Neuroscience & Medicine
Vol. 3 No. 4 (2012) , Article ID: 26224 , 10 pages DOI:10.4236/nm.2012.34049
Pinealectomy and Exogenous Melatonin Regulate Anxiety-Like and Depressive-Like Behaviors in Male and Female Wistar Rats
![]()
Laboratory of Genetics, Neuroendocrinology and Biotechnology, Unit of Nervous and Endocrine Physiology, Department of Biology, Faculty of Sciences, University Ibn Tofail, Kenitra, Morocco.
Email: *fatimaa93@hotmail.com
Received May 27th, 2012; revised July 16th, 2012; accepted August 16th, 2012
Keywords: Pinealectomy; Melatonin; Sex Dependent; Anxiety; Depression; Behavioral Tests
ABSTRACT
The main objective of this work was to 1) study the influence of endogenous melatonin (Mel) abolishment via pinealectomy and 2) explore exogenous Mel effect on anxiety-like and depressive-like behavior in male and female rats. Rats were shamoperated (Sh) or pinealectomized (Px) and following subgroups were selected 1) Px/NaCl (0.9%) and Sh/NaCl (0.9%) : rats injected subcutaneously, once daily for 8 weeks, with saline solution NaCl (0.9%) as vehicle; 2) Px/Mel (4 mg/Kg) and Sh/Mel (4 mg/Kg): rats similarly injected with 4 mg/Kg of Mel. All animals were housed under a photoperiod of (LD:16/8). After different treatments animals were tested in the open-field test (OFT), elevated plus maze test (EPM) to determine anxiety-like behavior, and forced swimming test (FST) to evaluate depressive-like level. Our results revealed that level of anxiety-like and depressive-like behavior are significantly higher in Px/NaCl (0.9%) when compared to Sh/NaCl (0.9%) group, suggesting that pinelectomy induced an anxiogenic and depressant effects. The Px effects would be due to the absence of endogenous Mel synthesis and release. Additionally, we clearly demonstrated that the level of anxiety-like and depressive-like behavior are higher in Px/Mel (4 mg/Kg) and Sh/Mel when compared respectively to Px/NaCl (4 mg/Kg) and Sh/NaCl groups suggesting an anxiolytic and antidepressant effects of exogenous Mel. Behavioral responses were sex dependent since the difference between females and males, especially, after melatonin administration, were statistically significant. These experiments provide evidence that pinealectomy and Mel regulated emotionally behavior in male and female rats.
1. Introduction
Pineal gland is located in the epithalamia of the brain. It synthesizes several hormones, especially Mel which is shown in all groups from plants [1] to vertebrates through to prasits and invertebrates [2]. In all investigated animals, Mel secretion follows a similar circadian rhythm independently of their nocturnal or diurnal activity [3].
Rhythmic Mel production in the rat is controlled by a circadian rhythm of the pineal N-acetyltransferase (NAT) activity which synthesizes the Mel precursor N-acetylserotonin from serotonin [4]. The enzyme rhythm is driven by a light-entrainable circadian pacemaking system in the suprachiasmatic nucleus (SCN), since its lesion abolishs the NAT rhythm [5]. Photic information is conveyed to the SCN via the retinohypothalamic tract [6] and then to pineal after several neural relays, leading to nocturnal release of norepinephrine from sympathetic nerve endings. This stimulates adrenergic receptors, activates pineal NAT and increases Mel release [7]. It results an alternation of low concentrations of Mel during the day and high concentrations during the night. The amphilicity of the Mel is allowing the molecule to enter any cell, and to attain any compartment or body fluid [8]. The main role of Mel is to inform the central nervous system about the environmental light and dark regimen [9] and consequently led to the synchronization of physiological and behavior rhythmic functions with the seasons [10-12].
The main experimental protocols used to understand the different Mel actions on the biological functions are surgical removal of the pineal gland, namely called pinealectomy and Mel administration. Mel is involved in the circadian regulation of many physiological functions such as reproduction, immunity, sleep, visual and cerebrovascular, functions [13-15]. In rodents, Mel is known to be implicated in several processes such as sedative and anticonvulsant [16], avoidance learning [17], short-term memory [18] and the velocity [19]. It also regulates some behavioral processes such as stressand anxiety-related behaviors [20], producing antidepressant [21,22], and anxiolytic [22-24] actions. In humans, disturbances in the circadian profile of Mel were often associated to mood disorders in depressed patients [25]. The role of Mel is also demonstrated by pinealectomy which abolishes Mel synthesis, removes its endogenous rhythmic pattern and completely inhibits the plasma levels of hormone [26]. Pinealectomy seems to be involved in regulation of emotional behavior, but studies in this issue are incoherent ranging from its insignificant role [27] to an evident implication in control of anxiety-and depressive like behaviors [28].
The biological effects of Mel may be mediated via its receptors (MT1, MT2 and MT3) widely distributed in the brain and peripheral organs. In the many mammals, MT1 are shown especially in SCN, pars tuberalis, paraventricular nucleus, cerebellar cortex, hippocampus and cortex. MT2 are characterized in dorsal thalamus, corpus cerebella and MT3 in hypothalamus, thalamus, frontal cortex, kidney, liver and lung [29-31]. MT1 and MT2 inhibit cyclic AMP synthesis [32], while MT3 sites activation increases phospholipid turnover [31,32].
Given the considerations mentioned above, an experimental research is needed for illuminating the role of pineal gland and Mel treatment in anxiety and depression. The administration of Mel with or without abolishment of Mel hormone via pinealectomy may produce different effects on anxiety and depression behavior. This allows us to understand the influence of endogenous and exogenous Mel on emotionally behavior in the both sex of rats.
2. Materials and Methods
2.1. Animals
This experimental study was performed on male and female Wistar rats initially weighing (80 ± 20) g. Animals were housed by six in cages (36 cm long, 20 cm wide and 15 cm high). All rats were maintained on a 12 h Light/12h dark cycle and at a standard temperature (21˚C ± 1˚C). Water and food were provided ad libitum. At the beginning of treatment, the colony room was maintained under a long photoperiod LD: 16/8 (16 h Light/8h Darkness).
2.2. Experimental Procedure
Rats were sham operated (Sh) or pinealectomized (Px) after being anesthetized with chloral hydrate (0.5 ml/100 g, sigma-aldrich, laborchemikalien Gmbh, Germany) according to the classical procedure described in literature. After anesthesia, the intact animals were placed in stereotaxic apparatus. The skin of the skull was incised along the suture lambda and the bone was excised in a circle of about 5 mm of diameter whose center corresponds to the lambda. The venous sinus was exposed and perforated with fine forceps and the pineal gland removed by suction. Dressing coagulant was immediately applied to the venous sinuses to stop the bleeding and close the wound, and then the skin was stitched. In sham operated animals, the bone was excised and then closed and the skin of skull was stitched. A dual test was adopted to ensure the success of pinealectomy 1) after each pinealectomy the presence of the pineal in the bottle of reception after aspiration is carefully checked and the content of bottle was emptied between two operations; 2) in addition, once behavioral study is completed, the animals were killed, brains and blood were collected for possible analysis. Before freezing the brain, the absence of the pineal in all pinealectomized animals was noted. Surgeries were performed in Dr. N. Lakhdar-Ghazal laboratory at the University of Mohammed V of Rabat (Morocco) and rats were transferred to University of Kénitra. The survival rate of animals after pinealectomy was about 60%.
One week after surgery, four subgroups were selected; each subgroup consists of six (6) animals. 1) Px/NaCl (0.9%) and Sh/NaCl (0.9%): rats injected subcutaneously, once daily for 8weeks, with saline solution NaCl (0.9%) as vehicle containing 5% ethanol; 2) Px/Mel (4 mg/Kg) and Sh/Mel (4 mg/Kg): rats similarly injected with 4 mg/Kg of Mel (N-acetyl-5-methoxytryptamine; Sigma Lot No. 112K0998 France), dissolved in 5% ethanol. All injections were made approximately at 4:00 pm.
A first experimental groups: Px/NaCl (0.9%); Sh/NaCl (0.9%) were designed to compare emotional behavior of intact and Px rats and thus to determine the effects of endogenous Mel. The second experimental groups: Px/Mel (4 mg/Kg) and Sh/Mel (4 mg/Kg)] were used to compare emotional behavior of intact and Px rats and thus to determine the effects of exogenous Mel treatment. At the end of treatment, the rats were subjected to different behavioral tests undertaken in following order: OFT, EPM and FST.
3. Behavioral Testing
3.1. Open Field Test
The OFT is used to measure the anxiety-like behavior in rodents. The maze adopted is made of wood (100 cm × 100 cm) enclosed with 40 cm high walls and placed under strong illumination (100 W, 2 m above the apparatus) [32]. The area was divided into 25 squares (20 cm × 20 cm), defined as 9 central and 16 peripheral squares. At the beginning of the 10-min test, the animal was placed in the centre of the apparatus and its behavior was videotaped for subsequent analysis. The quantified parameters were the time spent in the center of the area (TCA) and the number of returns to the center (NRC). Central perimeter residence time is used as a measure of anxiety [33]. The number of returns to the central area is also an indicator of the emotional reactivity [32,33]. The central area of a novel environment is anxiogenic and aversive and the behavioral inhibition appears therefore as an avoidance behavior towards the central zone of the OFT [34]. The apparatus was cleaned between each examination using 70% ethyl alcohol.
3.2. Elevated Plus-Maze Test
The EPM is an ethological model of anxiety in rodents provoked by the novelty and repulsion as a result of elevation and illumination of the maze [35]. This test is based on the creation of a conflict between the exploratory drive of the rat and its innate fear of open and exposed areas; it has been validated for the detection of emotional responses to anxiogenic and anxiolytic substances [36]. Thus, increased open-arms exploration indicates reduced anxiety-related behavior. The EPM consists of a wooden plus-shaped platform elevated 70 cm above the floor. Two of the opposing arms (50 cm × 10 cm) are closed by 40 cm high side and end walls, having an open roof. In order to avoid fall, the other two arms (open arms) were surrounded by 0.5 cm high edge, the four arms had at their intersection a central platform (10 cm × 10 cm). A 100-W lamp was placed exactly over the central platform. At the beginning of the test, the rats were placed on the central area of the maze facing an open arm. The following parameters of anxiety-related behavior were measured during the 5 min testing period: 1) entries into open arms (EOA), 2) time spent on the open arms (TOA), 3) and number of full entries into the arms (TAE). Decreased anxiety-like behavior is illustrated by a significant statistical increase of parameters in open arms (time and/or entries). The total number of the entries into all arms provides general hyperactivity [37]. To eliminate any lingering olfactory cues, the apparatus was cleaned between each examination using 70% ethyl alcohol.
3.3. Forced Swimming Test
The FST is an excellent maze used to assess the depressive-like behavior [38]. Swimming sessions were conducted by placing the rat in individual glass cylinders (height = 50 cm; diameter = 30 cm) containing 30 cm of water at (23˚C ± 2˚C). During the session, rats were forced to swim for 5 min and the duration of immobility was measured. The latency to the first bout of immobility was also recorded starting immediately after placing the rats in the cylinder. A rat was judged immobile when it ceased all active behaviors (i.e. struggling, swimming and jumping) and remained passively floating or making minimal movements necessary to maintain the nostrils above water. High percent time floating is interpreted as an increased depressive-like response [38].
3.4. Statistics
All data are expressed as the means ± standard error of the means (S.E.M.). To determine the differences between experimental groups statistical analysis was performed by analysis of variance (ANOVA) 1st/2nd order followed by a post-hoc tests (Fisher LSD) or Student test “t”. Differences were considered significant when p < 0.05, very significant when p < 0.01 and highly significant when p < 0.001.
4. Results
4.1. Effect of Pinealectomy on Anxiety and Depression Levels
4.1.1. Open Field Test
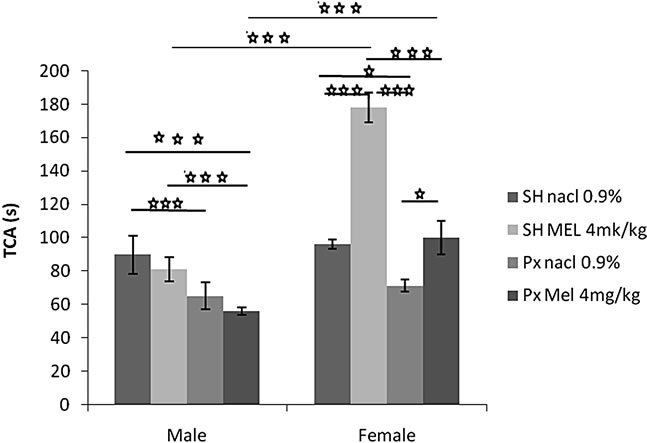
4.1.1.1. Time Spent in the Central Area (TCA) (Figure 1(a))
The sex factor (F(1.32) = 74.72, p < 0.001), and the treatment factor (F(1.32) = 37.76, p < 0.001) significantly affected the TCA. An interaction was found between treatment and sex F(1.32) = 24.64, p < 0.001). Indeed, the females of the groups Sh/Mel and Px/Mel showed a TCA significantly and respectively higher compared to males of similar groups (p < 0.001 and p < 0.0001).
A comparison between the groups showed that females of Px/NaCl group spend significantly less time in the central area compared to the animals of Sh/NaCl group (p = 0.04 < 0.05). Also, females of Px/Mel group spend significantly less time in the central area compared to the animals of Sh/Mel group (p = 0.000004 < 0.001). Similar or more pronounced results were obtained in males (p = 0.0001 < 0.001). Females of Px/NaCl group spend significantly less time in the central area compared to the animals of Sh/Mel (p = 0.00001 < 0.001). In contrast, no effect was obtained in males. Females of Px/Mel group spend significantly more time in the central area compared to the animals of Px/NaCl group (p = 0.02 < 0.05). Similarly, Females of Sh/Mel group spend significantly more time in the central area compared to the animals of Sh/NaCl group (p = 0.000002 < 0.001). However, no effect in males was observed. Males of Sh/NaCl group spend significantly more time in the central area compared to the animals of Px/Mel group (p < 0.001). No significant difference was observed between these groups in females.
4.1.1.2. Number of Returns to the Center (NRC) (Figure 1(b))
The sex factor (F(1.32) = 49.19, p < 0.001), and the treatment factor (F(1.32) = 16.91, p < 0.001) significantly affected the NRC. An interaction was found between treatment and sex (F(1.32) = 9.11, p < 0.001). Indeed, the females of the groups Sh/Mel and Px/Mel showed a NRC significantly and respectively higher compared to males of similar groups (p = 0.0001 < 0.001 and p < 0.0001).
The results of Figure 1(b) showed that females and males of Px/NaCl group visited central area significantly less than animals of Sh/NaCl group (p < 0.05 and p = 0.0004 < 0.001). Also, Females and males of Px/Mel group visited the central area less than animals of Sh/Mel group (p = 0.0005 < 0.001, p = 0.0001 < 0.001). Similar result was obtained between females Px/NaCl and Sh/Mel (p < 0.001). In contrast, no effect was obtained in males. Females of Sh/Mel group spend significantly more time in the central area compared to the animals of Sh/NaCl group (p = 0.00009 < 0.001), no significant difference was observed between these groups in males. However, a high significant effect between Px/NaCl and Px/Mel groups was observed (p = 0.000003 < 0.001).
4.1.1.3. Locomotors Activity (NTS) (Figure 1(c))
Locomotors activity was unaffected by any treatment (F(1.32) = 34.00, p > 0.05), and no effect of sex (F(1.32) = 1.49, p = 0.23 > 0.05) was noted. The interaction between sex and other treatments did not reach significance F(1.32) =1.63, p = 0.20 > 0.05). The values of all groups were comparable.
4.1.2. Elevated Plus Maze
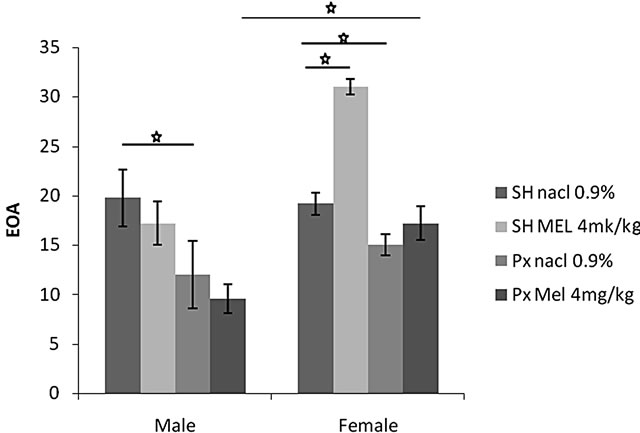
4.1.2.1. Entry to Open Arms (EOA) (Figure 2(a))
Statistical analysis showed that EOA was significantly affected by sex factor (F(1.20) = 15.39, p = 0.001 < 0.01)), and the melatonin treatment (F(1.20) = 7.47, p = 0.001 < 0.01). An interaction was found between treatment and sex (F(1.20) = 18.31, p = 0.0005 < 0.001). The females of Px group treated with Mel (Px/Mel) visited open arms significantly more than males of similar group (p = 0.01 < 0.05). Females and males of Px/NaCl groups visited open arms significantly less than animals of both sex of Sh/NaCl groups (p < 0.05). Additionally, females of Sh/Mel visited open arms significantly more than animals of Sh/NaCl (p < 0.05).
4.1.2.2. Time Spent in Open Arms (TOA) (Figure 2(b))
This parameter was affected by melatonin treatment (F(1.32) = 4.29, p = 0.01), but not by sex factor (F(1.32) = 3.65, p > 0.05). No interaction was found between treat-
 (a)
(a)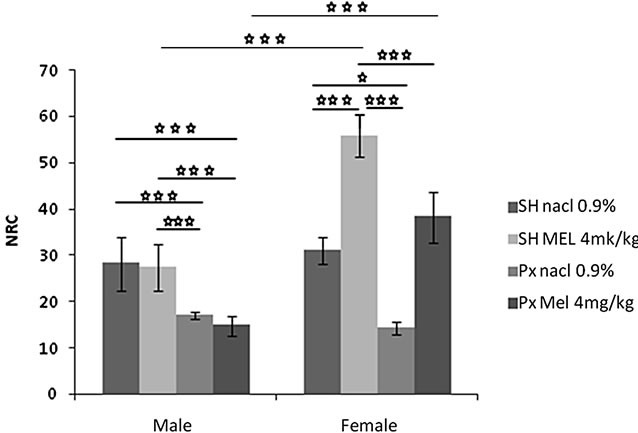 (b)
(b)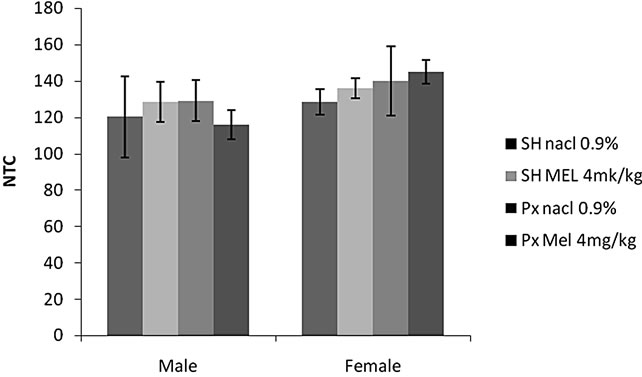 (c)
(c)
Figure 1. Mean (S.E.M.) (a) Total amount time spent in the center of the open field (TCA); (b) Number of return into center area of the arena in the open-field behavior apparatus (NRC); and (c) The number of total squares (NTS); by Sham/Px female and Sham/Px male rats treated by Mel (4 mg/kg) or (NaCl 0.9%) beginning at 8 weeks of age and during 8 weeks of treatment. Values at distal ends of horizontal bar differ, *p < 0.05, **p < 0.01, ***p < 0.001.
ment and sex F(1.32) = 2.47, p > 0.05). A comparison between the groups showed that females of Sh/Mel group spend significantly more time in the open arms compared to the animals of sh/NaCl (p = 0.01), Px/NaCl (p < 0.01)
 (a)
(a)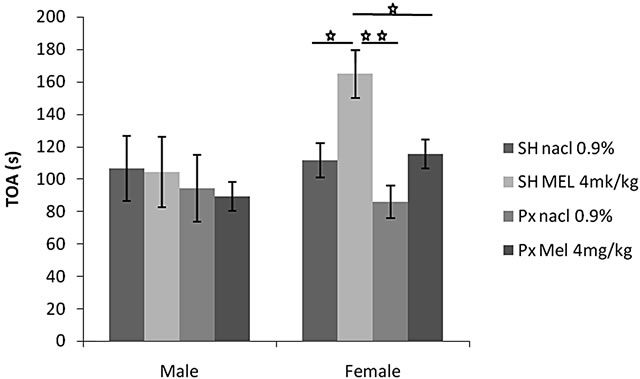 (b)
(b)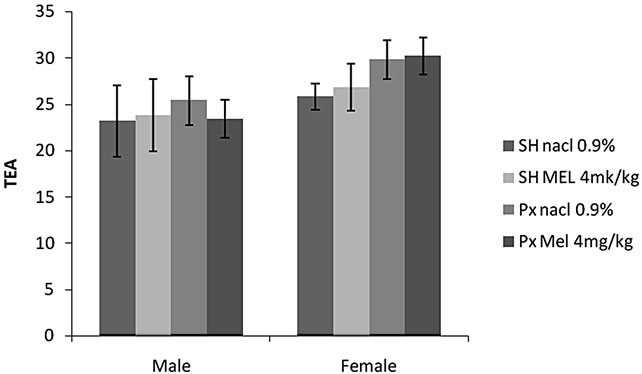 (c)
(c)
Figure 2. Mean (S.E.M.) (a) number of entries in the two exposed arms of elevated plus maze (EOA); (b) total amount of time spent exploring these arms (TOA) and (c) total number of arms entries (TEA) by Sham/Px female and Sham/Px male rats treated by Mel (4 mg/kg) or (NaCl 0.9%) beginning at 8 weeks of age and during 8 weeks of treatment. Values at distal ends of horizontal bar differ, *p < 0.05, **p < 0.01, ***p < 0.001.
and those Px/Mel (p < 0.05). Conversely, no significant effect was found in males.
4.1.2.3. Total Entries in Arms (TEA) (Figure 2(c))
Locomotors activity was unaffected by sex (F(1.32) = 6.64, p > 0.05) or by melatonin treatment (F(1.32) =
0.75, p > 0.05). No interaction was found between treatment and sex F(1.32) = 0.32, p = 0.8 > 0.05). There was no significant difference between all groups.
4.1.3. Forced Swimming Test
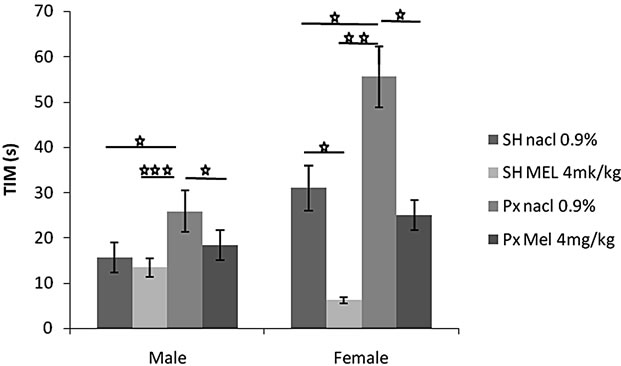
4.1.3.1. Immobility Time (TIM) (Figure 3(a))
Pinealectomy significantly increased immobility time in animals of both sex although the effects were more evident in females. Indeed, females of Px/NaCl group showed a TIM significantly higher than those of Sh/NaCl (p < 0.05) and higher than those of all other groups. A similar result was observed in males. Also, animals of Px/NaCl and Px/Mel group showed a TIM significantly higher than those of Sh/Mel (p < 0.01 and p < 0.05 respectively). In contrast, Mel decreased immobility time especially in females. Thus, animals of Px/Mel and Sh/Mel groups showed a TIM significantly and respectively lower than those of Px/NaCl (p < 0.05) and Sh/NaCl (p < 0.05).
4.1.3.2. Struggling Time (TST) (Figure 3(b))
Px rats exhibited a lower struggling behavior when compared to other groups in animals of both sex although the effects were more pronounced in females. Indeed, animals of Px/NaCl group showed a TST significantly lower than animals of Sh/NaCl (p < 0.001) as well as of all other groups. Also, animals of Px/Mel group showed a low struggling behavior than those of Sh/Mel (p < 0.001).
Conversely, animals treated by Mel exhibited vigorous struggling behavior in males as well as in females. Thus, animals of Px/Mel and Sh/Mel groups showed a TST significantly and respectively higher than those of Px/NaCl (p < 0.05) and Sh/NaCl. Similarly, in males, a significant difference was noted between Px/NaCl and Px/Mel (p < 0.05).
5. Discussion
The present study examines two important aspects: effect of pinealectomy and Mel administration on 1) anxietylike behavior in OFT and EPM and 2) depressive-like behavior in FST in male and female rats.
In OFT, Px rats showed a high anxiety-like behavior since the time passed at the center of the OFT and number of returns to the center was lower than sham operated animals. In this maze, the number of the entries to the edge of the OFT increased and the total distance travelled decreased if the anxiety of the animal is high [39,40].
In the EPM, Px rats visited open arms less frequently and spent less time in these arms than sham operated animals. In this test, the number of the entries to closed arms is increasing and the total distance travelled is decreasing when the anxiety level of the animal is high [41]. These findings suggest that anxiety measurement is more sensitive to the removal of pineal gland, as pinealectomy
 (a)
(a)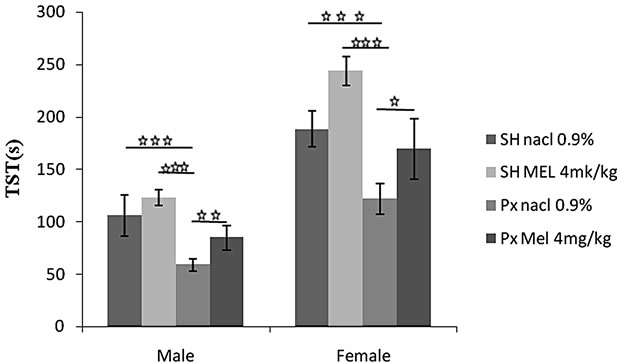 (b)
(b)
Figure 3. Mean (S.E.M.) (a) Immobility time in Forced swimming test (TIM); (b) Struggling time (TST) by Sham/Px female and Sham/Px male rats treated by Mel (4 mg/kg) or (NaCl 0.9%) beginning at 8 weeks of age and during 8 weeks of treatment. Values at distal ends of horizontal bar differ, *p < 0.05, **p < 0.01, ***p < 0.001.
treatment interacted with anxiety provoking test situations. An additional argument of the role of pineal gland was given by comparison of behavioral response of Sh/Mel and Px/Mel treated animals. Thus, in OFT, Px/Mel showed a high level of anxiety-like with comparison to Sh/Mel group. Such result was also observed in the EPM confirming that Px/Mel treated rats visited open arms less frequently and spent less time in these arms than those of sham operated treated by the pineal hormone.
Taking the observations mentioned above, our results showed that the anxiety-like behavior was significantly affected by the pinealectomy since the control subjects were more mobile in the anxiogenic environmental of the OFT and EPM than the Px subjects. On the other hand, Mel secretion in rats follows a circadian pattern which is high throughout the darkness [42]. However, in pinealectomy the rhythm of Mel is suppressed and the blood Mel levels drastically decreased [43]. Since the rhythm of the endogenous Mel release in the Px animals is abolished, and since this endogenous rhythm in the sham Px animals is intact; we hypothesized that the increase in anxiety level observed in Px rats would be due to the absence of Mel synthesis and release. This assumption is in agreement with the findings of the previous studies suggesting that pinealectomy was partially involved in anxiety behavior [43,44] and seems to be coherent with another report demonstrating an important role of both maternal and postnatal pinealectomy in organization of affective behaviors in another species, in this case the Siberian hamster [43,44]. Conversely, some studies reported insignificant effect of pinealectomy which is inconsistent with our study [44]. It is likely that this disagreement would be due to the experimental conditions which are different, especially the time of the day when the measurements are performed [44] or doses and mode of Mel administration adopted [43,44]. However, a complementary experimental research is needed for illuminating the role of pineal gland in anxiety behavior.
The assumption made above was verified by comparing Sh/Mel and Px/Mel groups which showed a lower anxiety-like behavior than their respective control subjects i.e. Sh/NaCl and Px/NaCl groups in the two anxiety-tests. This suggests that Mel is efficient in reducing the anxiety since the time passed at the center of the OFT and at the open arms of EPM was longer especially in the Mel treated animals. Mel was even capable to cancel or reverse the anxiogenic effect of pinealectomy since anxiety-like level was higher in Px/Mel vs Px/NaCl, such effect was approximately similar to than of intact animals (Sh/NaCl). The finding that Mel induced anxiolytic effect supported our previous observation [45]. Similar studies reported that exogenous treatment with Mel alone [45] or in conjunction with sub threshold doses of benzodiazepines had an anxiolytic effect in several anxiety tests [46]. Our result also concord with a study showing that after the removal of the pineal gland a high dose of Mel could show its effect on anxiety-like behavior [47]. This study reported that the high dose of Mel administration enhanced the time spent in open arms, while, after the pinealectomy, the low dose of Mel (1 µg/kg) administration decreased it.
The second finding concerns the influence of pinealectomy on depressive-like behavior measured in the FST. We demonstrated that Px rats showed a high level of depressive-like behavior since the immobility time and struggling behavior were lower than Sh animals, suggesting that the depressive-like measurement is more sensitive to the removal of pineal gland. The despair of rat is high when it ceased all active behaviors (struggling, swimming, and jumping) and remained passively floating. High percent time floating is interpreted as an increased depressive-like response [38]. Following the previous reasoning, these experiments provide evidence that: 1) depressive-like behavior was significantly affected by the pinealectomy since the control subjects were more mobile and struggling than the Px subjects and 2) the increase in depressive-like behavior observed in Px rats would be due to the absence of endogenous Mel synthesis and release. This finding is consistent with fact that antidepressive-like is regulated by both maternal and postnatal pinealectomy in forced swim test previously reported [47]. This assumption is concordant with the fact that Sh/Mel and Px/Mel rats showed a low depressive-like behavior than their respective control subjects, namely Sh/NaCl and Px/NaCl animals. This result, which confirms our previous study [48], showed the Mel efficiency to reduce depressive-like behavior. In the present study, the Mel treatment cancelled the anxiogenic effect of pinealectomy mentioned above (Px/Mel vs Px/NaCl) to attain a similar level of intact animals (Sh/NaCl). The results reported by some studies in this subject are conflicting and dependent on the mode of Mel administration. While it appears that acute Mel administration at high dose (30 mg/kg) does not alter the immobility duration [47], lower doses (10 mg/kg) reduce immobility times [48].
The third finding was the pinealectomy and Mel effects on anxiety-like and depressive-like behaviors were depending on the sex. Indeed, whether in anxiety or depressive measurement tests, females showed a positive and coherent response to different treatments, while the males were generally less active and showed partially significant effects. This confirmed our previous observation concerning the existence of a sexual dimorphism in the behavioral response [49]. These results are consistent with other studies on the effect of Mel injection on the anxiety-like behavior in two sexes [49-51]. With regard to depressive-like behavior, our report was in coherence with some studies previously reported [52]. In addition, intact male rats show higher levels of immobility [53,54], and a reduced sensitivity to antidepressants compared to females [54,55]. Such sexual dimorphism would be explained by an implication of male and female sexual hormones [55].
Otherwise, there is evidence in the literature that the depression and anxiety are characterized by decreased function in the noradrenergic locus coeruleus, serotoninergic dorsal and median raphe, and dopaminergic ventral tegmental area systems [56]. Also, degeneration of noradrenergic fibers from locus coeruleus has been associated with stress-induced depression in rats [57]. Anxiety is associated also dysfunction in gama aminobutyric acid (GABA) neurotransmission. As the Mel receptors are widely distributed in the different part of brain [58], the behavioral effects of Mel could be mediated by Mel receptor itself to modulate neurotransmitter systems [58-60]. Indeed, when Mel was applied in vivo, it increased the GABA levels in several brain regions in rats [58,60]. In our study, the reduced anxiety-like behavior after chronically Mel administration could be due to the increased in brain GABA levels, a such hypothesis could be demonstrated.
Finally, Melatonin is one hormone from a set of compounds synthesized by the pineal gland as 5-methoxytryptophol (5-ML), 5-methoxytryptamine (5-MT) and 5-methoxyindole acetic acid (5-MIAA) from a common substrate i.e. serotonin (5-HT). If the influence of melatonin on the emotional behavior is widely accepted, the role of other hormones of the pineal gland is not excluded [60].
6. Acknowledgements
The authors thank Paul Pévet from University of Strasbourg (France) for support and generously providing the Mel, Nouria Lakhdar-Ghazal and Bouhaddou from University Mohammed V of Rabat (Morocco) for technical assistance for pinealectomy realization and Samira Boulbaroud for helpful discussions and comments on an earlier version of this manuscript. This research was supported by PROTARS (D14/03, CNRST Morocco), GDRINeurosciences (France-Morocco) and Neuromed project.
REFERENCES
- R. Hardeland, “Melatonin in Unicellular Organisms, Fungi, Macroalgae and Angiosperms: Consequences of Its Metabolism, Mediation of Environmental Signals and the Search for Its Primary Roles,” Bulletin de la Société Française d’Écophysiologie, Vol. 24, 1998, pp. 13-14.
- B. Vivien-Roels, “Présence, Synthèse et Rôle Possible de la mElatonine Chez les Invertébrés,” Bulletin de la Socié- té Française d’Ecophysiologie, Vol. 24, 1998, pp. 14-19.
- J. Arendt, “Melatonin, Circadian Rhythms and Sleep,” The New England Journal of Medicine, Vol. 343, 2000, pp. 1114-1116. doi:10.1056/NEJM200010123431510
- H. Illnerova, J. Vaneek and K. Hoffmann, “Regulation of the Pineal Melatonin Concentration in the Rat (Rattus Norvegicus) and the Djungarian Hamster (Phodopus Sungorus),” Comparative Biochemistry and Physiology Part A: Physiology, Vol. 74, No. 1, 1983, pp. 155-159. doi:10.1016/0300-9629(83)90727-2
- D. C. Klein and R. Y. Moore, “Pineal N-Acetyltransferase and Hydroxyindole-O-Methyltransferase: Control by the Suprachiasmatic Nucleus,” Brain Research, Vol. 174, No. 2, 1979, pp. 245-262. doi:10.1016/0006-8993(79)90848-5
- R. Y. Moore, J. C. Speh and J. P. Card, “The Retinohypothalamic Tract Originates from a Distinct Subset of Retinal Ganglion Cells,” Journal of Comparative Neurology, Vol. 352, No. 3, 1995, pp. 351-366. doi:10.1002/cne.903520304
- D. C. Klein, D. Sugden and J. L. Weller, “Postsynaptic Alpha-Adrenergic Receptors Potentiate the Beta-Adrenergic Stimulation of Pineal Serotonin N-Acetyltransferase,” Proceeding of the National Academy of Sciences of the USA, Vol. 80, No. 2, 1983, pp. 599-603. doi:10.1073/pnas.80.2.599
- B. Poeggeler, S. Saarela, R. J. Reiter, D. X. Tan, L. D. Chen and L. C. Manchester, “Melatonin, a Highly Potent Endogeneous Radical Scavenger and Electron Donor, New Aspects of the Antioxidant Chemistry of this Indole Accessed in Vitro,” Annals of the New York Academy of Sciences, Vol. 738, No. XI-XII, 1994, pp. 419-420.
- D. C. Klein, “The Mammalian Melatonin Rhythm Generating System,” In: L. Watterberg, Ed., Light and Biological Rhythms in Man, Pergamon Press, New York, 1993, pp. 55-70.
- J. Arendt, “Melatonin and the Mammalian Pineal Gland,” In: J. Arendt, Ed., Melatonin and the Pineal Gland: Influence on Mammalian Seasonal and Circadian Physiology, Reviews of Reproduction, Vol. 3, 1998, pp. 13-22.
- B. D. Goldman and J. M. Darrow, “The Pineal Gland and Mammalian Photoperiodism,” Neuroendocrinology, Vol. 37, 1983, pp. 386-396. doi:10.1159/000123579
- P. Pévet, “The Role of the Pineal Gland in the Photoperiodic Control of Reproduction in Different Hamster Species,” Reproduction Nutrition Development, Vol. 28, No. 2B, 1988, pp. 443-458. doi:10.1051/rnd:19880310
- J. Borjigin, X. Li and S. H. Snyder, “The Pineal Gland and Melatonin: Molecular and Pharmacologic Regulation,” Annual Review of Pharmacology, Vol. 39, 1999, pp. 53-65. doi:10.1146/annurev.pharmtox.39.1.53
- A. Brzezinski, “Melatonin in Humans,” The New England Journal of Medicine, Vol. 336, 1997, pp. 86-95.
- J. Vanecek, “Inhibitory Effect of Melatonin on GnRH Induced LH Release,” Reviews of Reproduction, Vol. 4, No. 2, 1999, pp. 67-72.
- D. A. Golombek, P. Pévet and D. P. Cardinalli, “Melatonin Effects on Behavior: Posssible Mediation by the Central GABAergic System,” Neuroscience & Biobehavioral Reviews, Vol. 20, No. 3, 1996, pp. 403-412. doi:10.1016/0149-7634(95)00052-6
- G. L. Kovács, I. Gajari, G. Telegdy and K. Lissak, “Effects of Melatonin and Pinealectomy on Avoidance and Exploratory Activity in the Rat,” Physiology & Behavior, Vol. 13, No. 3, 1974, pp. 349-355. doi:10.1016/0031-9384(74)90087-0
- A. Argyriou, H. Prast and A. Philippu, “Melatonin Facilitates Short-Term Memory,” European Journal of Pharmacology, Vol. 349, No. 2-3, 1998, pp. 159-162. doi:10.1016/S0014-2999(98)00300-8
- A. Karakas, H. Coskun, A. Kayaa, A. Kücükc and B. Gündüzd, “The Effects of the Intraamygdalar Melatonin Injections on the Anxiety Like Behavior and the Spatial Memory Performance in Male Wistar Rats,” Behavioural Brain Research, Vol. 222, No. 1, 2011, pp. 141-150. doi:10.1016/j.bbr.2011.03.029
- F. Loiseau, C. L. Bihan, M. Hamon and M. H. Thiebot, “Effects of Melatonin and Agomelatine in Anxiety-Related Procedures in Rats: Interaction with Diazepam,” European Neuropsychopharmacology, Vol. 16, No. 6, 2006, pp. 417-428. doi:10.1016/j.euroneuro.2005.11.007
- M. Mantovani, R. Pertile, J. B. Calixto, A. R. Santos and A. L. Rodrigues, “Melatonin Exerts an AntidepressantLike Effect in the Tail Suspension Test in Mice: Evidence for Involvement of N-Methyl-D-Aspartate Receptors and the L-Arginine-Nitric Oxide Pathway,” Neuroscience Letters, Vol. 343, No. 1, 2003, pp. 1-4. doi:10.1016/S0304-3940(03)00306-9
- F. Z. El Mrabet, I. Lagbouri, A. Mesfioui, A. El hessni and A. Ouichou, “The Influence of Gonadectomy on Anxiolytic and Antidepressant Effects of Melatonin in Male and Female Wistar Rats: A Possible Implication of Sex Hormones,” Neuroscience & Medicine, Vol. 3, No. 2, 2012, p. 162.
- E. B. Naranjo-Rodriguez, A. Ortiz Orsornio, E. Hernandez-Avitia, V. Mendoza-Fernandez and A. Escobar, “Anxiolytic-Like Actions of Melatonin, 5-Methoxy-Tryptophan, 5-Hydroxytryptophol and Benzodiazepines on a Conflict Procedure,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol. 24, 2000, pp. 117-129.
- M. Papp, E. Litwa, P. Gruca and E. Mocaer, “AnxiolyticLike Activity of Agomelatine and Melatonin in Three Animal Models of Anxiety,” Behavioural Pharmacology, Vol. 17, No. 1, 2006, pp. 9-18.
- J. Arendt. “Jet-Lag and Shift Work: (2). Therapeutic Use of Melatonin,” Journal of the Royal Society of Medicine, Vol. 92, No. 8, 1999, pp. 402-405.
- R. A. Hoffman and R. J. Reiter, “Rapid Pinealectomy in Hamsters and Other Small Rodents,” The Anatomical Record, Vol. 24, No. 1, 1965, pp. 83-89.
- M. Juszcak, J. Drobnik, J. W. Guzek and H. Schwarzberg, “Effect of Pinealectomy and Melatonin on VasopressinPotentiated Passive Avoidance in Rats,” Journal of Physiology and Pharmacology, Vol. 47, No. 4, 1996, pp. 621-627.
- J. L. Workman, Z. M. Weil, C. R. Tuthill and R. J. Nelson, “Maternal Pinealectomy Increases Depressive-Like Responses in Siberian Hamster Offspring,” Behavioural Brain Research, Vol. 189, No. 2, 2008, pp. 387-391. doi:10.1016/j.bbr.2008.01.016
- M. L. Dubocovich, P. Delagrange, D. N. Krause, D. Sugden, D. P. Cardinali and J. Olcese, “International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, Classification, and Pharmacology of G ProteinCoupled Melatonin Receptors,” Pharmacological Reviews, Vol. 62, No. 3, 2010, pp. 343-380. doi:10.1124/pr.110.002832
- M. L. Dubocovich, M. I. Masana and S. Benloucif, “Molecular Pharmacology and Function of Melatonin Receptor Subtypes,” In: J. Olcese, Ed., Melatonin after Four Decades: An Assessment of Its Potential, Vol. 20, New York, 2000, pp. 181-190.
- D. Mazurais, I. Brierley, I. Anglade, J. Drew, C. Randall, N. Bromage, D. Michel, O. Kah and L. M. Williams, “Central Melatonin Receptors in the Rainbow Trout: Comparative Distribution of Ligand Binding and Gene Expression,” Journal of Comparative Neurology, Vol. 409, No. 2, 1999, pp. 313-324. doi:10.1002/(SICI)1096-9861(19990628)409:2<313::AID-CNE11>3.0.CO;2-1
- M. Durand, O. Berton, S. Aguere, L. Edno, I. Combourieu, P. Mormède and F. Chaouloff, “Effects of Repeated Fluoxetine on Anxiety-Related Behaviour,” Journanl of Neuropharmacology, Vol. 38, No. 6, 1999, pp. 893-907.
- L. Schramm, M. P. McDonald and L. E. Limbird, “The Alpha(2A)-Adrenergic Receptor Plays a Protective Role in Mouse Behavioral Models of Depression and Anxiety,” The Journal of Neuroscience, Vol. 21, No. 13, 2001, pp. 4875-4882.
- L. Meyer, J. Caston and A. G. Mensah-Nyagan, “Seasonal Variation of the Impact of a Stressful Procedure on Open Field Behaviour and Blood Corticosterone in Laboratory Mice,” Behavioural Brain Research, Vol. 167, No. 2, 2006, pp. 342-348. doi:10.1016/j.bbr.2005.09.023
- F. Clénet, E. Bouyon, M. Hasco and M. Bourin, “Light/ Dark Cycle Manipulation Influences Mice Behavior in the Elevated Plus Maze,” Behavioural Brain Research, Vol. 166, No. 1, 2006, pp. 140-149. doi:10.1016/j.bbr.2005.07.018
- S. Pellower, P. Chopin, S. E. File and M. Briley, “Validation of Open: Closed Arms Entries in an Elevated PlusMaze as a Measure of Anxiety in the Rat,” Journal of Neuroscience Methods, Vol. 14, No. 3, 1985, pp. 149-167. doi:10.1016/0165-0270(85)90031-7
- N. Benabid and A. Ouichou, “Affective Responses of Early Life Photoperiod in Male Wistar Rats,” Neurosciences and Medecine, Vol. 2, No. 3, 2011, pp. 185-191. doi:10.4236/nm.2011.23025
- R. D. Porsolt, G. Anton, N. Blavet and M. Jalfre, “Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments,” European Journal of Pharmacology, Vol. 47, No. 4, 1978, pp. 379-391. doi:10.1016/0014-2999(78)90118-8
- N. Benabid, A. Mesfioui and A. Ouichou, “Effects of Photoperiod Regimen on Emotional Behaviour in Two Tests for Anxiolytic Activity in Wistar Rat,” Brain Research Bulletin, Vol. 75, No. 1, 2008, pp. 53-59. doi:10.1016/j.brainresbull.2007.07.016
- L. M. Pyter and R. J. Nelson, “Enduring Effects of Photoperiod on Affective Behaviors in Siberian Hamsters (Phodopus Sungorus),” Behavioral Neuroscience, Vol. 120, No. 1, 2006, pp. 125-134. doi:10.1037/0735-7044.120.1.125
- G. R. Dawson and M. D. Tricklebank, “Use of the Elevated Plus Maze in the Search for Novel Anxiolytic Agents,” Trends in Pharmacological Sciences, Vol. 16, No. 2, 1995, pp. 33-36. doi:10.1016/S0165-6147(00)88973-7
- L. Pickavance, M. Tadayyon, G. Williams and R. G. Vernon, “Lactation Suppresses Diurnal Rhythm of Serum Leptin,” Biochemical and Biophysical Research Communications, Vol. 248, No. 1, 1998, pp. 196-199. doi:10.1006/bbrc.1998.8934
- R. A. Hoffman and R. J. Reiter, “Rapid Pinealectomy in Hamsters and Other Small Rodents,” The Anatomical Record, Vol. 153, No. 1, 1965, pp. 19-21.
- A. Kaya, A. Karakaş and H. Coşkun, “The Effects of the Time of the Day and the Pinealectomy on Anxiety-Like Behaviour in Male Wistar Rats,” Biological Rhythm Research, Vol. 42, No. 5, 2011, pp. 367-383. doi:10.1080/09291016.2010.525380
- C. Kopp, E. Vogel, M. C. Rettori, P. Delagrange, P. Renard, D. Lesieur and R. Misslin, “Regulation of Emotional Behaviour by Day Length in Mice: Implication of Melatonin,” Behavioural Pharmacology, Vol. 10, No. 8, 1999, pp. 747-752.
- B. Guardiola-Lemaitre, A. Lenegre and R. D. Porsolt, “Combined Effects of Diazepam and Melatonin in Two Tests for Anxiolytic Activity in the Mouse,” Pharmacology Biochemistry and Behavior, Vol. 41, No. 2, 1992, pp. 405-408.
- M. L. Dubocovich., E. Mogilnicka and P. M. Areso, “Antidepressant-Like Activity of the Melatonin Receptor Antagonist, Luzindole (N-0774), in the Mouse Behavioral Despair Test,” European Journal of Pharmacology, Vol. 182, No. 2, 1990, pp. 313-325. doi:10.1016/0014-2999(90)90290-M
- A. V. Shaji and S. K. Kulkarni, “Central Nervous System Depressant Activities of Melatonin in Rats and Mice,” Indian Journal of Experimental Biology, Vol. 36, No. 3, 1998, pp. 257-263.
- F. P. Valle and B. B. Gorzalka, “Open-Field Sex Differences Prior to Puberty in Rats,” Bulletin of the Psychonomic Society, Vol. 16, No. 6, 1980, pp. 429-431.
- A. L. Brotto, M. A. Barr and B. B. Gorzalka, “Sex Differences in Forced Swim and Open-Field Test Behaviours after Chronic Administration of Melatonin,” European Journal of Pharmacology, Vol. 402, No. 1-2, 2000, pp. 87-93. doi:10.1016/S0014-2999(00)00491-X
- M. S. Cohen, S. M. Kosslyn, H. C. Breiter, G. J. DiGirolamo, W. L. Thompson and A. K. Anderson, “Changes in Cortical Activity during Mental Rotation. A Mapping Study Using Functional MRI,” Brain, Vol. 119, No. 1, 1996, pp. 89-100. doi:10.1093/brain/119.1.89
- W. P. Pare and E. Redei, “Sex Differences and Stress Response to WKY Rats,” Physiology & Behavior, Vol. 54, No. 6, 1993, pp. 1179-1185. doi:10.1016/0031-9384(93)90345-G
- S. J. Alonso, M. A. Castellano, D. Afonso and M. Rodriguez, “Sex Differences in Behavioral Despair: Relationships between Behavioral Despair and Open Field Activity,” Physiology & Behavior, Vol. 49, No. 1, 1991, pp. 69-72. doi:10.1016/0031-9384(91)90232-D
- H. M. T. Barros and M. Ferigolo, “Ethopharmacology of Imipramine in the Forced-Swimming Test: Gender Differences,” Neuroscience & Biobehavioral Reviews, Vol. 23, No. 2, 1998, pp. 279-286. doi:10.1016/S0149-7634(98)00029-3
- C. M. Contreras, H. Lara-Morales, M. Molina-Hernandez, M. Saavedra and G. Arrellín-Rosas, “An Early Lesion of the Lateral Septal Nuclei Produces Changes in the Forced Swimming Test Depending on Gender,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, Vol. 19, No. 8, 1995, pp. 1277-1284. doi:10.1016/0278-5846(95)00266-9
- K. J. Ressler and C. B. Nemeroff, “Role of Serotoninergic and Noradrenergic Systems in the Pathophysiology of Depression and Anxiety Disorders,” Depression and Anxiety, Vol. 12, Suppl. 1, 2000, pp. 2-19. doi:10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4
- I. Kitayama, S. Nakamura and T. Yaga, “Degeneration of Locus Coeruleus Axons in Stress-Induced Depression Model,” Brain Research Bulletin, Vol. 35, No. 5-6, 1994, pp. 573-580. doi:10.1016/0361-9230(94)90171-6
- R. E. Rosenstein and D. P. Cardinali, “Melatonin Increases in Vivo Gaba Accumulation in Rat Hypothalamus, Cerebellum, Cerebral Cortex and Pineal Gland,” Brain Research, Vol. 398, No. 2, 1986, pp. 403-406.
- F. Xu, J. C. Li, K. C. Ma and M. Wang, “Effects of Melatonin on Hypothalamic Gamma-Aminobutyric Acid, Aspartic Acid, Glutamic Acid, Beta-Endorphin and Serotonin Levels in Male Mice,” Biological Signals, Vol. 4, No. 4, 1995, pp. 225-231.
- D. A. Golombek, P. Pevet and D. P. Cardinali, “Melatonin Effects on Behavior: Possible Mediation by the Central GABAergic System,” Neuroscience & Biobehavioral Reviews, Vol. 20, No. 3, 1996, pp. 403-412.
NOTES
*Corresponding author.

