin, Vol. 28, No. 3, 2005, pp. 481-484. doi:10.1248/bpb.28.481
- R. M. Rosa, M. I. Melecchi, R. da Costa Halmenschlager, F. C. Abad, C. R. Simoni, E. B. Caramao, J. A. Henriques, J. Saffi and A. L. de Paula Ramos, “Antioxidant and Antimutagenic Properties of Hibiscus tiliaceus L. Methanolic Extract,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 19, 2006, pp. 7324-7330. doi:10.1021/jf061407b
- A. Adetutu, O. A. Odunola, O. A. Owoade, O. A. Adeleke and O. A. Amuda, “Anticlastogenic Effects of Hibiscus sabdariffa Fruits against Sodium Arsenite-Induced Micronuclei Formation in Erythrocytes in Mouse Bone Marrow,” Phytotherapy Research, Vol. 18, No. 10, 2004, pp. 862-864. doi:10.1002/ptr.1554
- E. O. Farombi and A. Fakoya, “Free Radical Scavenging and Antigenotoxic Activities of Natural Phenolic Compounds in Dried Flowers of Hibiscus sabdariffa L.,” Molecular Nutrition and Food Research, Vol. 49, No. 12, 2005, pp. 1120-1128. doi:10.1002/mnfr.200500084
- B. H. Hartman, D. E. Kester and F. T. Davies, “Plant Propagation: Principles and Techniques,” Prentice Hall, Hoboken, 1994.
- S. P. Herald, T. Suzuki and K. Hattori, “Multiple Shoot Regeneration from Young Shoots of Kenaf (Hibiscus cannabinus)” Plant Cell, Tissue and Organ Culture, Vol. 77, No. 1, 2004, pp. 49-53. doi:10.1023/B:TICU.0000016497.79856.9a
- B. Christensen, S. Sriskandaraja, M. Serek and R. Muller, “In Vitro Culture of Hibiscus rosa-sinensis L.: Influence of Iron, Calcium and BAP on Establishment and Multiplication,” Plant Cell, Tissue and Organ Culture, Vol. 93, No. 2, 2008, pp. 151-161. doi:10.1007/s11240-008-9354-4
- M. G. Ostrolucka, G. Libiakova, E. Ondruskova and A. Gajdosova, “In Vitro Propagation of Vaccinium Species,” Acta Universitatis Latviensis, Vol. 676, 2004, pp. 207-212.
- R. Rashid and S. S. Bal, “Effect of Hormones on Direct Shoot Regeneration in Hypocotyl Explants of Tomato,” Notulae Scientia Biologicae, Vol. 2, No. 1, 2010, pp. 70-73.
- M. M. Bazargani, B. E. S. Tabatabaei and M. Omidi, “Multiple Shoot Regeneration of Cotton (Gossypium hirsutum L.) via Shoot Apex Culture System,” African Journal of Biotechnology, Vol. 10, No. 11, 2011, pp. 2005-2011.
- E. Ebrahimie, A. Hosseinzadeh and M. R. Nagavi, “Combined Direct Regeneration Protocols in Tissue Culture of Different Cumin Genotypes Based on Pre-Existing Meristems,” Pakistan Journal of Biological Sciences, Vol. 10, No. 9, 2007, pp. 1359-1369. doi:10.3923/pjbs.2007.1360.1370
- S. Bhalla, J. O. Abdullah, S. Sreenamanan and C. Karuthan, “Shoots Induction from Hibiscus rosa-sinensis Nodal Explant Using N6-benzylaminopurine (BAP),” Research Journal of Agriculture and Biological Sciences, Vol. 5, No. 4, 2009, pp. 403-410.
- C. Zapata, M. Srivatanakul, S. H. Park, B. M. Lee, M. G. Salas and R. H. Smith, “Improvements in Shoot Apex Regeneration of Two Fiber Crops: Cotton and Kenaf,” Plant Cell, Tissue and Organ Culture, Vol. 56, No. 3, 1999, pp. 185-191. doi:10.1023/A:1006238924439
- R. Ayadi, L. Hamrouni, M. Hanana, S. Bouzid, M. Trifi and M. L. Khouja, “In Vitro Propagation and Regeneration of an Industrial Plant Kenaf (Hibiscus cannabinus L.),” Industrial Crops and Products, Vol. 33, No. 2, 2011, pp. 474-480. doi:10.1016/j.indcrop.2010.10.025
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
- P. Misra and D. Chakrabarty, “Clonal Propagation of Rosa clinophylla Thory. Through Axillary Bud Culture,” Scientia Horticulturae, Vol. 119, No. 2, 2009, pp. 212- 216. doi:10.1016/j.scienta.2008.07.028
- O. I. Odutayo, R. T. Oso, B. O. Akinyemi and N. A. Amusa, “Microbial Contaminants of Cultured Hibiscus cannabinus and Telfaria occidentalis Tissues,” African Journal of Biotechnology, Vol. 3, No. 9, 2004, pp. 473-476.
- Z. Jabbarzadeh and M. Khosh-Khui, “Factors Affecting Tissue Culture of Damask Rose (Rosa damascena Mill.),” Scientia Horticulturae, Vol. 105, No. 4, 2005, pp. 475- 482. doi:10.1016/j.scienta.2005.02.014
- X. Liu and P. M. Pijut, “Plant Regeneration from in Vitro Leaves of Mature Black Cherry (Prunus serotina),” Plant Cell, Tissue and Organ Culture, Vol. 94, No. 2, 2008, pp. 113-123. doi:10.1007/s11240-008-9393-x
- G. Sujatha and B. D. R. Kumari, “Micropropagation, Encapsulation and Growth of Artemisia Vulgaris Node Explants for Germplasm Preservation,” South African Journal of Botany, Vol. 74, No. 1, 2008, pp. 93-100. doi:10.1016/j.sajb.2007.09.002
- G. Mahendran and V. N. Bai, “Mass propogation of Satyrium nepalense D.Don.—A Medicinal Orchid via Seed Culture,” Scientia Horticulturae, Vol. 119, No. 2, 2009, pp. 203-207. doi:10.1016/j.scienta.2008.07.029
- Y. H. Li, J. P. Gao and S. Z. Fei, “High Frequency Embryogenic Callus Induction and Plant Regeneration from Mature Caryposis of Big Bluestem and Little Bluestem,” Scientia Horticulturae, Vol. 121, No. 3, 2009, pp. 348- 352. doi:10.1016/j.scienta.2009.02.002
- M. Srivatanakul, S. H. Park, J. R. Sanders, M. G. Salas and R. H. Smith, “Multiple shoot Regeneration of Kenaf (Hibiscus cannabinus L.) from a Shoot Apex Culture System,” Plant Cell Reports, Vol. 19, No. 2, 2000, pp. 1165- 1170. doi:10.1007/s002990000256
- E. A. Jo, R. K. Tewari, E. J. Hahn and K. Y. Paek, “In Vitro Sucrose Concentration Affects Growth and Acclimatization of Alocasia amazonica Plantlets,” Plant Cell, Tissue and Organ Culture, Vol. 96, No. 3, 2009, pp. 307- 315. doi:10.1007/s11240-008-9488-4
- J. F. Gomez-Leyva, L. A. Martinez-Acosta, I. G. LopezMuraira, H. Silos Espino, F. Ramirez-Cervantes and I. Andrade-Gonzalez, “Multiple Shoot Regeneration of Roselle (Hibiscus sabdariffa L.) from a Shoot Apex Culture System,” International Journal of Botany, Vol. 4, No. 3, 2008, pp. 326-330. doi:10.3923/ijb.2008.326.330
- J. Govinden-Soulange, N. Boodia, C. Dussooa, R. Gunowa, S. Deensah, S. Facknath and B. Rajkomar, “Vegetative Propagation and Tissue Culture Regeneration of Hibiscus sabdariffa L. (Roselle),” World Journal of Agricultural Sciences, Vol. 5, No. 5, 2009, pp. 651-661.
- Q. Du, H. Wang and J. Xie, “Thiamin (Vitamin B1) Biosynthesis and Regulation: A Rich Source of Antimicrobial Drug Targets” International Journal of Biological Sciences, Vol. 7, No. 1, 2011, pp. 41-52. doi:10.7150/ijbs.7.41
- M. Airo, G. Giardina, G. Farruggia and G. V. Zizzo, “In Vitro Propagation of Hibiscus rosa-sinensis (L.),” Acta Horticulturae, Vol. 812, 2009, pp. 107-112.
- N. P. Makunga and J. Van Staden, “An Efficient System for the Production of Clonal Plantlets of the Medicinally Important Aromatic Plant: Salvia africana-lutea L.,” Plant Cell, Tissue and Organ Culture, Vol. 92, No. 1, 2008, pp. 63-72. doi:10.1007/s11240-007-9305-5
- M. M. Datta, A. Majumder and S. Jha, “Organogenesis and Plant Regeneration in Taxus wallichiana (Zucc.),” Plant Cell Reports, Vol. 25, No. 1, 2006, pp. 11-18. doi:10.1007/s00299-005-0027-z
- B. R. Shrestha, K. Tokuhara and M. Mii, “Plant Regeneration from Cell Suspension-Derived Protoplasts of Phalaenopsis,” Plant Cell Reports, Vol. 26, No. 6, 2007, pp. 719-725. doi:10.1007/s00299-006-0286-3
- Y. Guo, J. Bai and Z. Zhang, “Plant Regeneration from Embryogenic Suspension-Derived Protoplasts of Ginger (Zingiber officinale Rosc.),” Plant Cell, Tissue and Organ Culture, Vol. 89, No. 2-3, 2007, pp. 151-157. doi:10.1007/s11240-007-9223-6
- G. S. Pullman and S. Johnson, “Somatic Embryogenesis in Loblolly Pine (Pinus taeda L.): Improving Culture Initiation Rates,” Annals of Forest Science, Vol. 59, No. 5-6, 2002, pp. 663-668. doi:10.1051/forest:2002053
NOTES
*Corresponding author.

