Advances in Microbiology
Vol.3 No.1(2013), Article ID:29209,7 pages DOI:10.4236/aim.2013.31012
Effect of Stress-Adaptation on Antibiotic Sensitivity Profiles of Campylobacter jejuni
1Department of Poultry Science, University of Arkansas, Fayetteville, USA
2Department of Food Science and Technology, University of Tennessee, Knoxville, USA
Email: *gskumar@uark.edu
Received December 21, 2012; revised January 22, 2013; accepted February 20, 2013
Keywords: Campylobacter jejuni; Stress; Stress-Adaptation; Antibiotic Resistance; Antibiotics
ABSTRACT
Campylobacter jejuni is one of the leading causes of human gastroenteritis. Campylobacter jejuni requires special conditions and media in the laboratory for its growth. In nature, however, this organism is able to survive in very diverse and hostile environments and produce disease in humans and animals. The different mechanisms by which C. jejuni survives stressful conditions in the environment still remain unclear. Stress-adaptation may be one of the factors helping this organism to survive stresses. Some C. jejuni strains have been found to have increased antibiotic resistance in last several years. To determine the effect of acid adaptation on the antibiotic sensitivity profile of C. jejuni, 4 different isolates of C. jejuni (a human isolate and 3 poultry isolates) were exposed to an acid pH of 5.5 and then re-challenged with different stresses. The antibiotic sensitivity profiles of C. jejuni after stress-adaptation were compared with antibiotic sensitivity profiles of non-stressed C. jejuni using the Kirby Bauer agar disc diffusion assay. The antibiotic sensitivity profiles of the C. jejuni isolates used in this study were found to change when the acid-adapted bacteria were subjected to further stresses such as an acidic pH of 4.5, aerobic atmosphere and starvation. In the majority of the cases, antibiotic-resistant C. jejuni isolates were found to be more sensitive to antibiotics after stress-adaptation, but in a few cases C. jejuni showed increased resistance. These results indicate that increasing various stresses in a sequential pattern may, in some cases, reduce antibiotic resistance of C. jejuni isolates.
1. Introduction
Campylobacter jejuni is among the leading causes of foodborne bacterial diarrheal disease with approximately 850,000 cases per year in the United States with an associated economic loss estimated to be 1.5 billion dollars [1]. Other possible sequelae of C. jejuni infections include Guillain-Barré syndrome (GBS), reactive arthritis, and irritable bowel syndrome [2]. Sources of infection for C. jejuni are primarily associated with poultry and poultry products since C. jejuni are often found as commensals in large numbers in the gastrointestinal tracts of birds [3,4]. C. jejuni is predominantly found to cause gastrointestinal enteritis in humans and even a very low dose, as few as 500 organisms, can cause infection [5]. The incubation period of foodborne campylobacteriosis is usually 4 - 5 days but can range from 1 - 10 days [6]. The symptoms associated with this disease include fever, diarrhea, headache, abdominal pain, myalgia, vomiting and blood in feces [7]. In majority of the infections caused by C. jejuni, the use of antimicrobials is not necessary as infected persons usually recover within 5 - 8 days [5]. But in some of the cases, where the infections are not found to subside within 3 - 4 days, antibiotic therapy may be indicated. Erythromycin is the drug of choice, but others such as ciprofloxacin, doxycycline and tetracycline are also used in the treatment of C. jejuni infections. Recently, increases in the antibiotic resistance patterns of C. jejuni against antibiotics such as ciprofloxacin, tetracycline and erythromycin have been found in C. jejuni isolates from food and water sources [8,9]. Presence of such resistant strains in the food chain has raised concerns over the antibiotic treatment of Campylobacter infections.
Considering the highly fastidious growth requirements of C. jejuni, and the lack of genetic survival mechanisms, the ability of C. jejuni to survive outside the host and cause foodborne illness is perplexing. Research shows that some bacterial foodborne pathogens are capable of surviving many of the control measures employed in the food industry by a mechanism known as adaptive tolerance response. Exposure of bacteria to stressful environment induces a response called as the adaptive tolerance response (ATR) which helps the bacteria to survive further homologous or heterologous stresses [10,11]. C. jejuni has been found to induce an adaptive tolerance response when exposed to acid or aerobic conditions [12, 13], but research involving effects of stress-adaptation on antibiotic sensitivity profiles after C. jejuni is exposed to secondary stresses is limited. Understanding stress response on a global level may give insight into how this fastidious organism survives outside the host. When bacteria are exposed to environmental stresses they may undergo phenotypic and genotypic changes to enhance their survival in the stressful environment [14]. These changes may give rise to cross-protection when these bacteria are exposed to secondary stresses which may also subsequently change their antibiotic resistance profiles [15]. Exposure of foodborne pathogens such as E. coli, Salmonella Typhimurium and Staphylococcus to sublethal food preservation stresses was found to change their antibiotic sensitivity profiles [16]. By studying the effects of an adaptive tolerance response on the antibiotic sensitivity profiles of acid-adapted C. jejuni after exposure to various secondary stresses could provide information on the role of stress and stress-adaptation on antibiotic resistance. These results should help in developing better control strategies to reduce/eliminate C. jejuni in processing environments. The aim of this research was to determine whether the ATR induced by different isolates of C. jejuni on adaptation to a mild acid pH had any effects on the antibiotic sensitivity profiles after they were subjected to secondary stresses including acid, starvation and exposure to oxygen.
2. Materials and Methods
2.1. Bacterial Isolates and Growth Conditions
Four C. jejuni isolates were selected for the present study including a human isolate, 81 - 176, known to cause the disease in human volunteers and three poultry isolates. The human isolate 81 - 176 was donated by Dr. Michael Johnson, University of Arkansas, Fayetteville, AR. The poultry isolates used were PRCC 3 (pre-chilled chicken carcass), POCC 13 (post-chilled chicken carcass) and RECC 3 (retail chicken carcass), which were obtained and isolated in our laboratory from different stages of poultry processing. Each isolate killed over 92% of HeLa cells when previously tested in an in vitro cytotoxicity assay [17]. The isolates were stored at −80˚C in Campylobacter enrichment (CE) broth (Acumedia®) supplemented with glycerol and sub-cultured prior to the stress experiments. Frozen stock cultures were passed twice on Campylobacter blood agar plates and then inoculated into CE broth and incubated in a micro-aerobic atmosphere consisting of 5% oxygen, 10% carbon dioxide and 85% nitrogen at 42˚C for 18 h to obtain the early stationary phase cultures for the experiments.
2.2. Acid-Adaptation of C. jejuni and Exposure to Secondary Stresses
Early stationary phase (18 h) cultures in Campylobacter enrichment (CE) broth were divided into two portions and centrifuged at 8000 × g for 5 min and subsequently re-suspended either in acid broth (pH 5.5) to obtain acidadapted cells or in CE broth to obtain non-stressed cells. Acid broth was prepared by adding hydrochloric acid (HCl) directly to the CE broth. After an adaptation time of 2 h, the acid-adapted culture was divided into three portions, centrifuged and exposed to the following stresses for a period of 2 h: 1) acid stress (pH 4.5), by re-suspending in CE broth with a pH of 4.5 and incubating in microaerobic atmosphere; 2) acid and aerobic stress, by re-suspending in CE broth with a pH of 4.5 and incubating in aerobic atmosphere; 3) starvation stress by re-suspending cells in phosphate buffered saline (PBS) with a pH of 7.2. The non-stressed cultures of each isolate were centrifuged and re-suspended again in CE broth to form the control group with no stress. The experimental design is given in the form of a flow chart in Figure 1.
2.3. Determination of Antibiotic Sensitivity of C. jejuni Using Disc Diffusion Assay
The acid-adapted cultures exposed to different secondary stresses and the non-stressed cultures of C. jejuni were used to determine antibiotic sensitivity by the Kirby Bauer agar disc diffusion assay as described by the Clinical and Laboratory Standards Institute (CLSI) using Mueller-Hinton (MH) agar (Difco®) plates. Briefly, the agar disc diffusion assay was performed as follows. Prior to performing the assay, all the cultures were centrifuged and re-suspended in normal saline and the concentration of the cultures were determined by serial dilution and plating on CE blood agar plates as well as MH agar plates. For the assay, 100 µl of cell suspensions from each of the cultures were inoculated on to MH agar plates. Three to four antibiotic discs were evenly placed on the inoculated plates. Plates were incubated at 37˚C for 42 - 48 h under microaerobic conditions. Escherichia coli ATCC 25922, with known antimicrobial susceptibility, was used as the positive control for each of the antibiotics used. The antibiotic discs (BBLTM SensiDiscTM, Becton Dickinson) included in this study were ampicillin, 10 µg; chloramphenicol, 30 µg; ciprofloxacin, 5 µg; clindamycin, 2 µg; erythromycin, 15 µg; gentamicin, 10 µg; kanamycin, 30 µg; nalidixic acid, 30 µg; streptomycin 10 µg; tetracycline, 30 µg; and vancomycin, 30 µg. The antimicrobial inhibition zones were measured and interpreted as sensitive (S), intermediate (I) or resistant (R) according to CLSI standards and Huysmans and
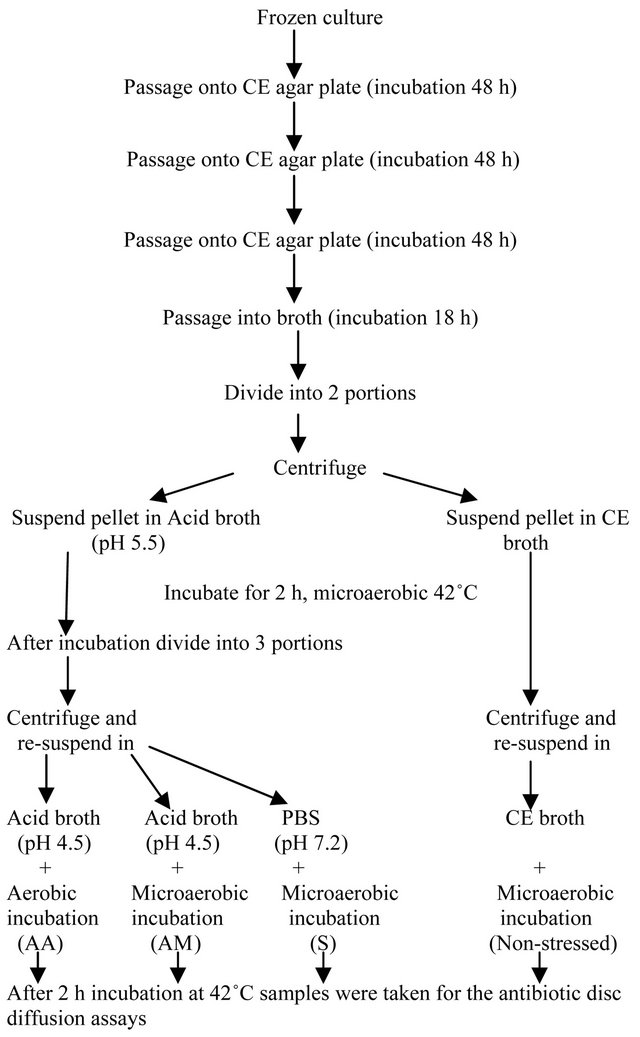
Figure 1. Flow chart of acid-adaptation and exposure of C. jejuni cultures to different secondary stresses.
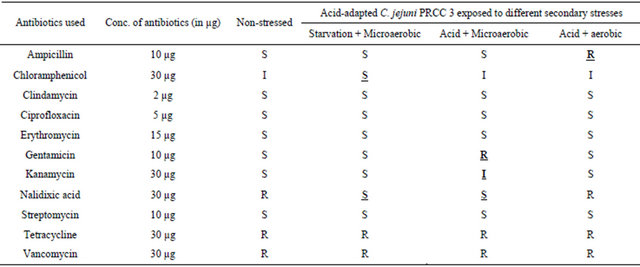
Turnidge, 1997 [18,19]. The measurement criteria used for measuring the antimicrobial inhibition zones for the different antibiotics used in this study are shown in Table 1.
2.4. Statistical Analysis
The experiments were conducted in three independent replicates and the mean antibiotic inhibition zones were calculated using the JMP statistical software package 9.0.2 and the means were compared using a student’s t-test. The results were considered statistically significant with p-values reported at p < 0.05.
3. Results
For each C. jejuni isolate antibiotic inhibition zones to the various antibiotics used were measured and recorded. Acid-adaptation and exposure to secondary stresses produced changes in the antibiotic sensitivity profiles of the human C. jejuni isolate, 81 - 176, for the antibiotics gentamicin, kanamycin, chloramphenicol and tetracycline compared to the control group with no stress (Table 2). All the stresses (starvation, acid and acid + aerobic exposure) changed the antibiotic sensitivity profiles of 81 - 176 for gentamicin and kanamycin from resistant and intermediate resistance to sensitive. However, for chloramphenicol and tetracycline the profiles were changed to sensitive from intermediate resistance and resistant in response to starvation and acid + aerobic exposure, respectively. Only with one antibiotic, nalidixic acid, did the sensitivity profile of 81 - 176 change to resistant from sensitive after starvation stress. The other two stresses did not produce any changes compared to the non-stressed C. jejuni 81 - 176.
For the C. jejuni poultry isolate from pre-processed chicken carcass (PRCC 3), the antibiotic sensitivity profiles were changed with 5 of the 11 antibiotics used in the study compared to the control group after acid-adapted C. jejuni were given the secondary stresses of either acid or starvation or acid + aerobic exposure (Table 3). The antibiotic sensitivity profile of PRCC 3 to ampicillin changed from sensitive to resistant with acid + aerobic exposure, whereas the acid stress produced resistance to gentamicin and intermediate resistance to kanamycin. Starvation stress was found to produce sensitive populations of PRCC 3 to chloramphenicol, whereas both starvation and acid stresses changed the antibiotic sensitivity profile for nalidixic acid from resistant to sensitive.
Similarly, for the C. jejuni poultry isolate from postprocessed chicken carcass (POCC 13), acid-adaptation and exposure to secondary stresses produced changes in the antibiotic sensitivity profiles for 5 of the 11 antibiotics
Table 1. Disc diffusion criteria used in this study. The antimicrobial inhibition zones for the 11 antibiotics used in this study were measured and recorded according to CLSI standards and Huysmans and Turnidge, 1997 [18,19].
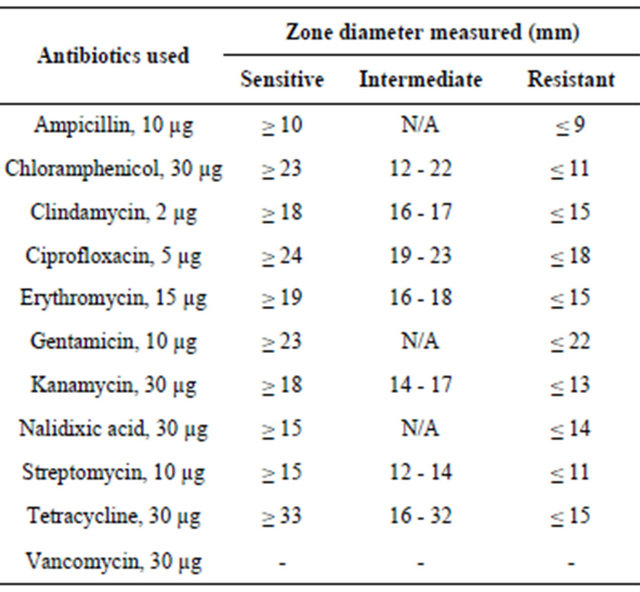
Table 2. Antibiotic profiles of C. jejuni human isolate 81 - 176 (S: sensitive, I: intermediate or R: resistant) after acid-adaptation and exposure to secondary stresses. The profiles were compared with the control group with no stress exposure.
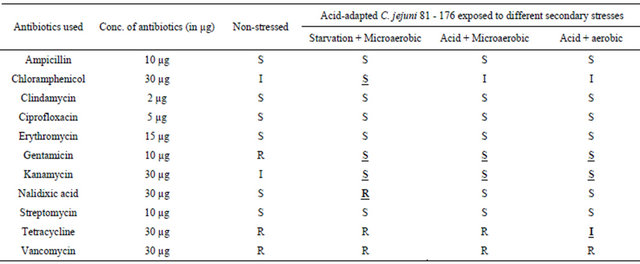
Table 3. Antibiotic profiles of C. jejuni poultry isolate PRCC 3 (S: sensitive, I: intermediate or R: resistant) after acidadaptation and exposure to secondary stresses. The profiles were compared with the control group with no stress exposure.
used in the study compared to the control group (Table 4). All stresses were found to change the resistant profile of non-stressed POCC 13 to sensitive with the antibiotic kanamycin. For the antibiotic nalidixic acid the profile was changed from resistant to sensitive with acid stress, whereas both acid and starvation stresses changed the profile from intermediate resistance to sensitive for streptomycin. Acid + aerobic stress changed the sensitive profile of POCC 13 towards gentamicin to resistant. The ciprofloxacin profile changed from sensitive to intermediate resistance with starvation and to resistant with acid + aerobic exposure.
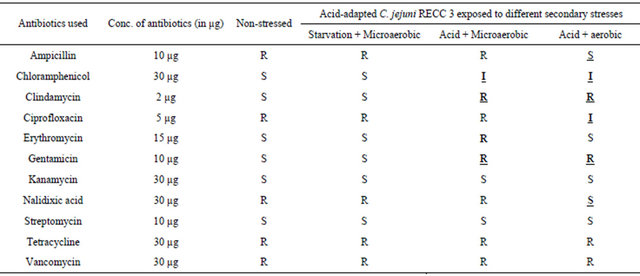
The antibiotic sensitivity profiles for RECC 3, the C. jejuni poultry isolate from retail chicken carcass, changed with 7 of the 11 antibiotics used in this study compared to the control group after acid-adaptation and exposure to secondary stresses (Table 5). Exposure to the secondary stress of starvation changed the antibiotic sensitivity profiles of non-stressed RECC 3 from resistant to sensitive for ampicillin and nalidixic acid and to intermediate resistance with ciprofloxacin. For the antibiotics gentamicin and clindamycin, the secondary stresses of acid and acid + aerobic exposure changed the profile from sensitive to resistant, whereas for starvation stress the profile remained sensitive as with the non-stressed control. Acid-adapted RECC 3 exposed to secondary stress changed the sensitive profile to resistant for erythromycin whereas exposure to the secondary stress of acid as well as acid + aerobic changed the profile to intermediate with chloramphenicol.
Table 4. Antibiotic profiles of C. jejuni poultry isolate POCC 13 (S: sensitive, I: intermediate or R: resistant) after acidadaptation and exposure to secondary stresses. The profiles were compared with the control group with no stress exposure.
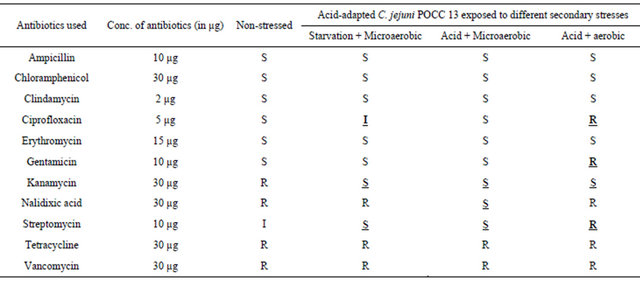
Table 5. Antibiotic profiles of C. jejuni poultry isolate RECC 3 (S: sensitive, I: intermediate or R: resistant) after acidadaptation and exposure to secondary stresses. The profiles were compared with the control group with no stress exposure.
4. Discussion
Foodborne bacteria encounter a variety of stresses in the processing environments, in foods and inside their hosts. These stresses have been found to induce adaptive responses in these bacteria along with changes in the cell which affects their innate antimicrobial susceptibility [15]. Sublethal food preservation stresses such as salt (>4.5%), reduced pH (<5.0) and high temperature (45˚C) were found to produce significantly different antibiotic sensitivity patterns for foodborne pathogens such as E. coli, Salmonella Typhimurium and Staphylococcus. Some of these stresses were found to induce permanent changes in antibiotic sensitivity profiles even after removal of the stresses [16]. However, research on the effects of stress-adaptation and subsequent exposure to secondary stresses on the antibiotic sensitivity profiles of foodborne bacteria is limited.
Various types of secondary stresses produce different types of responses in the bacteria including changes in their antimicrobial susceptibilities. All the four C. jejuni isolates showed differences in sensitivity profiles against all the antibiotics used in the present study after stressadaptation and exposure to secondary stresses except for vancomycin. These differences in the sensitivity profile were found to be dependent on the isolate, acid-adaptation, and subsequent exposure to secondary stresses.
Acid-adapted C. jejuni human isolate (81 - 176) was found to be resistant to nalidixic acid when it was exposed to the secondary stress of starvation. Starvation is known to activate a stringent response which produces a transcriptional switching resulting in reduced expression of genes affecting growth and increased expression of genes required for the survival of the bacteria [20]. Stringent response is also known to produce increased levels of alarmone guanosine 5’-(tri)diphosphate 3’-diphosphate [(p)ppGpp] which is known to affect the cell physiology in many ways including antimicrobial susceptibility [21]. Increase in ppGpp under starvation conditions has shown to increase the resistance of E. coli toward antibiotics like penicillin, trimethoprim, gentamicin and polymixin B [22]. The stringent response also decreases the production of pro-oxidant molecules such as 4-hydroxy-2-alkylquinolines (HAQs) and increases the antioxidant defenses which in turn increases oxidative killing by bactericidal antibiotics such as aminoglycosides, B-lactam, cationic and fluoroquinolone groups of antibiotics [23]. In our study, however, when the acid-adapted C. jejuni poultry isolate, PRCC 3, was exposed to starvation stress it was found to be more sensitive to nalidixic acid. Similarly acid-adapted C. jejuni poultry isolate, POCC 13, was found to be more sensitive to kanamycin when exposed to starvation. It might be assumed that some changes may be happening in these isolates during acid-adaptation which might be changing the resistance pattern when they are subsequently exposed to starvation. The C. jejuni poultry isolate, RECC 3, however did not show any difference in the sensitivity to the various antibiotics after exposure to the secondary stress of starvation compared to its control.
Exposure to oxygen or an oxidative stress was found to induce the genes of the multidrug efflux system, MaxXY-OprM of Pseudomonas aeruginosa that are known to be activated upon exposure to various antimicrobials [24]. C. jejuni also possess several drug efflux pumps of different families, but many of them are yet to be functionally characterized. The main drug efflux pump of C. jejuni CMeABC is an RND (Resistance Nodulation Division) type of efflux transporter which is known to confer resistance to several antibiotics [25]. This drug efflux pump was also found to be involved in bile resistance as well as colonization of C. jejuni in the chicken gut [26]. In this research, the poultry C. jejuni isolates were found to be resistant to ampicillin, gentamicin, ciprofloxacin and clindamycin when compared to their non-stressed controls. Hence, it might be assumed that similar to P. aeruginosa, the efflux systems of C. jejuni may be playing an important role in the antibiotic resistance when these bacteria are exposed to a secondary stress of acid and exposure to oxygen.
The exposure of acid-adapted C. jejuni poultry isolates to a sublethal pH of 4.5 was found to increase their sensitivity to antibiotics such as nalidixic acid, kanamycin and ampicillin. However, under this condition the retail isolate RECC 3 was found to be more resistant to erythromycin. These changes might be due to sudden mutations in the genome as stress-induced mutagenesis is found to increase when bacteria are exposed to stresses like starvation, exposure to oxygen, low pH, temperature extremes and exposure to antibiotics [27].
5. Conclusion
The results of this research indicate that stresses commonly encountered in the food and poultry processing environments when given in a sequential pattern may give rise to more antibiotic sensitive populations of C. jejuni. However, some stresses are found to produce a reverse effect by increasing the resistance of C. jejuni, which may help them to survive further stresses such as passage through the gastrointestinal tract. This type of a stress-adaptation could play a major role in the response of the bacteria towards antibiotics. Additional research is needed to determine the types of stresses that could increase the susceptibility of C. jejuni towards antibiotics. Further studies could also be conducted to determine the mechanisms involved in the response of C. jejuni towards antibiotics under the influence of multiple stresses.
6. Acknowledgements
The authors would like to thank the Arkansas Bioscience Institute (ABI) for funding this project. We also thank Dr. Mike Johnson for providing the C. jejuni strain 81 - 176 used in this study.
REFERENCES
- R. L. Scharff, “Economic Burden from Health Losses Due to Foodborne Illness in the United States,” Journal of Food Protection, Vol. 75, No. 1, 2012, pp. 123-131. doi:10.4315/0362-028X.JFP-11-058
- M. J. Blaser and J. Engberg, “Clinical Aspects of Campylobacter jejuni and Campylobacter coli Infections,” In: I. Nachamkin, C. M. Szymanski and M. J. Blaser, Eds., Campylobacter, 3rd Edition, ASM Press, Washington DC, 2008, pp. 99-121.
- W. Jacobs-Reitsma, “Campylobacter in the Food Supply,” In: I. Nachamkin and M. J. Blaser, Eds., Campylobacter, 2nd Edition, American Society for Microbiology, Washington DC, 2000, pp. 467-481.
- J. E. L. Corry and H. I. Atabay, “Poultry as a Source of Campylobacter and Related Organisms,” Journal of Applied Microbiology, Vol. 90, No. S6, 2001, pp. 96S-114S. doi:10.1046/j.1365-2672.2001.01358.x
- R. Black, M. Levine, M. Clements, T. Hughes and M. Blaser, “Experimental Campylobacter jejuni Infection in Humans,” Journal of Infectious Disease, Vol. 157, No. 3, 1988, pp. 472-479. doi:10.1093/infdis/157.3.472
- T. Humphrey, S. O’Brien and M. Madsen, “Campylobacters as Zoonotic Pathogens: A Food Production Perspective,” International Journal of Food Microbiology, Vol. 117, No. 3, 2007, pp. 237-257. doi:10.1016/j.ijfoodmicro.2007.01.006
- F. Poly, D. Threadgill and A. Stinzi, “Genomic Diversity in Campylobacter jejuni: Identification of C. jejuni 81 - 176 specific Genes,” Journal of Clinical Microbiology, Vol. 43, No. 5, 2005, pp. 2330-2338. doi:10.1128/JCM.43.5.2330-2338.2005
- B. Melero, P. Juntunen, M. L. Hänninen, I. Jaime and J. Rovira, “Tracing Campylobacter jejuni Strains along the Poultry Meat Production Chain from Farm to Retail by Pulsed-Field Gel Electrophoresis and the Antimicrobial Resistance of Isolates,” Food Microbiology, Vol. 32, No. 1, 2012, pp. 124-128. doi:10.1016/j.fm.2012.04.020
- B. Garin, M. Gouali, M. Wouafo, A. M. Perchec, P. M. Thu, N. Ravaonindrina, F. Urbès, M. Gay, A. Diawara, A. Leclercq, J. Rocourt and R. Pouillot, “Prevalence, Quantification and Antimicrobial Resistance of Campylobacter spp. on Chicken Neck-Skins at Points of Slaughter in 5 Major Cities Located on 4 Continents,” International Journal of Food Microbiology, Vol. 157, No. 1, 2012, pp. 102-107.
- A. E. Yousef and P. D. Courtney, “Basics of Stress Adaptation and Implications in New-Generation Foods,” In: A. E. Yousef and V. K. Juneja, Eds., Microbial Stress Adaptation and Food Safety, CRC Press LLC, Boca Raton, 2003, pp. 1-30.
- B. Ray and A. Bhunia, “Fundamental Food Microbiology,” 4th Edition, Taylor & Francis Group, New York, 2008.
- C. Murphy, C. Carroll and K. N. Jordan, “Induction of an Adaptive Tolerance Response in the Foodborne Pathogen Campylobacter jejuni,” FEMS Microbiology Letters, Vol. 223, No. 1, 2003, pp. 89-93. doi:10.1016/S0378-1097(03)00348-3
- Y. Ma, I. Hanning and M. Slavik, “Stress-Induced Adaptive Tolerance Response and Virulence Gene Expression in Campylobacter jejuni,” Journal of Food Safety, Vol. 29, No. 1, 2009, pp. 126-143. doi:10.1111/j.1745-4565.2008.00147.x
- G. Storz and R. Hengge-Aronis, “Bacterial Stress Responses,” ASM Press, Washington DC, 2000.
- K. Poole, “Bacterial Stress Responses as Determinants of Antimicrobial Resistance,” Journal of Antimicrobial Chemotherapy, Vol. 67, No. 9, 2012, pp. 2069-2089.
- M. A. S. Mc Mahon, I. S. Blair, J. E. Moore and D. A. Mc Dowell, “The Rate of Horizontal Transmission of Antibiotic Resistance Plasmids Is Increased in Food Preservation-Stressed Bacteria,” Journal of Applied Microbiology, Vol. 103, No. 5, 2003, pp. 1883-1888. doi:10.1111/j.1365-2672.2007.03412.x
- C. Gilbert and M. Slavik, “Determination of Toxicity of Campylobacter jejuni Isolated from Humans and from Poultry Carcasses Acquired at Various Stages of Production,” Journal of Applied Microbiology, Vol. 97, No. 2, 2004, pp. 347-353. doi:10.1111/j.1365-2672.2004.02302.x
- CLSI, “Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard,” 8th Edition, Clinical Laboratory Standards Institute, Wayne, 2003.
- M. B. Huysmans and J. D. Turnidge, “Disc Susceptibility Testing for Thermomphilic Campylobacters,” Pathology, Vol. 29, No. 2, 1997, pp. 209-216. doi:10.1080/00313029700169884
- D. Chatterji and A. K. Ojha, “Revisiting the Stringent Response, ppGpp and Starvation Signaling, Current Opinion in Microbiology, Vol. 4, No. 2, 2001, pp. 160-165. doi:10.1016/S1369-5274(00)00182-X
- K. Potrykus and M. Cashel, (p)ppGpp: Still Magical?” Annual Review of Microbiology, Vol. 62, No. 1, 2008, pp. 35-51. doi:10.1146/annurev.micro.62.081307.162903
- D. L. A. Greenway and R. R. England, “The Intrinsic Resistance of Escherichia Coli to Various Antimicrobial Agents Requires ppGpp and σS,” Letters in Applied Microbiology, Vol. 29, No. 5, 1999, pp. 323-326. doi:10.1046/j.1472-765X.1999.00642.x
- D. Nguyen, A. Joshi-Datar, F. Lepine, E. Bauerle, O. Olakanmi, K. Beer, G. McKay, R. Siehnel, J. Schafhauser, Y. Wang, B. E. Britigan and P. K. Singh, “Active Starvation Responses Mediate Antibiotic Tolerance in Biofilms and Nutrient-Limited Bacteria,” Science, Vol. 334, No. 6058, 2011, pp. 982-986.
- S. Fraud and K. Poole, “Oxidative Stress Induction of the mexXY Multidrug Efflux Genes and Promotion of Aminoglycoside Resistance Development in Pseudomonas Aeruginosa,” Antimicrobial Agents and Chemotherapy, Vol. 55, No. 3, 2010, pp. 1068-1074.
- J. Lin, L. O. Michel and Q. Zhang, “CmeABC Functions as a Multidrug Efflux System in Campylobacter jejuni,” Antimicrobial Agents and Chemotherapy, Vol. 46, No. 7, 2002, pp. 2124-2131. doi:10.1128/AAC.46.7.2124-2131.2002
- J. Lin, O. Sahin, L. O. Michel and Q. Zhang, “Critical Role of Multidrug Efflux Pump CmeABC in Bile Resistance and in Vivo Colonization of Campylobacter jejuni,” Infection and Immunity, Vol. 71, No. 8, 2003, pp. 4250- 4259. doi:10.1128/IAI.71.8.4250-4259.2003
- P. L. Foster, “Stress-Induced Mutagenesis in Bacteria,” Critical Reviews in Biochemistry and Molecular Biology, Vol. 42, No. 5, 2007, pp. 373-397. doi:10.1080/10409230701648494
NOTES
*Corresponding author.

