American Journal of Analytical Chemistry
Vol. 4 No. 7A (2013) , Article ID: 34446 , 8 pages DOI:10.4236/ajac.2013.47A012
Binding Ability of Corn Cobs Hemicellulose toward Cadmium
1Faculty of Pharmacy, University of North Sumatera, Medan, Indonesia
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of North Sumatera, Medan, Indonesia
Email: *muchlisyam@gmail.com
Copyright © 2013 Muchlisyam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 27, 2013; revised April 28, 2013; accepted June 15, 2013
Keywords: Corn Cobs; Hemicellulose; Cadmium Solution; Binding Ability; In Vitro; In Vivo
ABSTRACT
Non-Starch Polysaccahride (NSP) is an agricultural byproduct containing of cellulose, hemicelluloses, and lignin. Hemicellulose has a hydroxyl functional group and carboxylic function on the monomer hemicellulose used as a binding ability for cadmium ion and hence as a pharmaceutical active ingredient to prevent cadmium toxicity. The purpose of this study is to isolate and evaluate hemicelluloses from corn cobs as a binding ability toward cadmium ion. The study is conducted by isolating the corn cobs in such way using 0.2 M NaOH, characterization of hemicellulose from corn cobs produced by Infra Red Spectrofotometry. Binding ability of corn cobs hemicellulose (CCH) was done in 3 ways. The first, it was by titrimetric with cadmium 3 mg/cm3 as a titrant and indicator of 0.05 N NaOH. The second, it was by in vitro test at pH 2 as a comparison to use pectin. The third, the in vivo test was conducted in 3 variations of treatment covering CCH 10 mg, 100 mcg of cadmium for 10 weeks. Assay of cadmium was conducted using atomic absorption spectrophotometry with flame at a wavelength of 228.8 nm. The research result showed that the highest yield of hemicellulose (12.04%) was obtained from delignication with 0.03 M NaOH in 60% ethanol and 3% H2O2, hemicellulose isolation with 500 cm3 of 0.2 M NaOH, and precipitation with 1:4 ratio of 10% acetic acid in 95% ethanol. Characteristics of CCH on infrared vibration methods provide vibrational hemicellulose in the region of 1820 - 1600 cm−1. It meant that the functional group carbonyl was present, and the vibration widened near 3400 - 2400 cm−1. It indicated that there was the functional group vibration region of hydroxyl. It also meant that there was carboxylic group and finger print at 1500 - 500 cm−1. Test results of the titrimetric holding ability showed that CCH was binding 100 mg of cadmium (46.17 ± 0.9256) mg or 46.17%. Binding ability test results at pH 2 showed that 300 mg of CCH yield was binding 30 mg cadmium of (26.68 ± 0.1490) mg or 88.93%. The results of in vivo tests showed that cadmium levels decreased by 95.05%. Based on the exposure, it can be concluded that the CCH isolation yields of 12.04% and can reduce cadmium levels in the blood. It means that the CCH can be used as a chelating agent of cadmium ions by in vitro and in vivo.
1. Introduction
Cadmium is a heavy metal in a certain amount of time and can affect the development of neural and biological function, and health in general as well. Cadmium toxicity depends on several factors such as the way of how to get into the body, numbers, time exposed to body, age, and health condition of a person. Body exposure to cadmium can be through food, drink, medicine, environment, or at work [1]. Cadmium metal is a dangerous element because it tends to undergo a process of bioaccumulation. The potential toxic amount accumulates within sensitive organs and tissues. In humans, poisoning by most of these metals causes severe dysfunction in the kidneys, reproductive system, liver, brain, and central nervous system [1-3]. The occurrence of various types of diseases above is caused by the levels of cadmium in the body that are above the average level. In relation to this, it needs to be done to reduce the body’s exposure to cadmium using the supplemental inert material with no pharmacological effect, meanwhile has the ability to reduce cadmium levels. One effort that can be developed to reduce the amount of cadmium in the body is to harness the potential of natural resources such as agricultural by-product in Indonesia, which acts as a chelating agent for toxic metal in the body.
Non-Starch Polysaccarides (NSP) is a byproduct of the agricultural material processing which is amounted to around 30% - 50% of the total number of processed agricultural products. The largest component of the NSP is the fiber, consisting of components and non polysaccharide, hetero-polysaccharides such as cellulose (20% - 35%), hemicellulose (20% - 35%), pectin, alginate and lignin (10% - 25%). By conducting the process of extraction and careful fractionation of biomass byproduct of this material, it will result in various compounds which can be converted into high value products [4,5]. Hemicellulose in the pharmaceutical field could potentially be developed as a dietary supplement or as a drug formulation excipient such as chelating agents, disintergrator, thickeners, and stabilizers. In addition, several important applications of hemicellulose have been done by making the derivatives which have pharmacological effects such as lowering cholesterol, and inhibitors of HIV [5-8].
Several researchers have investigated the functional group in organic compounds which are present in nature, such as amine and hydroxyl groups in chitosan, hydroxyl carboxylate groups of sodium alginate, and pectin-containing carboxylic and hydroxyl groups found to adsorb various metals such as aluminum, iron, lead and copper [9-11]. Hemicellulose is one of the most abundant polymer after cellulose. However, its potential has yet to be completely recognized. Hemicellulose comprises a variety of monomers including xylose, mannose, arabinose, glucose, glukoronic acid having carboxylic and hydroxyl group on each monomer [12]. In the qualitative chemical analysis of organic compounds, the hydroxyl group in both cyclical aliphatic will be able to bind to the metal in order to form complex salts.
Corn cob contains a considerable resource of hemicellulose. It contains approximately 12.5% of hemicellulose and is the largest amount in comparison with other species [12]. Several researches have been performed toward the separation of hemicellulose from different plant byproduct with different methods of isolation. The lignification process was conducted among others by NaOH in 70% ethanol, chlorine, sodium hypochlorite and 30% H2O2. The Isolation of hemicellulose used alkaline compounds such as KOH or NaOH with various concentrations, whereas hemicellulose was used for purification of HCl and 90% ethanol [5,6,12].
Based on the terms described above, it is necessary for the research groups found on Corncobs hemicellulose (CCH) to do with an infrared spectrophotometer [13]. Further testing of the groups bonded ability of CCH as binding metal ions, the acid-base titration between cadmium and hemicellulose at alkaline pH using 0.05 N NaOH indicators [14]. The study made use of hydroxyl and carboxylic groups at corn cobs hemicellulose as a ligand to the metal ion to test its ability as a binding ability towards cadmium ions at pH 2 compared to the pectin, through determination of cadmium levels in the supernatant which was performed by the method of atomic absorption spectrophotometry (AAS) with flame at a wavelength of 228.8 nm [3,15,16].
Furthermore, this study will also be conducted with trial CCH binding ability in vivo toward cadmium using a rabbit for a period specified [17].
2. Experimental
2.1. Materials
Corn Cobs were obtained from local corns in Medan, Indonesia. Pectin Technical grade (China), NaOH (E. Merck), 35% hydrogen peroxide (E.Merck), 96% ethanol (E.Merck), acetic acid 98% (E.Merck), cadmium sulfate (E.Merck), Cadmium ion 1000 ppb/cm3 (E.Merck), and all other chemicals used were of analytical grade.
2.2. Methods
2.2.1. Isolation of Hemisellulose from Corn Cobs
The extraction method was modified from the combination methods described by [5,6,12]. The corn cobs powder of 50 grams was added to 500 cm3 of 0.03 M NaOH in 70% ethanol and heated at 60˚C, then stirred for 2 hours to dissolve the lignin. The suspension was allowed to cool to room temperature and filtered through Whatman filter paper. The precipitate was added 500 cm3 of 0.2 M NaOH and stirred for 8 hours at room temperature to dissolve hemicellulose, and then filtered. The filtrate was heated at a temperature of 65˚C, and added 137 cm3 of 3% H2O2 in stages. Each addition of 1 cm3 3% H2O2 to the filtrate was stirred constantly. Stirring was performed until the entire 3% of H2O2 was used and continued to a clear solution. Solution of 10% acetic acid in 95% ethanol with a ratio of 1:4 (v/v) was added to the sample solution and left at room temperature for 6 hours until the precipitate was formed. The suspension was centrifuged at a rate of 10.000 rpm for 15 minutes, and the filtrate was discarded. The precipitate was washed with 96% ethanol, and dried in vacuum dryer. The washed precipitate is hemicellulose.
2.2.2. Characterization of Corn Cobs Hemicellulose Using Infrared Spectrophotometry (FTIR)
Weighed amount of 1 mg hemicellulose and added 200 mg of potassium bromide, ground to a homogeneous, then analyzed the vibration in the range of wave number 4000 - 500 cm−1, Infrared spectra recorded the fingerprint spectrum and the spectrum of functional groups resulting from the hemicellulose [13].
2.3. Test to CCH Power Strap Respect for Cadmium (Cd) Ion with Titrimetric in the pH Bases
Procedure:
The CCH was weighed 100 mg and then put in six erlenmeyer, then dissolved in 1 cm3 of 0.05 N NaOH, diluted with distilled water to 10 cm3, and titrated with a solution 3 mg/cm3 cadmium ion. The end point of the titration is indicated by the formation of white precipitate [14].
2.4. Identify the Cadmium CCH Titration Results with the Color Reaction
The results of titration with cadmium 10 mg/cm3 were centrifuged at 10,000 rpm for 15 minutes. Then, the filtrate was separated from sediment, and the sediment was washed with aquades. After that, it was suspended in 1 cm3 of concentrated nitric acid and diluted with 10 cm3 of distilled water and stirred for 2 minutes, then centrifuged and separated. The results of hemicellulose filtrate titration appear and the first reaction solution of potassium sulfide is added to cadmium forming a yellow color [16].
2.5. Identification by FTIR
Procedure:
Titration of the resulting solution was centrifuged and the filtrate was used for identification power strap ability of cadmium by as many as 10 cm3 hemicellulose. Titration results with fried dryer and dry powder weighed as much as 1 mg and were identified by FTIR. The results were compared to the yield of vibrational CCH with infra red.
2.6. Cadmium Absorption
The absorption method was modified from the methods described by [10]. The hemicellulose and pectin respectively weighed 100 mg, 200 mg and 300 mg. Then, each was dissolved into erlenmeyer containing 25 cm3 solution 30 mg/cm3 of cadmium in 0.1 N nitric acid and the pH adjusted to acid pH was performed at pH 2. The solution was stirred with a magnetic stirrer at room temperature for 2 hours.
Then, each was centrifuged at 10,000 rpm for 30 minutes. The supernatant was taken and diluted with 0.5 cm3 to 50 cm3 aquabidest; then 0.25 cm3 of dilution was taken and diluted to 25 cm3 with aquabidest and cadmium levels were measured. The cadmium in the supernatant were estimated using flame atomic absorption spectroscopy (Hitachi Analyst 100) at 228.8 nm [3,15,16]. The results are presented as mean values ± standard deviation.
2.7. The in Vivo CCH Testing in Rabbits
The test is performed with the following steps:
1) As many as 15 rabbits were weighed and their blood was drawn to determine the initial level of cadmium in Blood.
2) Rabbits were divided into 3 groups and each group consisted of 4 rabbits. They were given to drink 3 cm3 aquabides and then treated as follows:
Group I
Rabbits were given 100 mcg of cadmium orally every 2 days for 2 weeks and then as much as 3 cm3 of it was drawn. A total of 1 cm3 was used to determine the levels of cadmium in the blood. Furthermore, it was given 10 mg CCH together with 100 mcg of cadmium every 2 days. After 2 weeks, as much as 3 cm3 of blood was drawn. It is used to test the results of the implementation of this by checking the cadmium levels in blood samples. And it was held for 10 weeks.
Group II
Rabbits were given 10 mg CCH and 100 mcg of cadmium through their mouths every 2 days for 2 weeks and as many as 3 cm3 of blood were drawn to determine the results of treatment by determining the levels of cadmium. The treatment was carried out for 10 weeks.
Group III
Rabbits were given 10 mg CCH through their mouths every 2 days for 10 weeks and as many as 3 cm3 of blood were taken every 2 weeks to determine the results of treatment by determining the levels of cadmium. The treatment was carried out for 10 weeks.
Determination of Cadmium Levels in Blood of Rabbits
One cm3 of blood was added to 5 cm3 of concentrated nitric acid and heated on a hot plate at a temperature of 150˚C for 30 minutes. Then, it was added 0.2 cm3 of 50% perchloric acid solution and 0.4 cm3 of concentrated sulfuric acid and heated in a row at a temperature of 150˚C, 200˚C and 250˚C respectively within 15 minutes. Once it was heated at a temperature of 320˚C for 20 minutes. The results were obtained by the destruction of the white and absorbed in 6 N HNO3 (v/v) at a temperature of 90˚C for 30 minutes until dissolved, and cooled and diluted with mineral-free water [15]. The determination of cadmium levels in a sample of the destruction was done by flame AAS at a wavelength of 228.3 nm [3,16,17].
3. Results and Discussion
3.1. Isolation of Hemicellulose
The modified isolation of corn cobs hemicellulose used 500 cm3 of 0.03 M NaOH in 70% ethanol and precipitation used the solution of 10% acetic acid in 95% ethanol with a ratio of 1:4 (v/v) and corn cobs hemicellulose reached the levels of 12.04%. It is in accordance with the results of Richana’s research in 2006 that reached the levels of 12.5% [12].
3.2. Characterization of Corn Cobs Hemicelluloses
The characterization of corn cobs hemicellulose includes functional groups and vibrational fingerprint with FTIR and retention time, peak height, area and type symetris by high performance liquid chromatography [12,14,18]. The results can be seen in the Table 1.
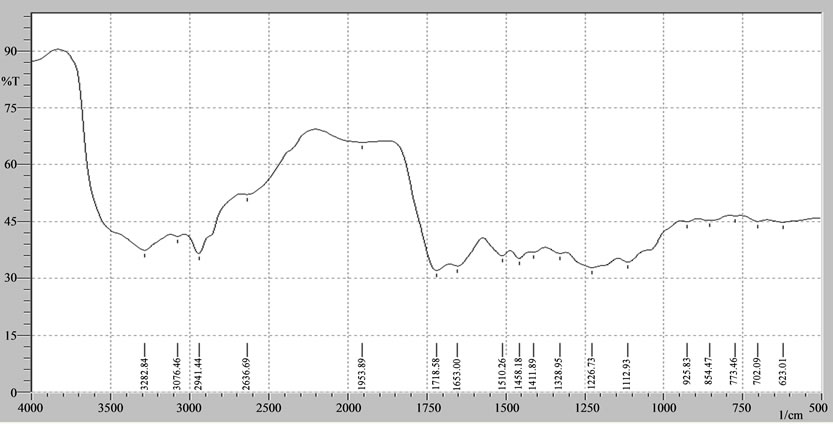
FTIR characterization of the hemicellulose in Table 1 above shows that the sample of the insulation has a C=O group and OH group. The functional group is present in the monomer on the hemicellulose and the right catchment area of 1500 - 500 cm−1 is an absorption area of fingerprint region [14] and the sample gives the of vibrations in the fingerprint region which is shown in Figure 1(a).
The HPLC characterization in Table 1 above shows that the sample of retention time, peak height, area and symetric which in Figure 1(b).
At the characterization by HPLC in Table 1 is illustrated in Figure 1(b) indicates that the yield of hemicellulose appears to have hydrophilic properties of hemicellulose by HPLC testing using aquabidest as mobile phase, using a C18 column, with a flow rate of 0.8 cm3/ min and Ultra Violet light detector at a wavelength of 280 nm produces retention time of 1.8, peak height of 5.11558 and symmetrical area of 0.82.
3.3. The CCH Bind Power Test Results for Cadmium (Cd) with Titrimetric in the Bases pH
The test of CCH ability as a chelating agent metal titrimetric with titration was done indirectly and as a solvent, 0.05 N NaOH as much as 1 cm3 was used. This is based on hemicellulose of hydroxyl groups that react
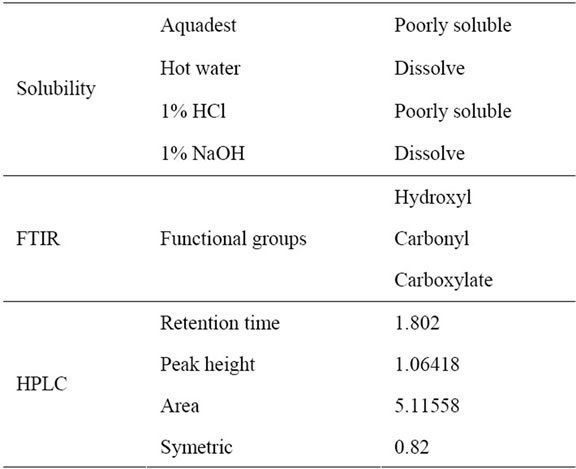
Table 1. Characterization of corn cob hemicelluloses with Solubility, FTIR and HPLC.
with the sodium salt into alkaline salts which are soluble in water. This is in accordance with the legal principles that reactions between Lewis acids and bases will form water-soluble salts [16,19]. The titration results of CCH bond between the metal ions cadmium are shown in Table 2.
Based on Table 2, it appears 100 mg of CCH was bond cadmium at (46.88 ± 0.5094) mg. That’s mean, that the cadmium ions can release sodium from hemicellulose. Because based on their group in the periodic system, the sodium contained in the class 1A and had a low electronegativity compared to cadmium ions in group II. This is reinforced by laws developed by [19], the law of the Hard Soft Acid Base stating the bonding that occurs in hemicellulose also occurs at hydroxyl groups contained in the carboxylate groups and hydroxyl CCH. Although the reaction between sodium, a strong acid and a hydroxyl compound of hemicellulose, a strong base can be replaced by other cations, there are soft acid such as cadmium ions [16].
This indicates that the titration performed in the alkaline pH of the hemicellulose can bind cadmium with endpoint white precipitation of Cd(OH)2.
3.3.1. The Identification of Cadmium CCH Bond with Color and FTIR
The titration reaction of cadmium with CCH was tested through the identification of specific reactions to cadmium, filtrate, precipitate and the final titration [14]. It appears that there has been a reaction between cadmium and hemicellulose as indicated by the specific reaction of cadmium in the filtrate titration results. Filtrate titration results indicate that sediment has specific color for cadmium. This proves that there has been an ionic bond between the cadmium and CCH and they form watersoluble complexes. This is also evidenced by the sediment test titration results showing a similar reaction. It means that the deposits were formed in the precipitate titration of cadmium hydroxide. The comparison between infrared vibrational forms of CCH and the vibrations reaction of CCH with cadmium can be seen in Figures 1(a) and 2.
Based on Figure 2, it appears that there is a change in the form of vibrations of the corn cobs hemicellulose before (Figure 1) and after the reaction with cadmium (Figure 2). This shows that the inclusion of cadmium ions at CCH will change the layout of existing vibrations in the corn cobs hemicellulose group. Thus, it generates the vibration of the bonds between hemicellulose and cadmium. OH vibrations in the region of 3700 cm−1 to 3000 cm−1 change the amount of vibration and the vibration between hemicellulose bond and cadmium in the form of vibrations extends to 3382.84 cm−1 which is a carboxylate vibrations of hydroxy groups. But, it is no longer in bonds
 (a)
(a)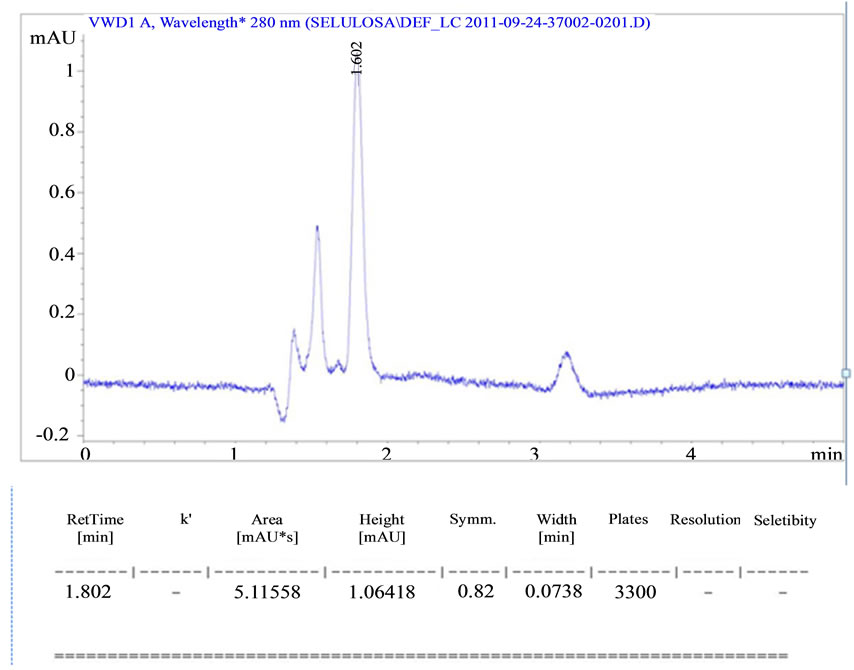 (b)
(b)
Figure 1. (a) Characterization infra red vibration of corn cobs hemicellulose; (b) Characterization corn cobs hemicellulose by high performance liquid chromatography (HPLC).
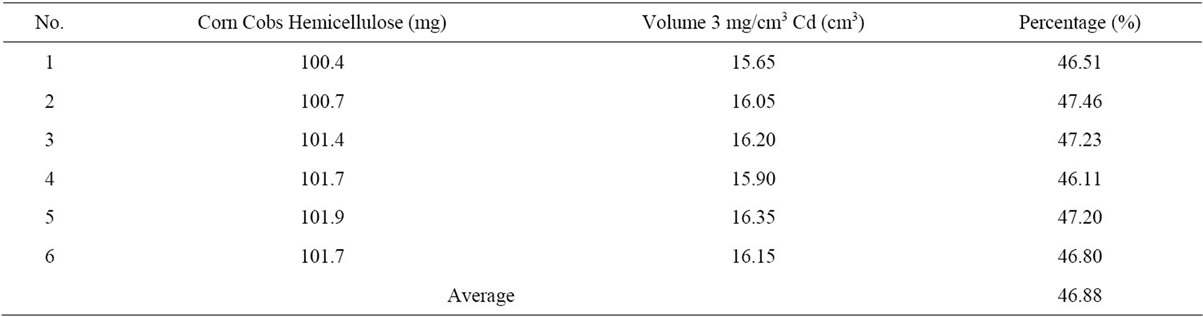
Table 2. Titration results corn cobs hemicellulose with cadmium 3 mg/cm3 as a titrant.
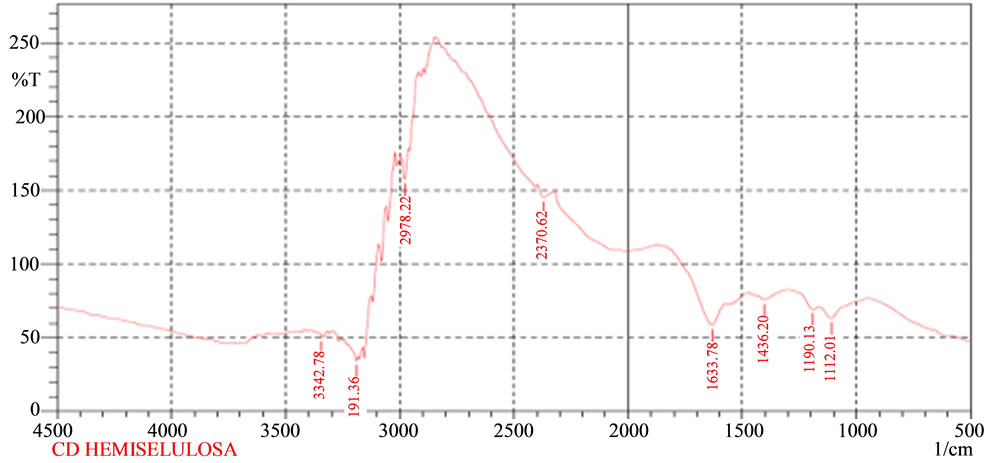
Figure 2. Characterization infrared vibration of CCH and cadmium complex.
between hemicellulose and cadmium. It means that carboxylic groups on the hemicellulose has been filled with cadmium bond vibrations present in areas adjacent to 3623.44 cm−1 and 3677.45 cm−1 [13] showing that there is a reaction of carboxylic functional groups on the corncobs hemicellulose with cadmium.
3.4. Chelating Ability of Corn Cobs Hemicellulose and Pectin toward Cadmium in pH 2
Chelating ability of corn cobs hemicellulose and pectin toward cadmium ion is shown in Table 3. The administration of chelating agent to bind distinct cadmium in the pH 2 gives different results.
From Table 3, it can be seen that there is an influence of the type and weight of chelating agent applied. But, in contrast to pectin, with the addition of pectin, the concentration will also increase properly the strap to cadmium ions. On the 100 mg pectin, there has a binding ability of (20.86 ± 0.3924) mg cadmium ion or 69.53%, but the 200 mg pectin will give a greater ability around (25.30 ± 0.6599) mg cadmium ion or 84.33% and 300 mg of pectin bound by an increase of (26.89 ± 0.5817) mg of cadmium ions or 89.63%. Compared to corn cobs hemicellulose, the average increase of the binding ability is around 4.33% - 6.61%. The overall number of increased binding ability of pectin is still below the binding ability. It means that a chelating effect of the corn cobs hemicellulose is greater than pectin. The higher the amount of chelating agent showed the higher increase of chelating agent ability. But, in corn cobs hemicellulose, the increase was not in proportional with its amount. It is in accordance with the principle of absorption that the absorption process will end if there is equilibrium between the absorbents combination [18]. Therefore, the weight does not affect a significant absorbance with the
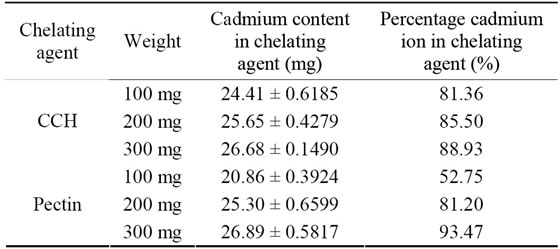
Table 3. Various binding effect of CCH and pectin for 30 mg in 25 cm3 cadmium ions.
increasing amount of binding ability of corn cobs hemicellulose for each treatment.
On statistical calculations [20,21], it can be seen that the addition of corn cobs hemicellulose will give an increase to binding effect for cadmium ion. But, it can be proved here that the CCH given as binding ability to a solution of 30 mg in 25 cm3 of cadmium causes a decline in cadmium content in the solution, meaning that there is a chemical function of the CCH that can bind cadmium ions. This suggests that the hydroxyl and carboxylic group of the corn cobs hemicellulose causes attraction for cadmium ions to be present in solution.
Based on Table 3, it can be stated that the levels of cadmium are tied with chelating agent in the treatment of the type of interaction and the variation of weight. The chelating agent type shows the same thing with the sole influence on the differences that in chelating agent type there is also difference in the binding of cadmium levels and can be maximized by a given weight of 300 mg.
The profile of chelating effect of corn cobs hemicellulose and pectin in Figure 3 shows that the weight will increase the ability of the chelating agent to modify isolation corn cobs hemicellulose and pectin as a whole. It can be seen in Figure 3 that the corn cobs hemicellulose has greater ability than pectin.
Based on Figure 3 above it can be seen that with the weight of 300 mg, pectin has the ability to absorb cadmium ions greater than the CCH. But, in the terms of pharmacology, pectin is used as anti-diarrhea only in doses below 165 mg, and if given in doses of 300 mg weight, constipation most likely occurred. Besides as a fiber, CCH has pharmacological effects to improve the digestive system and is not toxic.
3.5. Binding Ability Corn Cobs Hemicellulose toward Cadmium with in Vivo
The corn cobs hemicellulose test with three treatments was carried out for 10 weeks and the results of the test treatments A1, A2 and A3 are shown in Table 4.
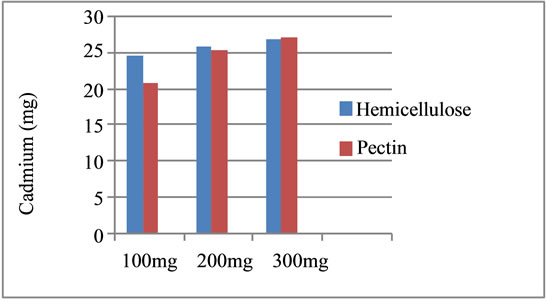
Figure 3. Charts comparison of CCH and pectin bind to cadmium ions.
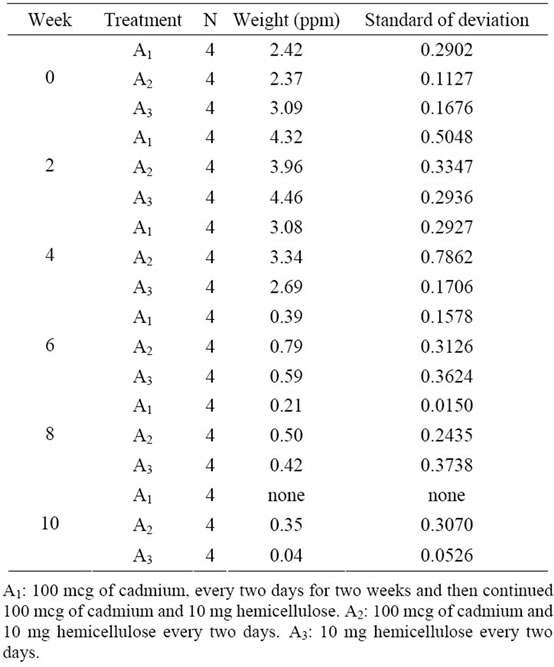
Table 4. Effect of treatment for 10 weeks on the average blood cadmium levels.
Based on the Table 4, it appears from the blood samples taken prior to treatment (W0 ) that the average blood cadmium level is at (2.63 ± 0.3893) ppm/cm3, and after the third treatment every other day for 2 weeks there was a decrease in blood cadmium levels as proved in treatment A1, A2 and A3. This is caused by the influence of carrots eaten an average of 200 grams per day has contained cadmium at 0.02 ppm. Thus, if affects the content of cadmium in the blood of rabbits. Under the same conditions, the examination of the fourth week of a decline in cadmium levels in all treatments showed a decrease of blood cadmium levels in rabbits. In other same conditions, results showed a decline in cadmium levels and continued until measurements in ten weeks. The treatment was then stopped because the results of the analysis of blood cadmium levels in some rabbits were not detected and the mean cadmium levels were (0.13 ± 0.2299) ppm/cm3. This means that the administration of 10 mg corn cobs hemicellulose for 10 weeks can reduce cadmium levels of 2.50 ppm or 95.05%. On the other hand, F test [21] results show that the statistical analysis before treatment (W0) to check on W10 shows no significant difference. This means that the process of the treatment of three types of treatment for 10 weeks can reduce blood cadmium levels in rabbits. In other words, corn cobs hemicellulose can be used as an ingredient to reduce cadmium levels in the blood as shown graphically in Figure 4.
Based on the Figure 4, it appears that duration of treatment affects the levels of cadmium in the blood. The results of blood tests at the end of W2 show an increase in levels of cadmium on, A1, A2 and A3 treatment. But, in the W4 week there had been a decrease in the blood and the decline continued until the W10. Treatment was stopped due to blood cadmium levels in several rabbits are not detected meaning that the administration of 10 mg corn cobs hemicellulose with a period of 10 weeks can reduce exposure to cadmium levels in the blood. It also means
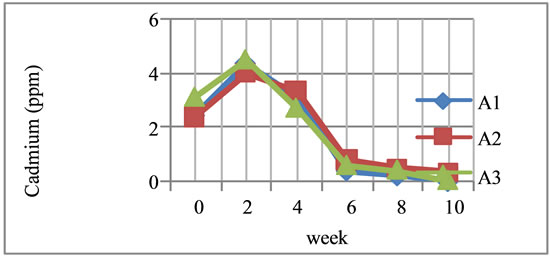
Figure 4. Graphics the relationship between time of administration and reduction of cadmium content in the blood. Description: A1: 100 mcg cadmium, every two days for two weeks and then continued 100 mcg of cadmium and 10 mg corn cobs hemicellulose. A2: 100 mcg of cadmium and 10 mg corn cobs hemicellulose every two days. A3: 10 mg corn cobs hemicellulose g every two days.
when corn cobs hemicellulose is given in greater weight, it will affect the essential minerals needed by the body in large quantities such as calcium, iron, zinc, sodium, potassium, etc. Therefore, it is worried that it would also reduce levels of these essential minerals.
4. Conclusions
Modified isolation of corn cobs hemicellulose yields 12.04% of hemicelluloses and characteristics of the hydroxyl group with vibrational infrared (FTIR) methods providing vibrational hemicellulose in the region of 1820 - 1600 cm−1. The carbonyl group provides vibrational hemicellulose widened near 3400 - 2400 cm−1 and has finger print at 1500 - 500 cm−1. Corn cobs hemicelluloses have the ability to bind cadmium in pH 2 and to increase ability influenced by the weight. The highest ability is shown by the 300 mg corn cobs hemicelluloses with binding ability at (26.68 ± 0.1490) mg to 30 mg of cadmium solution, or 88.93%. Corn cobs hemicellulose can be used as a chelating agent of metal ions.
The results of in vivo tests showed that 10 mg of CCH, given every other day for 10 weeks, was able to reduce cadmium levels of 2.50 ppm or 95.05% from the initial levels of (2.63 ± 0.3893) ppm/cm3. It means that corn cobs hemicellulose can be used to reduce levels of blood cadmium.
REFERENCES
- W. C. Prozialeck, J. R. Edward, W. N. Nebert, J. M. Woods, A. Barchowsky and W. D. Atchison, “The Vascular System as a Target of Metal Toxicity,” Toxicology Sciences Journal, Vol. 102, No. 2, 2008, pp. 207-218. doi:10.1093/toxsci/kfm263
- S. J. Casas and J. Dan Sordo, “Lead Chemistry, Analytical Aspects,” Environmental Impact and Health Effects, 2006, pp. 3-5.
- S. Jickel and A. Negrusz, “Clarke’s Analytical Forensic Toxicology,” 3th Edition, Pharmaceutical Press, Chichago, 2008.
- R. Caprita, A. Caprita and C. Dan Julean, “Biochemical Aspect of Non-Polysaccarides,” Animals Sceinces and Biotechnologies, Vol. 43, No. 1, 2010, pp. 1-4.
- A. M. Karaaslan, M. A. Tshabalala and D. G. Buschle, “Wood Hemisellulose/Chitosan-Basedsemi-Interpenetrating Networks Hydrogels: Mechanical, Swelling and Control Drug Release Properties,” Journal of Bioresources, Vol. 5 No. 2, 2010, pp. 1036-1054.
- M. P. Yadav, D. B. Johnston and K. B. Hicks, “Structural Characterization of Corn Fiber Gums from Coarse and Fine Fiber and a Study of Their Emulsifying Properties,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 15, 2007, pp. 6366-6371. doi:10.1021/jf070024q
- C. B. Saha, “Hemicellulose Bioconversion,” Journal of Microbiologie Biotechnologie, Vol. 30 No. 16, 2003, pp. 279-291.
- A. E. Silva, H. R. Marcelino, M. C. S. Gomes, E. E. Oliveira, T. Nagashima Jr. and E. S. T. Egito, “Xylan, a Promising Hemicellulose for Pharmaceutical Use,” Products and Applications of Biopolymers Journal, 2008, pp. 60- 82.
- E. Purwoningsih, “Effect of Molecular Weight Chitosan against Levels Plumbum (Pb) Blood and Enzyme Activity-Alad δ (Delta Aminolevulinic Acid Dehydratase) Albino Mice (Mus Musculus L) Healthcare,” Master Thesis, University of North Sumatra, Medan, 2008.
- I. M. Tarigan, “Analysis of the Applicability of Chitosan and Chitosan Beads as Adsorbents Standard Solution to Lower Levels of Iron (Fe) and Aluminum (Al) by Atomic Absorption Spectrophotometry,” Master Thesis, University of North Sumatra, Medan, 2008.
- W. W. Wong, F. M. A. Abbas, M. T. Liong and M. E. Azhar, “Modification of Durian Ring for Improved Biosorbent Ability,” International Food Research Journal, Vol. 15, No. 3, 2008, pp. 363-365.
- S. Dumitriu, “Polysaccharides: Structural Diversity Dan Functional Versatility,” Marcel Dekker, New York, 2005.
- N. Richana, T. T. Irawadi and A. M. Nur, “The Extraction of Hemicellulose from Corn Cobs,” Postharvest Journal, Vol. 4, No. 1, 2007, pp. 38-43.
- H. G. Britthin, “Spectroscopy of Pharmaceutical Solids,” Center for Pharmaceutical Physis Taylor and Francis Group, New York, 2006. doi:10.1201/9780849361333
- C. A. Moffat, D. M. Osellton and B. Widdop, “Clarke’s Analysis of Drugs and Poisons,” Pharmaceutical Press, London, 2005.
- J. Szkoda and J. Zmudzki, “Determination of Lead and Cadmium in Biological Material by Graphite Furnace Atomic Absorption Spectrometry Method,” Buletin Veterinair Institute, Vol. 49, 2005, pp. 89-92.
- J. B. Smith and S. Mangkoewidjojo, “The Maintenance, Breeding and Use of Experimental Animals in the Tropics,” University of Indonesia Publisher, Jakarta, 1988.
- J. Basset, R. C. Denny, B. H. Jeffrey and J. Mendham, “Vogel’s Textbook of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis,” Translation, EGC Book Medical Publishers, Jakarta, 1994.
- W. Haryadi, “Analytical Chemistry,” PT Gramedia, Jakarta, 1990.
- R. G. Pearson, “Chemical Hardness and Density Functional Theory,” Journal of Chemicals Sciences, Vol. 117, No. 5, 2005, pp. 369-377. doi:10.1007/BF02708340
- R. G. D. Steel and J. H. Torrie, “Principles and Procedures Statistics A Biometric Approach,” No. 2 PT Gramedia Pustaka Husada, Jakarta, 1991.
NOTES
*Corresponding author.

