American Journal of Plant Sciences
Vol.3 No.2(2012), Article ID:17580,8 pages DOI:10.4236/ajps.2012.32032
Regio- and Substrate-Specific Oxidative Metabolism of Terpinolene by Cytochrome P450 Monooxygenases in Cupressus lusitanica Cultured Cells
![]()
1Department of Sustainable Bioresources Science, Faculty of Agriculture, Kyushu University, Fukuoka, Japan; 2Department of Environment, Faculty of Agriculture, Yamagata University, Tsuruoka, Japan.
Email: *koki-fujita@agr.kyushu-u.ac.jp
Received November 14th, 2011; revised December 6th, 2011; accepted December 27th, 2011
Keywords: Terpenoid Metabolism; Chytocrome P450; Cupressus Lusitanica; β-Thujaplicin; Oxidase
ABSTRACT
Many of monoterpenes produced in plants contribute to defenses against herbivores, insects and microorganisms. Among those compounds, β-thujaplicin formed in Cupressaceae plants has a unique conjugated seven-membered ring and some useful biological activities, e.g. fungicide, repellent, insecticide and so on. The biosynthesis pathway of β- thujaplicin has not yet been revealed; we have been trying to uncover it using Cupressus lusitanica cultured cells as a model. In our previous study, terpinolene was identified as a potential β-thujaplicin intermediate at the branching point to terpenoids. In this article, terpinolene metabolism in C. lusitanica cultured cells was investigated, and it was shown that the microsomal fraction from cells oxidized terpinolene into the hydroxylated compound, 5-isopropylidene-2-methylcyclohex-2-enol (IME). Then, IME was further oxidized by microsomal fraction to the epoxidized compound, 1,6- epoxy-4(8)-p-menthen-2-ol (EMO). These were the only two products detected from the microsomal reactions, respecttively. Moreover, microsomal reactions with monoterpenes other than terpinolene produced nothing detectable. These results show that the enzymes of these reactions had strict substrate specificity and regio-selectivity. Experiments on kinetics and with specific inhibitors confirmed that these reactions were caused by cytochrome P450 monooxygenases, respectively. These results support our hypothesis that terpinolene is a putative intermediate of β-thujaplicin biosynthesis and show that IME and EMO are also putative intermediates.
1. Introduction
Terpenoids are a group of compounds consisting of 5- carbon isoprene units. Their reactions result in a huge number of compounds, over 40,000 substances [1]. The commercial importance of terpenes is based on their flavors and fragrances in cosmetics, foods, soaps and so on. Therefore, agricultural research on terpenoids in fruits and flowers are in progress [2]. In addition, terpenes have various pharmacological roles-antimalarial and anti-inflammatory activities, etc. and ecological influences related to the plant defense system: antifungal activity, attraction of natural enemies of predators and signaling to other plants [3,4].
Some Cupressaceae trees produce β-thujaplicin (also called hinokitiol in Japan) as a defensive compound. This substance is known to have an important role in preserveing its heart wood, and exerts strong activity as fungicide, insecticide and/or their repellent [5,6]. In humans, it has been reported to have activity against some viruses and anticancer activity [7,8]. The structure of β-thujaplicin is based on a unique conjugated seven-membered ring called a tropolone ring.
In our laboratory, we have been trying to reveal the biosynthetic pathway of β-thujaplicin in Cupressus lusitanica (Mexican Cypress) cultured cells. This cell culture system is a very convenient model for metabolic analysis of β-thujaplicin and related terpenes. C. lusitanica cultured cells does not produce β-thujaplicin, until when it is exposed to an elicitor or other stress; therefore, they have been used to investigate the β-thujaplicin biosynthesis pathway and its related enzymes. So far, our group has shown by some feeding experiments that β-thujaplicin is synthesized from geraniol (actually, geranyl diphosphate (GDP)), which indicates that β-thujaplicin is a monoterpene; further, implied that th intermediate of β-thujaplicin biosynthesis would have a menthane-like carbon skeleton [9]. Elicitor-stressed cells showed extremely high terpinolene synthase activity, and its time course profile corresponds to that of β-thujaplicin concentration [10]; additionally, 1,6-epoxy-4(8)-p-menthen-2-ol (EMO) and 4(8)-p-menthen-1,2-diol existed in elicitor-treated cells [11]. From these results, we have speculated that the terpinolene metabolism pathway (Figure 1) and these substances comprise the potent intermediates of β-thujaplicin biosynthesis. Tropolonoid biosynthesis pathways have been shown in fungi [12] and bacteria [13], however, the pathways in plants have not been uncovered yet, except for the colchicine derivatives in Colchicum plants. Those are composed from dopamine and 4-hydroxycinnamic acid [14]; therefore, the origin and the pattern of ring formation must be different from those of β-thujaplicin.
In this paper, crude enzyme reactions with some monoterpenes found in C. lusitanica cultured cells were performed and the reaction products were analyzed and characterized. Hydroxylations of allylic positions and epoxidation of double bonds of monoterpenes have previously been reported in plants of some cytochrome P450 monooxygenases reacting with monoterpenes [15-18]; hence, the involvement of cytochrome P450s in this terpinolene metabolism was investigated. As a result, we discovered the two-step regioand substrate-specific oxygenation of terpinolene by cytochrome P450 monooxygenases and describe those processes here.
2. Experimental Procedures
2.1. Cell Culture Conditions
Callus cultures of C. lusitanica were maintained in Gamborg B5 medium [19] supplemented with 0.01 mM benzyl aminopurine, 10 mM-naphthyl acetic acid, 20 g/L sucrose and 2.7 g/L gellan gum at pH 5.5 for more than 10 years. No terpene including β-thujaplicin was produced under this growth condition. For microsomal fraction preparation, ca. 5 g of C. lusitanica cells growing in solid B5 medium for 4 weeks was transferred to 30 ml of liquid production medium [20]. To initiate terpene production, 2 ml of partially purified yeast extract solution was added as an elicitor [5]. After 24 h of 25˚C, 70 rpm shaking,
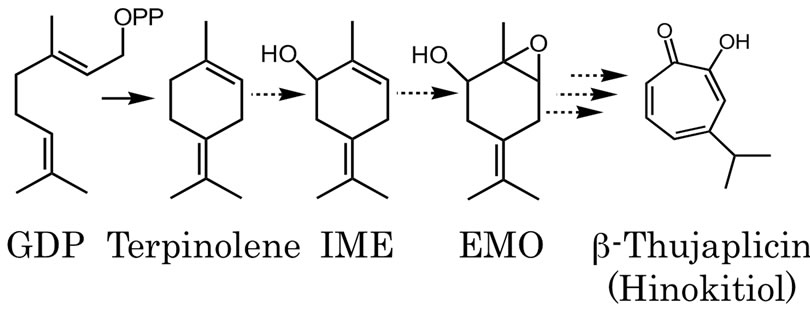
Figure 1. Expected oxidized monoterpene biosynthesis pathway in C. lusitanica cultured cells initiated from GDP and terpinolene. The dotted arrows indicate unrevealed reactions and the several arrows indicate multiple enzymatic steps.
dark incubation, cells were separated from medium and stored at –80˚C.
Preparation of microsomal fraction—Eight g of cells were ground in a pre-chilled mortar and pestle with liquid nitrogen, and suspended in 200 ml of pre-chilled buffer under the conditions reported by Bouwmeester et al. [21]. The homogenate was transferred to a small bottle, sonicated for 3 min in 10 s pulses at 35% power (USP-400A SHIMADZU, Kyoto, Japan) and stirred for 12 min. The homogenate was centrifuged at 20,000 g for 20 min, followed by filtration with a glass fiber filter, and then at 150,000 g for 90 min. Microsomal pellets were either used directly for assay or stored at –80˚C.
2.2. Microsomal Reaction
Microsomal pellets were resuspended in assay buffer [21]. One ml of microsomal suspension was incubated in a 20 ml teflon-lined screw cap vial flushed with O2 gas, and the reaction was started by the addition of 1 mM NADPH, an NADPH-regenerating system (5 mM glucose-6-phosphate, 1 i.u. ml–1 glucose-6-phospate dehydrogenase) and 550 nmol of terpinolene or 315 nmol of 5-isopropylidene-2-methyl-cyclohex-2-enol (IME) (10 l of acetone stock, respectively). Control assays were performed with the enzyme solution boiled for 15 min before incubation or the enzyme reaction without substrates. After incubation for 30 min or 1 h at 30˚C, reactions were stopped by addition of Et2O or ethyl acetate. As an internal standard, 1.5 nmol dodecyl alcohol was added to the reaction mixtures, then the mixtures were extracted twice with Et2O or ethyl acetate. The organic phases were transferred to a fresh vial, washed with water, dehydrated with anhydrous MgSO4 and passed over a short silica gel column. The fractions of reaction products were concentrated by a stream of N2 and analyzed with GC/ MS (HP 5890 series II and HP 5972; Hewlett Packard, Palo Alto, CA). A fused silica capillary column InertCap 5 (length, 30 m; i.d., 0.25 mm; film thickness, 0.25 μm, GL Sciences, Tokyo, Japan) was used. Helium was used as the carrier gas with a column head pressure of 100 kPa. The oven temperature was kept at 70˚C for 3 min and increased with a gradient of 10˚C/min up to 250˚C. The temperature of the inlet port was 220˚C and that of the interface to MS was 250˚C. Enzyme activity rates were linear for over 30 min for terpinolene oxygenase and for over 60 min for IME oxygenase.
O2-free atmosphere reactions were performed in vials flushed with argon immediately before addition of substrate and cofactors. To distinguish from peroxidase, the concentration of 25 i.u. ml–1 of catalase was employed. Quantitative analysis with GC/MS was performed with selected ion-monitoring mode: for IME m/z, 109, 119, 134, 152; for EMO m/z, 91, 107, 135, 168; and for internal standard m/z, 55, 68. Other conditions were the same as before. The assays were performed biologically in duplicate or triplicate.
2.3. Reactions with Inhibitors
For CO atmosphere reactions, reaction mixtures were bubbled with CO for 5 min before addition of NADPH and NADPH-regenerating systems.
Cytochrome P450 monooxygenase-specific inhibitors were also tested. Miconazole and clotrimazole were added to the reaction mixture in 4 to 8 l of DMSO and incubated 15 min at 30˚C prior to the addition of substrate and cofactors. The assays were performed in triplicate.
2.4. Preparation of Authentic IME
Authentic IME was chemically synthesized by Motherwell’s method [22]. Briefly, α-Pinene oxide (2.4 ml, 15 mmol) was added to a stirred solution of p-toluene sulfonic acid (2.85 g, 15 mmol) in DMF (100 ml), and the reaction mixture was stirred at room temperature for 1 h to overnight. Reaction products were derived with water and Et2O. Then the organic phase was washed with water, dehydrated and concentrated under a vacuum. The reaction products were separated with alumina (aluminum oxide 90 active neutral, Merck, Darmstadt, Germany) column chromatography (hexane: ethyl acetate = 95:5). The purity and structure of synthesized IME was confirmed by analysis of GC/MS, 1Hand 13C-NMR.
2.5. Preparation of Authentic EMO
Because EMO has already been discovered in extracts of C. lusitanica cells and medium [11], authentic EMO was obtained by extraction and purification of the cells and medium. Cells were incubated in production medium with elicitor for three days, separated from the medium, and ground with a mortar and pestle. Then cells and medium were extracted with ethyl acetate together. The organic phase was washed, dehydrated and concentrated under vacuum. EMO was isolated from the extracts by silica gel column chromatography (hexane: ethyl acetate = 30:70). The purity of the obtained EMO was confirmed by GC/MS analysis.
3. Results
3.1. Terpinolene Metabolism
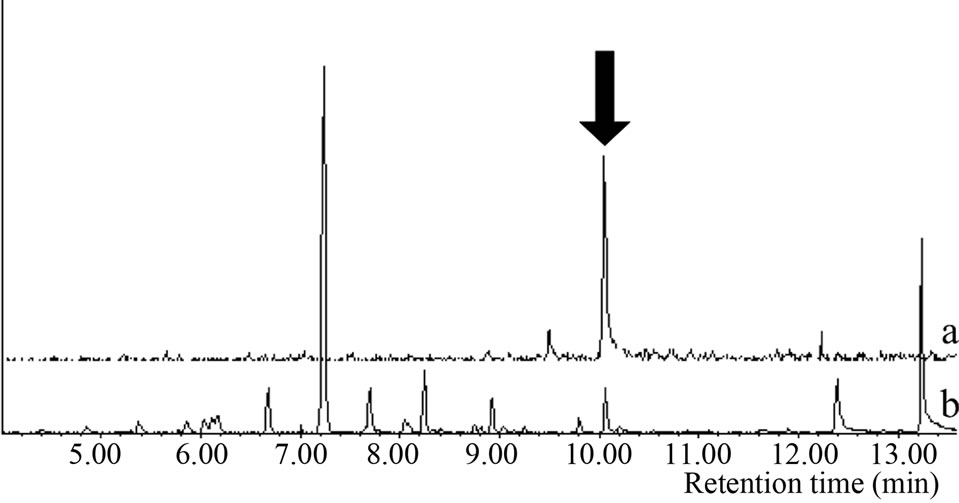
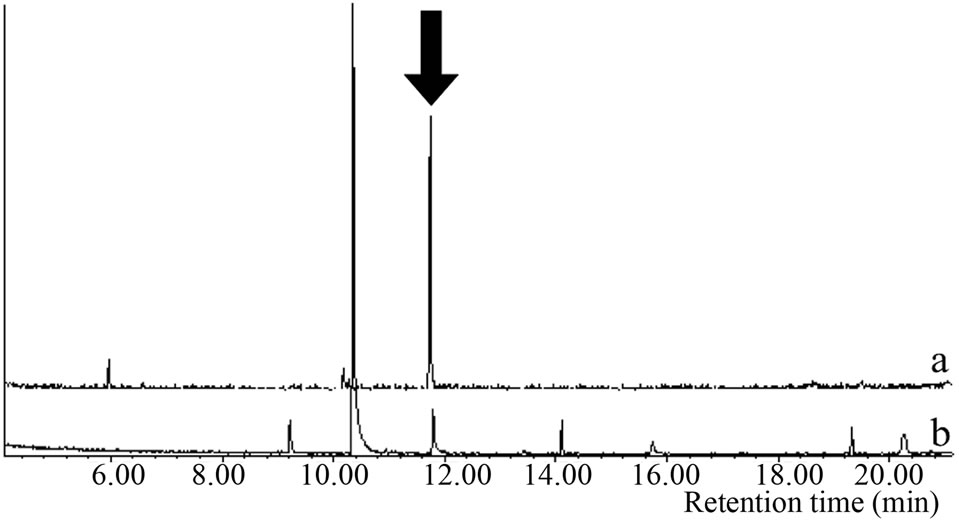
Microsomal reaction with terpinolene was carried out first. The reaction products were analyzed with GC/MS and the peak of 10.1 min was newly appeared only in the complete condition; there was no such peak for negative controls (Figure 2(a)). The retention time and mass spectrum of the peak agreed with chemically synthesized IME
 (a)
(a)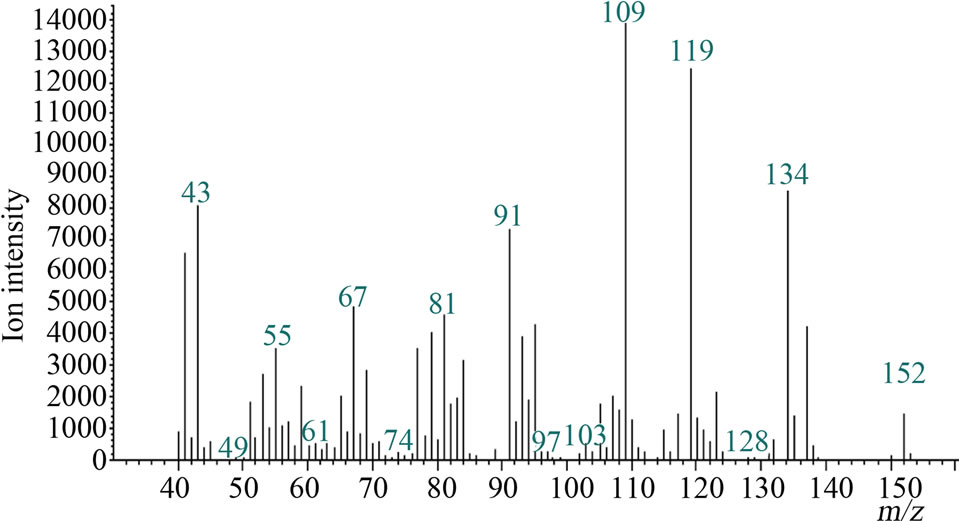 (b)
(b)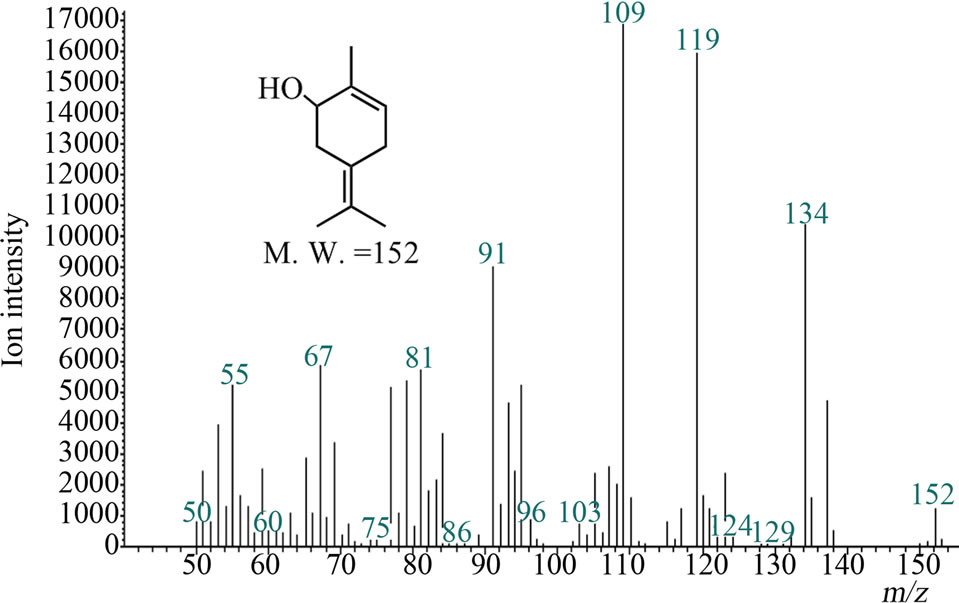 (c)
(c)
Figure 2. Gas chromatograms and mass spectra of microsomal reaction product of terpinolene and authentic IME: (a) Chromatograms of authentic IME “a” and reaction product “b”. The peak at 7.2 min was terpinolene. The peak in negative controls; (b) Mass spectrum of 10.1-min peak of microsomal reaction product; (c) Mass spectrum of authentic IME.
(Figures 2(a)-(c)). Other products regarded as being produced from terpinolene (e.g. hydroxylated at another position or epoxidized terpinolene) were not detected. Heat-deactivated enzyme solution and the reaction without terpinolene did not produce the substance (data not shown). From these results, it was shown that terpinolene was hydroxylated to IME by enzymes in a microsomal fraction of C. lusitanica cultured cells and that no other reaction occurred.
3.2. Characterization of Terpinolene Hydroxylase
Hydroxylations of cyclic and acyclic monoterpene olefins at allylic positions by cytochrome P450 monooxygenases were previously reported [15-18]; therefore, it was confirmed that the oxidation of terpinolene to IME was catalyzed by cytochrome P450s. To prove the involvement of a P450 enzyme, O2- and NADPH-removing reactions, CO atmosphere reactions, catalase-applying reactions and P450-specific inhibitor reactions (using miconazole and clotrimazole) were performed. Results are summarized in Table 1. The microsomal reaction without O2, performed under an Ar atmosphere, did not produced detectable level of IME. The value of IME produced under CO atmosphere was about 30% less than that of the O2 atmosphere reaction. Then, the microsomal reaction with catalase was performed to rule out the involvement of hydrogen peroxide. Catalase did not inhibit production of IME, i.e. denying the possibility of peroxidase reaction. Cytochrome P450-specific inhibitors confirmed its involvement; the IC50 values of miconazole and clotrimazole were 52 μM and 47 μM, respectively (Table 1). These are similar to previously reported IC50 values in and spearmint monoterpene hydroxylases [23]. These results showed that terpinolene was metabolized to IME by a cytochrome P450 monooxygenase in C. lusitanica cell culture. The KM value given by the Lineweaverbark plot was 55 μM (data not shown), suggesting that terpinolene was an adequate substrate. This is similar to the values of other plants’ monoterpene hydroxylases previously reported: limonene hydroxylases were from 11 to
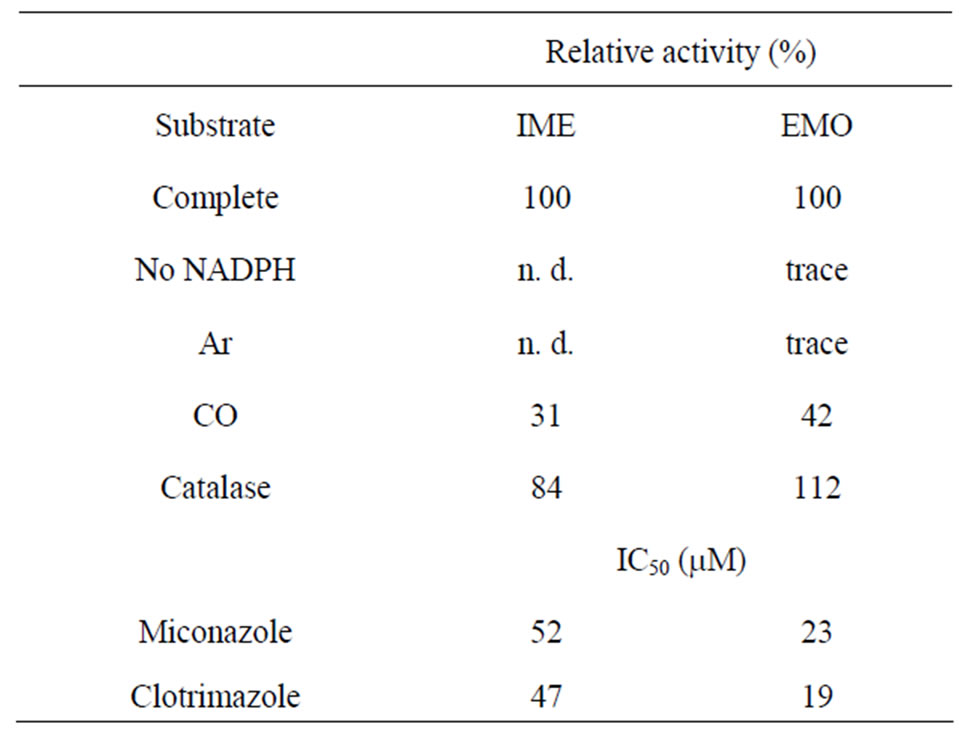
Table 1. Characteristics of terpinolene hydroxylase and IME epoxidase: Relative activity indicates the rate of substrate production as complete reaction was 100%. Complete activityincludes all elements of reaction. No NADPH: NADPH and regenerating system was removed. Ar and CO: replacement of air in reaction vials with each gas. Catalase: complete system with 25 i.u. ml–1 catalase. n. d.: not detected.
21 μM in caraway, peppermint, spearmint and perilla, and geraniol and nerol hydroxylases in Vinca rosea were 5.5 and 11 μM [21-24].
3.3. Microsomal Reactions with Other Monoterpene Olefins
C. lusitanica culture cells produce monoterpenes when they are exposed to elicitor and mechanical stress [5,6].
The cells emit various volatile monoterpenes such as terpinolene, sabinene, limonene, β-ocimene, α-terpinene, myrcene, p-cymene, γ-terpinene, α-pinene, β-pinene and α-terpineol [10] (Figure 3). In order to confirm whether these monoterpene olefins were possible substrates, they were treated with a microsomal fraction from C. lusitanica cells. For this experiment, 2-carene and (±)-3-carene were also used because these two compounds were theoretically proposed by Erdtman as intermediates of β-thujaplicin biosynthesis by the idea from organic chemistry [24].
From the results of GC/MS analysis of the reactions with monoterpenes above, there was no peak thought to be made by oxidized monoterpenes (data not shown). Therefore, oxidation of terpinolene by cytochrome P450s was the dominant reaction in C. lusitanica cells among the monoterpenes emitted from elicitor-treated cells.
3.4. Metabolism of IME
To reveal the metabolism of IME in C. lusitanica culture cells, microsomal reactions were performed with IME, in the same manner as above. There was a newly appeared peak whose retention time and MS agreed with authentic EMO previously identified from cell and medium extracts [11] (Figure 4). In the case of deactivation by heat or IME-free reaction, no EMO peak was observed. From the chromatogram of the enzyme reaction product, no other peaks estimated to be produced from IME were
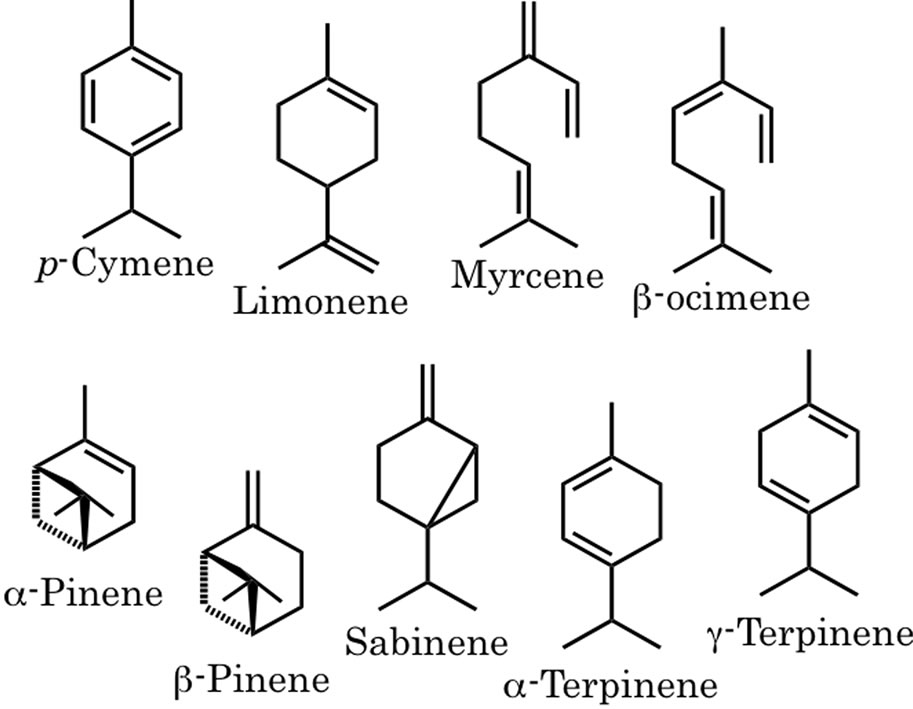
Figure 3. Monoterpene structures other than terpinolene emitted form C. lusitanica cells by initiation of elicitor.
 (a)
(a)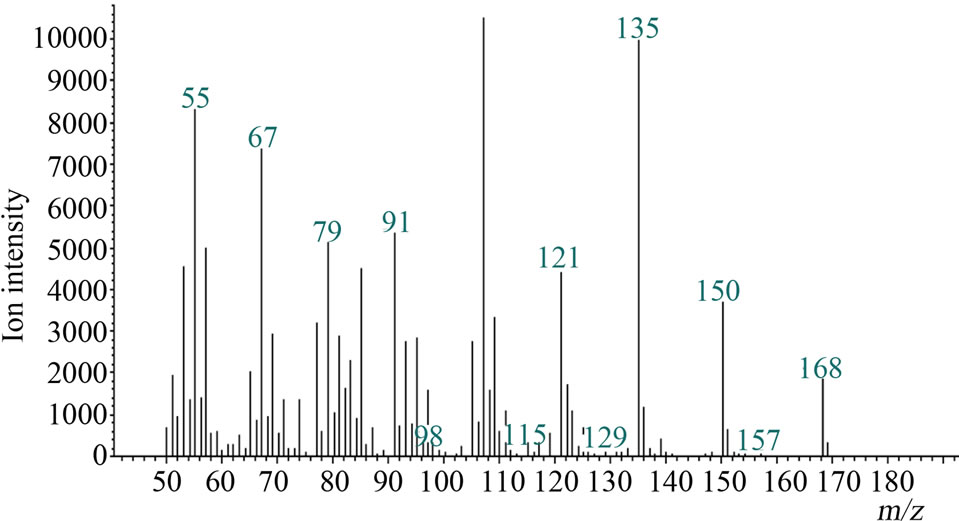 (b)
(b)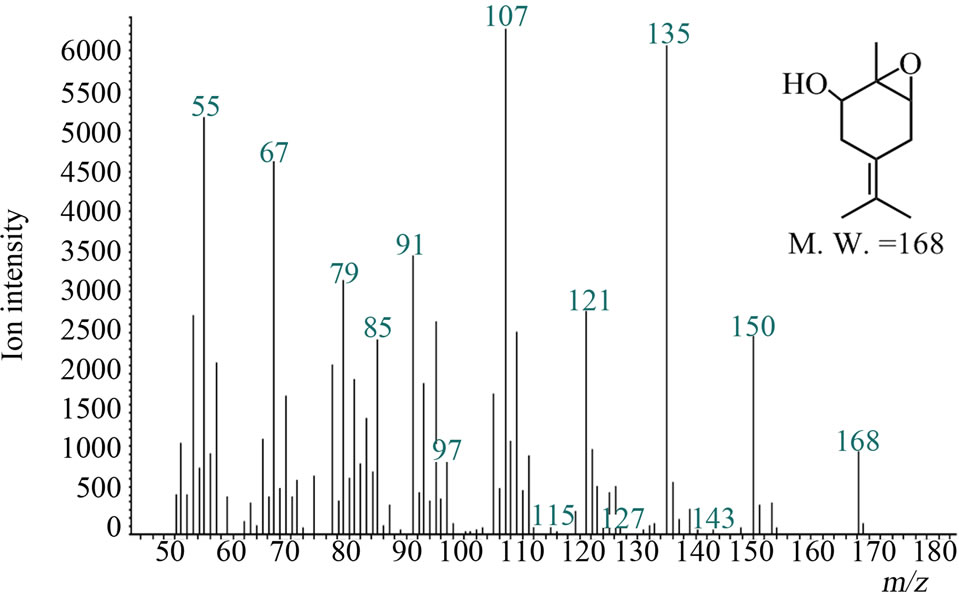 (c)
(c)
Figure 4. Gas chromatograms and mass spectra of microsomal reaction product of IME and authentic sample of EMO: (a) Chromatograms of authentic EMO “a” and reaction product “b”. The peak at 10.4 min was IME. The peak at 11.8 min appeared after the reaction and wasn’t detected in negative controls; (b) Mass spectrum of 11.8-min peak of microsomal reaction product; (c) Mass spectrum of authentic EMO.
found: for example, further hydroxylation of IME, epoxidation at the C4-8 double bond or further oxidation of the hydroxyl group to ketone. These results indicated that IME was the sole substrate for EMO production and also that EMO was the sole product of IME.
3.5. Characterization of IME Oxygenase
Because this reaction was also expected to be caused by cytochrome P450s, the characterizations were performed as described above. Trace amounts of EMO were detected in the NADPHand O2-removed reaction, but source of this EMO would be from the cell and medium used for crude enzyme solution. The value of EMO produced under a CO atmosphere was about 40% less than that in an O2 atmosphere reaction. The KM value given by the Lineweaver-bark plot was 31 μM. The cytochrome P450 enzyme in avocado catalyzing epoxidation of the double bond in nerol was up to 50 μM [26]. IC50 values of the reaction with miconazole and clotrimazole were 23 μM and 19 μM, respectively. These values were similar to those of other cytochrome oxygenations of plant monoterpenes catalyzed by P450 [23]. Then, the microsomal reaction with catalase also ruled out the involvement of peroxidase reaction. The amount of produced EMO was in the range of a complete reaction. These results showed that IME oxygenase was a kind of cytochrome P450 monooxygenase.
4. Discussion
Metabolism of terpenes starts at the production of hydrocarbon terpenes by various terpene synthases and follow many kinds of steps, such as oxidation, reduction and isomerization for final products [27]. However, the many pathways that have been proposed have no experimental evidence expect for those of some comercially useful terpenes such as taxol [28]. We have researched monoterpene biosynthesis in C. lusitanica cultured cells. When the cells were treated with elicitors made from yeast extracts, they produced various volatile and non-volatile monoterpenes. In ether extracts from the cells and the medium, some oxidized monoterpenes (4- terpineol, α-terpineol, 4-hydroxyphellandric acid methyl ester, 1,6-epoxy-4(8)-p-menthen-2-ol, p-ment-4(8)-en-1, 2-diol and β-thujaplicin) were identified. On the other hand, 10 hydrocarbon monoterpenes (α-pinene, sabinene, β-pinene, myrcene, p-cymene, α-terpinene, limonene, β- ocimene, γ-terpinene and terpinolene) were previously identified only as the emitted volatiles [10,11]. Although the amount of emitted terpinolene was smaller than that of other emitted monoterpenes, terpinolene synthase activity was very dominant among the monoterpene synthases, so it was assumed that terpinolene was metabolized very quickly to another compound, then probably eventually to β-thujaplicin [9]. In this paper, the oxidative metabolism of terpinolene, the product of terpinolene synthase, was researched using a crude enzyme extracted from C. lusintanica cultured cells.
From our previous results, some oxidized monoterpenes were identified in the ether extracts of elicitortreated cells and medium, for example, 4(8)-p-menthen-1, 2-diol and 1,6-epoxy-4(8)-p-menthen-2-ol [11]. The next reaction of terpinolene was thought to be oxygenation because there have been some reports about oxygenation of monoterpenes by cytochrome P450 monooxygenases [15-18]. In the present study, a microsomal reaction with terpinolene was tried and showed that microsomal fractions extracted from C. lusitanica cells hydroxylated terpinolene to IME, and that IME was further oxygenated to EMO. These results indicated a two-step oxidation process from terpinolene to EMO in C. lusitanica culture cells.
When terpinolene is used as a substrate for the microsomal reaction, IME was produced but EMO was not. This is because the amount of IME was not enough to react and produce EMO. In addition, the enzymes that take the reactions after EMO would exist in other parts of C. lusitanica cells than microsomal fractions, therefore, β-thujaplicin was detected neither the reaction products from terpinolene nor IME.
Elicitor-treated C. lusitanica cells produced some monoterpenes other than terpinolene: sabinene, limonene, p-cymene, α-pinene, β-pinene, α-terpinene, and β-ocimene [10]. These monoterpenes also have the possibility of oxidative metabolism with the microsomal reaction. However, peaks thought to have M. W. of 152 or 150 (hydroxy or ketone monoterpenes) were not detected on GC/ MS. In this research, the monoterpenes 2-carene and 3- carene were also tried as substrates [25], but no peak thought to be produced by oxygenation of these compounds was detected. The pathway from terpinolene was the only one in evidence, so terpinolene was found to be the sole substrate of the microsomal fraction enzyme among the monoterpenes produced in elicitor-treated cells. This result showed that the enzyme of the reaction had strict substrate selectivity.
The hydroxylase of terpinolene and the epoxygenase of IME were each experimentally characterized. An O2- removing reaction by Ar atmosphere and an NADPHremoving reaction gave reduced amounts of oxidized products of them. Some specific inhibitor reactions and KM values were similar to those found in previous works on other plants’ cytochrome P450 monooxygenases [21, 23,24,26], so the present reactions were assumed to be likewise caused by a cytochrome P450. Mau and Croteau reported that the oxidations of hydrocarbon monoterpenes by several cytochrome P450 monooxygenases resulted in hydroxylation at allyllic positions and multiple products [18]. Terpinolene has some allyllic positions, and IME also has some allyllic positions because of its two double bonds. The nerol oxidase of avocado made regio-isomers from nerol [26], so it was possible that regio-isomers of IME and EMO were produced by these reactions. However, the terpinolene oxidase in the C. lusitanica microsomal fraction hydroxylated specifically at the 3rd carbon and did not produce isomers of IME (there was no peak considered to be made by hydroxylation of terpinolene other than IME); i.e., the enzyme had strict site-regulation. Similarly, when EMO was produced by the enzyme reaction, no other reaction products were detected. Sheet [29] and Karp [23] reported that N-substituted imidazoles, such as miconazole and clotrimazole, may be useful to distinguish between different forms of cytochrome P450s. Enzymes that catalyze oxygenation of a single substrate at different positions were differently inhibited by various reagents. In this work, miconazole and clotrimazole were equally effective against terpinolene hydroxylase and IME epoxidase; therefore, these reactions should be expected to perform site regulation. These results indicated that this reaction cascade strictly regulates the position of oxidation and selects certain structures of substrates and products.
Some P450s in plant monoterpene production produce only one stereo-isomer while others do not act in that fashion. IME and EMO do have stereo-isomers, but the EMO produced in C. lusitanica cells had a single, absolute configuration (1S, 2S, 6R-1,6-epoxy-4(8)-p-menthen-2-ol) [11]. According to the previous report [11], the biosynthetic pathway from terpinolene in C. lusitanica culture cells would have stereo-regulation. The configurations of the hydroxyl unit of IME and of epoxy oxygen of EMO should have only one direction, respecttively. Therefore, our next work will be a further analysis of stereo-regulations of the cascade of reactions discussed in this article.
C. lusitanica cultured cells produce a characteristically large amount of β-thujaplicin, over 10% in weight of ethyl acetate extracts. As previously reported from our isotope feeding experiments, it is expected that β-thujaplicin is produced via a menthane-type intermediate [9]. Namely, EMO and 4(8)-p-mentene-1,2-diol were produced only when the cells were exposed to elicitor stress, suggesting that these substances were related to a direct defensive response or to the biosynthesis of defensive compounds. Considering the relationship between the timecourse profile of terpinolene synthase activity and that of β-thujaplicin concentration, terpinolene is expected to be an intermediate of β-thujaplicin biosynthesis [10]. From the present results, IME and EMO, enzyme reaction products from terpinolene and IME, are expected to be intermediates, too. Therefore, the pathway shown here was expected to be the most possible cascade to produce β-thujaplicin; the metabolism after EMO production will become our next target in our efforts to reveal the biosynthetic pathway of β-thujaplicin.
5. Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (15780127 & 19580191), Japan Society for the Promotion of Science.
The costs of publication were supported in part by the Research Grant for Young Investigators of Faculty of Agriculture, Kyushu University.
REFERENCES
- J. Buckinghum, “Dictionary of Natural Products, Web Version 2004,” Chapman & Hall, London, 2004. http://dnp.chemnetbase.com
- A. Aharoni, M. A. Jongsma and H. J. Bouwmeester, “Volatile Science? Metabolic Engineering of Terpenoids in Plants,” TRENDS in Plant Science, Vol. 10, No. 12, 2005, pp. 594-602. doi:10.1016/j.tplants.2005.10.005
- F. Yu and R. Utsumi, “Diversity, Regulation, and Genetic Manipulation of Plant Monoand Sesquiterpenoid Biosynthesis,” Cellular and Molecular Life Science, Vol. 66, No. 18, 2009, pp. 3043-3052. doi:10.1007/s00018-009-0066-7
- X. Cheng, Y. G. Lou, Y. B. Mao, S. Lu, L. J. Wang and X. Y. Chen, “Plant Terpenoids: Biosynthesis and Ecological Functions,” Journal of Integrative Plant Biology, Vol. 49, No. 2, 2007, pp. 179-186. doi:10.1111/j.1744-7909.2007.00395.x
- S. Inada, Y. Tsutsumi and K. Sakai, “Elicitor of the βThujaplicin Accumulation in Callus Cultures of Cupressus lusitanica,” Journal of the Faculty of Agriculture Kyushu University, Vol. 38, No. 1, 1993, pp. 119-126.
- K. Sakai, K. Kusada, Y. Tsutsumi and T. Shiraishi, “Secondary Metabolites in Cell Culture of Woody Plants. III. Formation of β-Thujaplicin in Cupressus lusitanica Callus Culture Treated with Fungal Elicitor,” Mokuzai Gakkaishi, Vol. 40, No. 1, 1994, pp. 1-5.
- R. Bentley, “A Fresh Look at Natural Tropolonoids,” Natural Product Reports, Vol. 25, No. 1, 2008, pp. 118-138. doi:10.1039/b711474e
- J. Zhao, “Plant Troponoids: Chemistry, Biological Activity, and Biosynthesis,” Current Medicinal Chemistry, Vol. 14, No. 24, 2007, pp. 2597-2621. doi:10.2174/092986707782023253
- K. Fujita, T. Yamaguchi, R. Itose and K. Sakai, “Biosynthetic Pathway of β-Thuaplicin in Cupressus lusitanica Cell Culture,” Journal of Plant Physiology, Vol. 156, No. 4, 2000, pp. 462-467. doi:10.1016/S0176-1617(00)80160-1
- R. D. Alwis, K. Fujita, T. Ashitani and K. Kuroda, “Volatile and Non-Volatile Monoterpenes Produced by Elicitor-Stimulated Cupressus lustanica Cultured Cells,” Journal of Plant Physiology, Vol. 166, No. 7, 2009, pp. 720- 728. doi:10.1016/j.jplph.2008.09.009
- Y. Matsunaga, K. Fujita, J. Yamada, T. Ashitani and K. Sakai, “Monoterpenes Produced by Cupressus lusitanica Culutured Cells Including a Novel Monoterpene (1s, 2s, 6s)-(+)-1,6-Epoxy-4(8)-p-Menthen-2-Ol,” Natural Product Research, Vol. 17, No. 6, 2003, pp. 441-443. doi:10.1080/1478641031000111525
- M. C. O’Sullivan and J. M. Schwab, “Verification of the Mechamist of Oxidative Ring Expansion in the Biosynthesis of Stipitatic Acid by Talaromyces stipitatus,” Bioorganic Chemistry, Vol. 23, No. 2, 1995, pp. 131-143. doi:10.1006/bioo.1995.1011
- D. E. Cane, Z. Wu and J. E. V. Epp, “Thiotropocin Biosynthesis. Shikimate Origin of a Sulfur-Containing Tropolone Derivative,” Journal of the American Chemical Society, Vol. 114, No. 22, 1992, pp. 8483-8489. doi:10.1021/ja00048a019
- R. B. Herbert, A. E. Kattah and E. Knagg, “The Biosynthesis of the Phenetylisoquinoline Alkaloid Colchicine. Early and Intermediate Stages,” Tetrahedron, Vol. 46, No. 20, 1990, pp. 7119-7138. doi:10.1016/S0040-4020(01)87895-9
- F. Karp, J. L. Harris and R. Croteau, “Metabolism of Monoterpenes: Demonstration of the Hydroxylation of (+)-Sabinene to (+)-cis-Sabinol by an Enzyme Preparation from Sage (Salvia officinalis) Leaves,” Archives of Biochemistry and Biophysics, Vol. 256, No. 1, 1987, pp. 179-193. doi:10.1016/0003-9861(87)90436-X
- F. Karp, C. A. Mihaliak, J. L. Harris and R. Croteau, “Monoterpene Biosynthesis: Specificity of the Hydroxylations of (-)-Limonene by Enzyme Preparations from Peppermint (Mentha piperita), Spearmint (Mentha spicata), and Perilla (Perilla frutescens) Leaves,” Archives of Biochemistry and Biophysics, Vol. 276, No. 1, 1990, pp. 219-226. doi:10.1016/0003-9861(90)90029-X
- M. Wüst, D. B. Little, M. Schalk and R. Croteau, “Hydroxylation of Limonene Enatiomers and Analogs by Recombinant (-)-Limonene 3- and 6-Hydroxylases from Mint (Mentha) Species: Evidences for Catalysis within Sterically Constrained Active Sites,” Archives of Biochemistry and Biophysics, Vol. 387, No. 1, 2001, pp. 125- 136. doi:10.1006/abbi.2000.2248
- C. J. D. Mau and R. Croteau, “Cytochorome P450 Oxygenases of Monoterpene Metabolism,” Phytochemistry Reviews, Vol. 5, No. 2-3, 2006, pp. 373-383. doi:10.1007/s11101-006-9008-2
- L. Gamborg, R. A. Miller and K. Ojima, “Nutrient Requirements of Suspension Cultures of Soybean Root Cells,” Experimental Cell Research, Vol. 50, No. 1, 1968, pp. 151-158. doi:10.1016/0014-4827(68)90403-5
- R. Itose and K. Sakai, “Improved Culture Conditions for the Production of β-thuaplicin by Suspension Cell Cultures of Cupressus lusitanica,” Plant Biotechnology, Vol. 14, No. 3, 1997, pp. 163-167.
- H. J. Bouwmeester, M. C. J. M. Konings, J. Gershenzon, F. Karp and R. Croteau, “Cytochrome P450 Dependent (+)-Limonene-6-Hydroxylation in Fruits of Caraway (Carum carvi),” Phytochemistry, Vol. 50, No. 2, 1999, pp. 243-248. doi:10.1016/S0031-9422(98)00516-0
- W. B. Motherwell, M. J. Bingham, J. Pothier and Y. Six, “A Study of Some Molecularly Imprinted Polymers as Protic Catalysts for the Isomerisation of α-Pinene Oxide to Trans-Carveol,” Tetrahedron, Vol. 60, No. 14, 2004, pp. 3231-3241. doi:10.1016/j.tet.2004.02.016
- F. Karp, C. A. Mihaliak, J. L. Harris and R. Croteau, “Monoterpene Biosynthesis: Specificity of the Hydroxylations of (-)-Limonene by Enzyme Preparations from Peppermint (Mentha piperita), Spearmint (Mentha spicata), and Perilla (Perilla frutescens) Leaves,” Archives of Biochemistry and Biophysics, Vol. 276, No. 1, 1990, pp. 219-226. doi:10.1016/0003-9861(90)90029-X
- K. M. Madyastha, T. D. Meehan and C. Coscia, “Characterization of a Cytochrome P-450 Dependent Monoterpene Hydroxylase from the Higher Plant Vinca rosea,” Biochemistry, Vol. 15, No. 5, 1976, pp. 1097-1102. doi:10.1021/bi00650a023
- H. Erdtman, “Chemistry of Some Heartwood Constituents of Conifers and Their Physiological and Taxonomic Significance,” Progress in Organic Chemistry, Vol. 1, 1952, pp. 22-53.
- D. L. Hallahan, S. C. Lau, P. A. Harder, D. W. M. Smiley, G. W. Dawson, J. A. Pickett, R. E. Christoffersen and D. P. O’Keefe, “Cytochrome P-450-Catalysed Monoterpenoid Oxidation in Catmint (Nepeta racemosa) and Avocado (Persea americana); Evidence for Related Enzymes with Different Activities,” Biochimica et Biophysica Acta—General Subjects, Vol. 1201, No. 1, 1994, pp. 94-100.
- J. Bohlmann and C. Keeling, “Terpenoid Biomaterials,” The Plant Journal, Vol. 54, No. 4, 2008, pp. 656-669. doi:10.1111/j.1365-313X.2008.03449.x
- M. Malik, R. M. Cusidó, M. H. Hirjailli, E. Moyano, J. Palasòn and M. Bonfill, “Production of the Anticancer Drug Taxol in Taxus baccata Suspension Cultures: A Review,” Process Biochemistry, Vol. 46, No. 1, 2011, pp. 23-34. doi:10.1016/j.procbio.2010.09.004
- J. J. Sheets, J. I. Mason and C. A. Wise, “Inhibition of Rat Liver Microsomal Cytochrome P-450 Steroid Hydroxylase Reactions by Imidazole Antimycotic Agents,” Biochemical Pharmacology, Vol. 35, No. 3, 1986, pp. 487- 491. doi:10.1016/0006-2952(86)90224-8
NOTES
*Corresponding author.

