International Journal of Clinical Medicine
Vol.06 No.03(2015), Article ID:55060,9 pages
10.4236/ijcm.2015.63027
Evaluating the p-FOXO1/FOXO1 Ratio: An Alternative Strategy for Endometrial Cancer Diagnosis
Mohsen Korani1, Soudabeh Fallah2*
1Department of Biochemistry, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran
2Department of Biochemistry, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
Email: *fallah.s@iums.ac.ir
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 7 March 2015; accepted 21 March 2015; published 26 March 2015
ABSTRACT
Background: The FOXO subfamily of Forkhead transcription factors plays a central role in promoting expression of proapoptotic and cell cycle regulatory genes. FOXO1 expression has an important role in human endometrium homeostasis. Therefore, reduced FOXO1 protein and its inactivation by phosphorylation might, play a role in progression of human endometrial cancer. Methods: Current study was designed to investigate the changes of the FOXO1, phosphorylated- FOXO1 (p-FOX1) proteins levels and the p-FOXO1/FOXO1 ratio in 30 patients with endometrial cancer and 20 subjects with normal endometrium, surgically using excised human endometrial tissue specimens, quantitative real time PCR and western blot methods. Results: FOXO1 protein level in patients with endometrial cancer significantly reduced in comparison with control group (0.17 ± 0.15 vs. 1 ± 0.14; p < 0.001). Difference between p-FOXO1 level in patients with endometrial cancer and the control group was not significant (0.77 ± 0.65 vs. 1 ± 0.19; p = 0.13). It was noteworthy that P-FOXO1/FOXO1 ratio in patients with endometrial cancer was elevated (4.43 ± 0.38 vs. 1 ± 0.18; p < 0.01). Conclusion: Findings of this study indicate the importance of FOXO1 activity in endometrial cancer cell biology and suggest that enhancing FOXO1 function may be a compelling strategy to combat endometrial cancer progression.
Keywords:
Endometrial Cancer, FOXO1, p-FOXO1, Transcription Factor

1. Introduction
Endometrial cancer is the most common type of gynecologic cancer in the United States. The majority (75% - 80%) of endometrial adenocarcinomas are known as estrogen-dependent, or type I carcinomas, and consist of the endometrioid histologic subtype. PTEN, a tumor suppressor gene, is known to be mutated in 40% - 50% of type I endometrial cancers [1] . Loss or mutation of the PTEN is the earliest detectable genetic defect in type I endometrioid endometrial cancer. PTEN encodes a lipid phosphatase that specifically dephosphorylates the D3 position of phosphatidylinositol 3,4,5-triphosphate and thereby, functionally antagonizes the phosphatidylinositol-3-kinase/Akt (PI3K/Akt) signaling pathway. Consequently, loss of PTEN function results in constitutive activation of the PI3K/Akt signal transduction pathway, a hallmark of many cancers [2] . This constitutive activation results in the inhibition of several downstream proapoptotic targets through phosphorylation, including the Forkhead boxO (FOXO) proteins. The FOXO subfamily of Forkhead transcription factors is a direct downstream target of the PI3K/Akt pathway [2] .
FOXO has been associated with a multitude of biological processes. Previous studies have revealed that FOXO family members play a crucial role in embryonic development, stress responses, DNA repair, carcinogenesis and aging; they are also involved in the maintenance of the balance between cell survival, proliferation, apoptosis, and growth arrest [3] -[9] . Their activity is negatively regulated by AKT-mediated phosphorylation. Phosphorylation of forkhead family members, FOXO1, FOXO3a, and FOXO4, which are all Akt substrates, interferes with DNA binding of these transcription factors and leads to their binding to 14-3-3 proteins in the cytoplasm, thereby preventing them from inducing transcription of FasL, resulting in the decreased activation of caspase 8 and suppression of apoptosis [10] . Cell homeostasis is maintained via the constant shuttling of FOXO1 between the nucleus and cytoplasm. FOXO1 phosphorylation by AKT at Thr24, Ser256 and Ser319 causes ex- port of FOXO1 from the nucleus to the cytoplasm. A PTEN loss of function mutation therefore results in the constitutive action of AKT, the phosphorylation, inactivation, and nuclear export of FOXO1. The inability to restrain cell cycle progression is thought to contribute to carcinogenesis [1] . Previous studies have shown that the antitumor activity of FOXOs comes from their proapoptotic and inhibitory cell cycle effects [11] .
FOXO1 expression in human endometrium increases during the secretary phase of the cycle. In contrast, FOXO3a is repressed in differentiating endometrium and FOXO4 appears not to be expressed in this tissue, at least not at protein level. Recently, it has been demonstrated that FOXO1 plays a major role in endometrial homeostasis by regulating cyclic differentiation (decidualization) and apoptosis of stromal cells in response to the increase and decrease in ovarian progesterone levels. FOXO1 is also abundantly expressed in endometrial epithelial cells [2] . Therefore, current study was designed to explore the changes of the FOXO1 and phosphorylated-FOXO1 (p-FOX1) proteins levels, the p-FOXO1/FOXO1 ratio in patients with endometrial cancer.
2. Material and Methods
2.1. Biological Samples
Tissue samples were obtained from 30 premenopaused patients diagnosed with endometrioid endometrial cancer and 20 premenopaused women with no clinically documented abnormalities of the endometrium undergoing hysterectomy at Arash Hospital of Tehran University of Medical Sciences (TUMS). Inclusion criteria included age (25 - 50 years) and no previous cancer diagnoses risk-related information such as age, parity, oral contraceptive pill (OCP) use, and body mass index (BMI), were collected from each subject. Endometrial tumor (endometrial carcinoma; EC) and non-tumor (normal endometrium; NE) tissues were excised by a surgical pathologist. Collected tissues were flash frozen in liquid nitrogen and stored in a freezer at −80˚C until analysis. A portion of the EC for each subject was analyzed at the Arash Pathology Core Laboratory as part of the routine cancer diagnosis, confirmation, grade and staging.
2.2. Western Blot
Western blot analysis of FOXO1 was performed as bellow: tissue samples were homogenized in a lysis buffer [10 mM HEPES (pH 7.6), 1.5 mM MgCl2, 0.5 mM DTT, 10 mM KCl, 10 mM NaF, 1 mM Na3VO4 and 10 µL Protease inhibitor cocktail (Sigma)]. After incubation at 4˚C for 15 min, NP-40 was added to a final concentration of 2%, total protein was extracted and following centrifugation (12,000 rpm for 30 min at 4˚C) the supernatant was collected and protein concentrations were determined, using Bradford method [12] . 75 µg of protein was subjected to western blot analysis and transferred to polyvinylidene difluoride (PVDF) membrane.
Nonspecific binding sites on PVDF were blocked by blocking buffer (5% BSA in TBS-Tween) at 4˚C for overnight. Membranes were probed with FOXO1 primary antibodiy (dilution 1:1000, FOXO1 L-17, Cell Signaling) and exposed to horseradish peroxidase-lableld secondary antiserum. The immunoreactive proteins were detected with western blotting substrate. The blots were developed in a chemiluminescence system (Pierce) and visualized by exposure to Kodak X-ray film. Membranes were stripped with restore plus western blot stripping buffer (Thermo scientific) and p-FOXO1 level was measured by reprobing of blots with primary antibody (dilution 1:1000) generated against p-FOXO1 (ser 256, Cell Signaling). β-actin was used for normalization and loading control. For this purpose, the membranes were stripped and blocked again and then reprobed by primary β-actin antibody (Biovision, dilution 1:1000). Densitometry was used in order to determine band density by using image J software.
2.3. Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was used to assess the normality of data distribution. Data were expressed as means ± S.D and were statistically analyzed by using independent sample t-test and one-way ANOVA. It is worth to report that nonparametric Mann-Whitney and Kruskal-Wallis analysis was used for FOXO1 and p- FOXO1 assay because the achieved data for FOXO1 and p-FOXO1 level did not have normal distribution. The differences of nominal variables between two groups were assessed by χ2 test. Statistical significance was set at p < 0.05.
3. Results
Table 1 summarizes the demographic features of women in present study. As it shown, the differences between age, BMI, age of menarche, pregnancy number, diabetes and OCP consumption in two groups were not statistically significant (p > 0.05). Base on endometrial cancer stage, patients were divided to four groups. There were 11, 9, 5 and 5 patients in stages I, II, III and IV respectively.
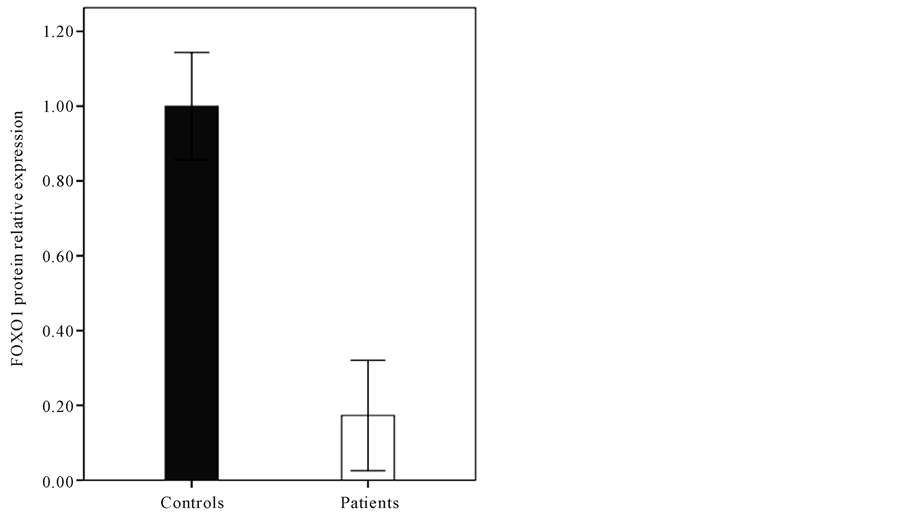
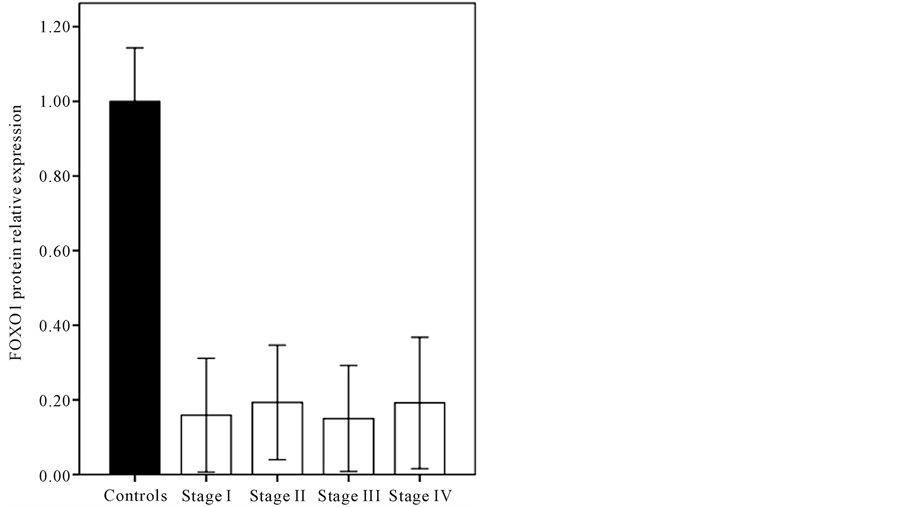
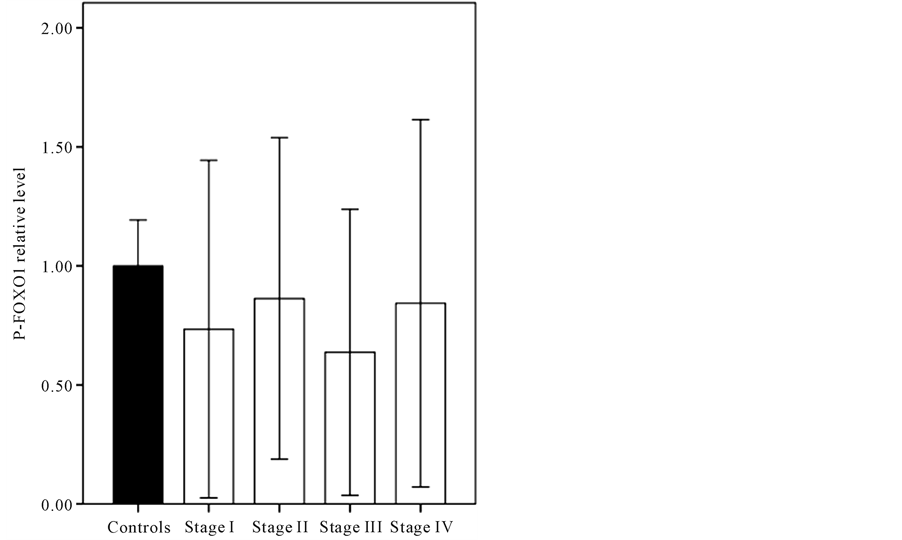
To investigate the role of FOXO1 mRNA, FOXO1 and phosphorylated FOXO1 proteins with endometrial cancer, we first profiled and determined their expression in extract endometrial tissue of the control group and patients with endometrial cancer. The mRNA level was examined in the endometrium of patients with cancer and control subjects by quantitative real-time PCR (data are not shown). The results of our previous study [13] showed that in endometrial cancer, the FOXO1 transcripts were 5.5 fold less abundant than normal endometrium. We also showed that, no significant difference was found for FOXO1 mRNA level of patient’s endometrial tissue in the different stages of cancer. As shown by western blot analysis, according to Figure 1 the FOXO1 protein level of the endometrial tissue sections of subjects with endometrial cancer, is lower (0.17 ± 0.15) significantly (p < 0.001) when compared with controls (1 ± 0.14). As it shown in Figure 2 the difference between endometrial tissue FOXO1 levels of the various stages (I-IV) of patients with endometrial cancer was not significant (p > 0.05). Depending on the frequency of FOXO 1 protein level, the study population was divided into two groups: patients with FOXO1 protein (+FOXO1group) and patients without FOXO1 protein (−FOXO1 group)
Table 1. Demographic features of studied women in this study.
Body Mass Index (BMI); Oral Contraceptive Pill (OCP); Data are mean ± SD.
Figure 1. The relative FOXO1 protein level of the endometrial tissue sections of subjects with endometrial cancer (0.17 ± 0.15) and control (1 ± 0.14) (p < 0.001).
Figure 2. The comparison between FOXO1 protein levels of the various stages I-IV of endometrium cancer (p > 0.05). β- actin was used for normalization and loading control.
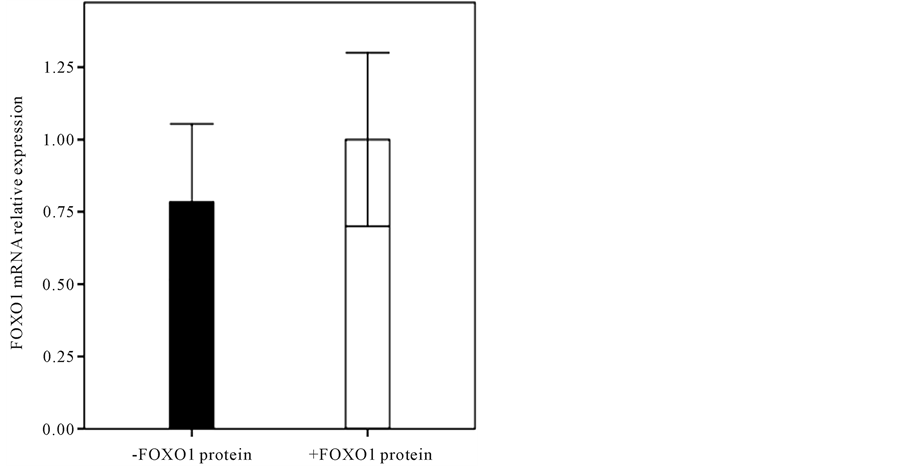
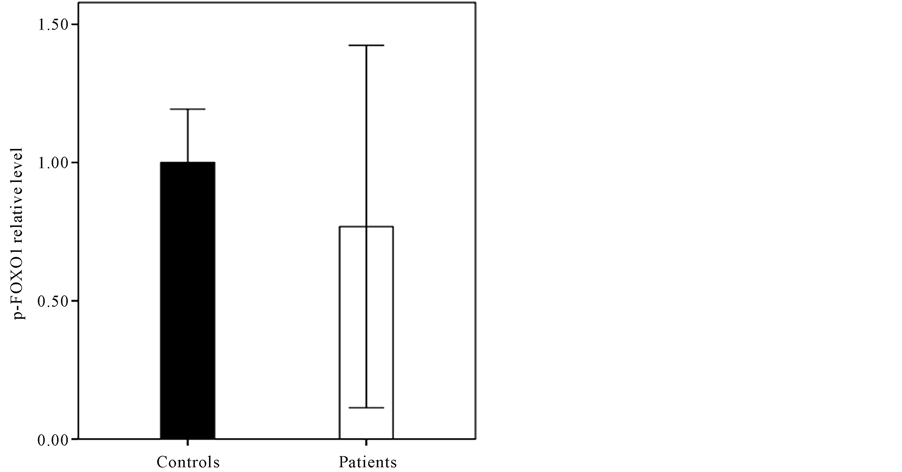
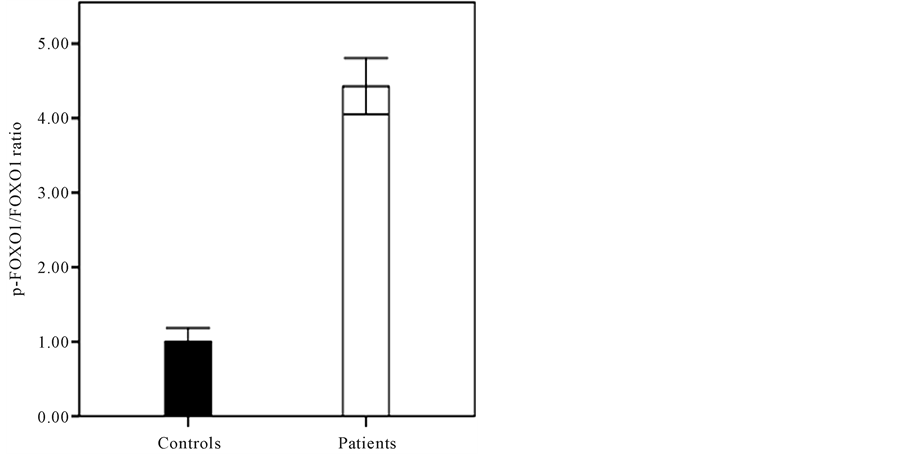
and then compared in terms of FOXO1 mRNA level. As it shown in Figure 3, the endometrial FOXO1 mRNA level of -FOXO1 group (0.78 ± 0.27) was lower than +FOXO1 group (1 ± 0.3) significantly (p < 0.05). The western blot analysis (Figure 4) showed that difference between endometrial tissue p-FOXO1 level of patients with endometrium cancer (0.77 ± 0.65) and the control group (1 ± 0.19) was not significant (p = 0.13). It was also found that difference between endometrial tissue p-FOXO1 levels of the various stages (I-IV) of endometrial cancer was not significant (p > 0.05) (Figure 5). It is noteworthy that FOXO1 and p-FOXO1 proteins were not detected in the 12 of patients. As it shown in Figure 6 the p-FOXO1/FOXO1 ratio of patients with endometrial cancer was higher (4.43 ± 0.38) than controls (1 ± 0.18) significantly (p < 0.01).
Figure 3. Comparison of FOXO1 mRNA level of +FOXO1 group (1 ± 0.3) with −FOXO1 group (0.78 ± 0.27) (p < 0.05).
Figure 4. The p-FOXO1 protein level of the endometrial tissue sections of subjects with endometrial cancer (0.77 ± 0.65) and controls (1 ± 0.19) (p = 0.13).
Figure 5. The comparison between endometrial tissue p- FOXO1 levels of the various stages of endometrium cancer (p > 0.05). β-actin was used for normalization and loading control.
Figure 6. The p-FOXO1/FOXO1 ratio of patients with endometrial cancer (4.43 ± 0.38) and controls (1 ± 0.18) (p < 0.01).
4. Discussion
Cancer is a complex, multistep process, which is tightly controlled by a balance between pro- and anticancer factors. Although it has been previously reported that FOXO1 has an anticancer function [14] , the correlation between FOXO1 and p-FOXO1 levels and endometrial cancer remains unknown. In our previous study [13] , we showed that FOXO1 expression decreases in cancerous endometrial tissue. The correlation between FOXO1, p-FOXO1 levels and endometrial cancer were investigated. We found that both mRNA and FOXO1 protein levels were decreased significantly in the endometrial tissue sections of subjects with endometrial cancer when compared with controls, suggesting that endometrial FOXO1 level alteration is involved in cancer incidence. The results of several studies are agreement with the findings obtained by present study. Goto et al. showed that loss of FOXO1 protein level perturbs endometrial homeostasis, promotes uncontrolled cell proliferation and increases susceptibility to genotoxic insults [2] . It was important that there was no significant difference between FOXO1 protein level of the various stages of cancer. The obtained results suggest that decreased expression of FOXO1 protein involved in cancer incidence and this decrease is mainly due to the decrease in the expression of the FOXO1 mRNA level.
As it shown in Figure 3, to investigate the relationship between FOXO1 protein and mRNA levels, patients divided in to two groups. In a group of patients who had significantly lower mRNA level (0.78 times, p < 0.05) in comparison with other group, the FOXO1 protein level was very small and undetectable. It seems that when mRNA reaches a critical level, will no longer be translated into protein. This process may indicate the presences of some microRNA, whose function in addition to degrading the mRNA, preventing its translation into protein. Therefore, our results also suggest that the lower FOXO1 protein level relative to its relevant FOXO1 mRNA level might be due to numerous causes such as: a decrease in stability of FOXO1 protein, a decrease in termination rate constant or the irreversible initiation elongation rate constant increase during the endometrial cancer or a decrease in the number of the free ribosomes of the cancerous state than in the reference state [15] . On the other hand, Ziginq et al. demonstrated that miR-370 can downregulates expression of FOXO1 by directly targeting the FOXO1 3’-untranslated region. They suggested that miR-370 plays an important role in the proliferation of human prostate cancer cells, by directly suppressing the tumor suppressor FOXO1 [16] . Stephen et al. demonstrated that expression of miR-9, -27, -96, -153, -182, -183, and -186, mimetic in HEC-1B cells were sufficient to reduce the abundance of FOXO1. Conversely, they also showed that FOXO1 expression was efficiently restored in the Ishikawa cell line upon simultaneous inhibition of miR-9, -27, -96, -153, -183, and -186. They identified that induction of FOXO1 in Ishikawa Cells by miR inhibitors was accompanied with G1 cell cycle arrest and cell death, and was attenuated by the siRNA-mediated downregulation of FOXO1 expression [17] . Kim et al. [11] showed that FOXO1 expression was inhibited by infecting gastric cancer cells (SNU-638) with a shRNA expressing lentivirus. They showed that constitutive phosphorylation of the FOXO1 transcription factor in gastric cancer cells correlates with microvessel area and the expressions of angiogenesis-related molecules. Therefore according to our results, it is proposed that FOXO1 protein serves as a tumor suppressor in endometrial cancer cells and is involved in normal growth control and maintenance of genomic stability. It is suggested that various specific miRs probably have a role in inducing the abnormal human endometrial cells proliferation, by directly suppressing the tumor suppressor FOXO1. It might be due to the reduction of FOXO1 expression in cancerous endometrial cells upon simultaneous elevation of different miRs. These observations revealed that the loss of FOXO1 protein perturbs endometrial homeostasis and promotes uncontrolled cell proliferation. In the present study, a decrease in FOXO1 mRNA and protein levels were identified for the first time in the endometrial tumor samples in Iranian women. As it shown in the results, no significant difference was detected for the FOXO1 mRNA and FOXO1 protein levels of the endometrial tissue of patients with cancer at different stages. It seems that alteration in gene expression profiles of FOXO1 and FOXO1 protein level, which occur in human endometrial cancer, likely play a crucial in initiation of cancer. In contrast to some studies that showed the p-FOXO1 protein level was increased in some cancers, in our study, a slightly decrease was found for p-FOXO1 level in the endometrial tissue of patients. We also found that p-FOXO1/FOXO1 ratio in the patients was significantly higher than controls (p < 0.01), suggesting that both FOXO1 and p-FOXO1 proteins levels alteration are involved in endometrial cancer cells.
Compared to the controls, in the patients a remarkable decrease was found for FOXO1 protein level, and even in 12 patients the FOXO1 protein level was not detectable. Therefore it can be concluded that reduction of p- FOXO1 protein level may also be due to decreased FOXO1 protein. p-FOXO1/FOXO1 ratio in all the patients was significantly higher than controls, suggesting that FOXO1 phosphorylation probably has an effective role in endometrial cancer incidence. It also suggests that increased growth factors signaling activity might be involved in underlying endometrial cancer. Since western blot analysis was performed with two antibodies against the total FOXO1 protein and inactivated form of FOXO1 (p-FOXO1) separately, an increase in p-FOXO1/FOXO1 ratio in the patient group in comparison with controls could be interpreted as an increase in FOXO1 inactivation. In a study, Kim et al showed that p-FOXO1 expression was closely and positively correlated with the expression of the active form of AKT [11] . They also showed that the phosphorylated inactive form of FOXO1 (p-FOXO1) was constitutively expressed in gastric cancer which was clinically significant. They also suggested that p-FOXO1 in combination with several pro-angiogenic molecules, could induce the angiogenic phenotypes of gastric cancer. The A-class of FOX proteins, especially FOXA1, functions as a pioneer factor to facilitate the androgen receptor (AR) transactivation and PCa growth. FOX family members not only have a tight relationship with AR, but also represent a pivotal group of proteins to be targeted for prostate cancer (PCa) therapy [18] . shyam et al. [19] showed that activation of FOXO1 and its nuclear sequestration is critical in the regulation of cell proliferation, cell viability and apoptosis in cervical cancer. They suggested that PI3K/AKT pathway may be a potential molecular target for cervical cancer therapy [19] . In agreement with our results, Jinju et al. have previously shown that phosphorylated FOXO1 is overexpressed in gastric cancer specimens and that
FOXO1 inactivation is related to better prognosis of gastric cancer patients. In other study, they also showed that FOXO1 inhibits CDDP-induced apoptosis in gastric cancer cells via activating PI3K/Akt pathway. Thus, FOXO1 may be an useful pharmacological indicator to predict CDDP (Cisplatin) efficacy in gastric cancer treat- ment [20] .
Therefore, the results of present study suggest that elevated p-FOXO1/FOXO1 ratio in the patients with endometrial cancer plausibly in combination with several tumorigenesis molecules and mechanisms could induce the endometrial cancer incidence or promotion. In the present study, reduction of FOXO1 and elevation of the p-FOXO1/FOXO ratio were identified. In our analysis of the combined status of the FOXO1 and p-FOXO1 profile in relation to endometrial cancer diagnosis or prognosis, a remarkable decrease in endometrial FOXO1 profile and a high p-FOXO1/FOXO1 ratio could be introduced as measures of carcinogenic activity in the endometrial cancer.
5. Conclusion
A significant decrease is found in mRNA and protein levels of FOXO1 in the patients’ endometrial tissues as comparison to controls. No significant difference is detected for in the mRNA and protein levels of FOXO1 in the patients with endometrial cancer at different stages. It is suggested that these levels of alteration in gene expression profiles of FOXO1 which occur in the primary stage of the cancers play a crucial role in initiation of human endometrial cancer. Following a significant decrease of FOXO1 expression in the endometrial cancer cells of some patients, a constitutive decrease is found for p-FOXO1, whereas those are lacking in nuclear FOXO1 expression originally. They are considered to exhibit no p-FOXO1 levels. It is noteworthy that p-FOXO1/ FOXO1 ratio in patients with endometrial cancer is elevated.
Acknowledgements
This study was supported by a grant from the Tehran University of Medical Sciences. The authors would like to thank Arash Hospital in the east of Tehran for providing the samples.
Study Limitations
There were several limitations in our study. Firstly, this was a small study, and these observations must be confirmed in a larger sample of patients with more analysis works. Nevertheless, a large scale prospective study should be launched to confirm the association of the studied gene expression profiles with the incidence of human endometrial cancer. Despite this promising implication, there are still a number of crucial steps to be performed to fully understand the original causes for endometrial human cancer. Long-term studies on more samples are needed to determine if this gene expression profile changes translate into human endometrial cancer. Our study is also limited by the non-representative nature of our study sample, because patients with endometrial cancer of eastern Tehran who were referred to Arash Hospital may have different social and economic status rather than other locations of Tehran or even Iran. Therefore, further studies are required to elucidate mechanism underpinning gene expression profiles associated with human endometrial cancer.
Conflict of Interest
There are no conflicts of interest.
Authors’ Contribution
Mohsen Korani designed the study and analyzed the data. Soudabeh Fallah contributed to the study design and wrote the paper. All authors read and approved the final manuscript.
References
- Berry, E., Hardt, J.L., Clardy, J., Lurain, J.R. and Kim, J.J. (2009) Induction of Apoptosis in Endometrial Cancer Cells by Psammaplysene A Involves FOXO1. Gynecologic Oncology, 112, 331-336. http://dx.doi.org/10.1016/j.ygyno.2008.10.017
- Goto, T., Takano, M., Albergaria, A., Briese, J., Pomeranz, K.M., Cloke, B., et al. (2008) Mechanism and Functional Consequences of Loss of FOXO1 Expression in Endometrioid Endometrial Cancer Cells. Oncogene, 27, 9-19. http://dx.doi.org/10.1038/sj.onc.1210626
- Essers, M.A., de Vries-Smits, L.M., Barker, N., Polderman, P.E., Burgering, B.M. and Korswagen, H.C. (2005) Func- tional Interaction between Beta-Catenin and FOXO in Oxidative Stress Signaling. Science, 308, 1181-1184. http://dx.doi.org/10.1126/science.1109083
- Tran, H., Brunet, A., Grenier, J.M., Datta, S.R., Fornace Jr., A.J., DiStefano, P.S., et al. (2002) DNA Repair Pathway Stimulated by the Forkhead Transcription Factor FOXO3a through the Gadd45 Protein. Science, 296, 530-534. http://dx.doi.org/10.1126/science.1068712
- Weigel, D., Jurgens, G., Kuttner, F., Seifert, E. and Jackle, H. (1989) The Homeotic Gene Fork Head Encodes a Nuc- lear Protein and Is Expressed in the Terminal Regions of the Drosophila Embryo. Cell, 57, 645-658. http://dx.doi.org/10.1016/0092-8674(89)90133-5
- Kim, D.H., Park, M.H., Lee, E.K., Choi, Y.J., Chung, K.W., Moon, K.M., et al. (2014) The Roles of FoxOs in Modu- lation of Aging by Calorie Restriction. Biogerontology.
- Lukas, J., Lukas, C. and Bartek, J. (2004) Mammalian Cell Cycle Checkpoints: Signalling Pathways and Their Organi- zation in Space and Time. DNA Repair (Amst), 3, 997-1007. http://dx.doi.org/10.1016/j.dnarep.2004.03.006
- Bhonde, M.R., Hanski, M.L., Budczies, J., Cao, M., Gillissen, B., Moorthy, D., et al. (2006) DNA Damage-Induced Expression of p53 Suppresses Mitotic Checkpoint Kinase hMps1: The Lack of This Suppression in p53 MUT Cells Contributes to Apoptosis. The Journal of Biological Chemistry, 281, 8675-8685. http://dx.doi.org/10.1074/jbc.M511333200
- Bhonde, M.R., Hanski, M.L., Notter, M., Gillissen, B.F., Daniel, P.T., Zeitz, M., et al. (2006) Equivalent Effect of DNA Damage-Induced Apoptotic Cell Death or Long-Term Cell Cycle Arrest on Colon Carcinoma Cell Proliferation and Tumour Growth. Oncogene, 25, 165-175.
- Cai, D., McCarron, R.M. and Hallenbeck, J. (2004) Cloning and Characterization of a Forkhead Transcription Factor Gene, FoxO1a, from thirteen-Lined Ground Squirrel. Gene, 343, 203-209. http://dx.doi.org/10.1016/j.gene.2004.09.003
- Kim, S.Y., Yoon, J., Ko, Y.S., Chang, M.S., Park, J.W., Lee, H.E., et al. (2011) Constitutive Phosphorylation of the FOXO1 Transcription Factor in Gastric Cancer Cells Correlates with Microvessel Area and the Expressions of Angiogenesis-Related Molecules. Cancer, 11, 264-271. http://dx.doi.org/10.1186/1471-2407-11-264
- Bradeord, M.M. (1975) A Rapid and Sensitive Method for the Quantitation of Microgram of Protein Utilizing the Principle of Protein Binding. Analytical Biochemistry, 72, 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3
- Korani, M., Fallah, S., Tehranian, A., Nourbakhsh, M., Samadikuchaksaraei, A., Pour, M.S. and Maleki, J. (2013) The Evaluation of FOXO1, KLF9 and YT521 Genes Expression in Human Endometrial Cancer. Clinical Laboratory, 59, 483-489.
- Zhang, Y.Q., Gan, B.Y., Liu, D. and Paik, J.-H. (2011) FOXO Family Members in Cancer. Cancer Biology & Therapy, 1, 253-259. http://dx.doi.org/10.4161/cbt.12.4.15954
- Mehra, A., Lee, K.H. and Hatzimanikatis, V. (2003) Insights into the Relation between mRNA and Protein Expression Patterns: I. Theoretical Considerations. Biotechnology and Bioengineering, 84, 822-832. http://dx.doi.org/10.1002/bit.10860
- Wu, Z., Sun, H., Zeng, W., He, J. and Mao, X. (2012) Upregulation of mircoRNA-370 Induces Proliferation in Human Prostate Cancer Cells by Down-Regulating the Transcription Factor FOXO1. PLoS ONE, 7, 1-11. http://dx.doi.org/10.1371/journal.pone.0045825
- Myatt, S.S., Wang, J., Lara, J.M., Christian, M., Ho, K.K., Fusi, L., et al. (2010) Repression of FOXO1 by microRNAs in Endometrial Cancer. Cancer Research, 70, 367-377. http://dx.doi.org/10.1158/0008-5472.CAN-09-1891
- Zhao, Y., Tindall1, D.J. and Huang. H.J. (2014) Modulation of Androgen Receptor by FOXA1 and FOXO1 Factors in Prostate Cancer. International Journal of Biological Sciences, 10, 614-619. http://dx.doi.org/10.7150/ijbs.8389
- Shyam, B.P., Suresh, S.Y., Mitali, D., Govardhan, H.B., Lakshmi, K.P., Sunita, S.S. and Gopeshwar, N. (2014) Down Regulation of FOXO1 Promotes Cell Proliferation in Cervical Cancer. Journal of Cancer, 5, 655-662. http://dx.doi.org/10.7150/jca.6554
- Park, J., Ko, Y.S., Yoon, J., Kim, M.A., Park, J.W., Kim, W.H., Choi, Y., Kim, J.H., Cheon, Y. and Lee, B.L. (2014) The Forkhead Transcription Factor FOXO1 Mediates Cisplatin Resistance in Gastric Cancer Cells by Activating Phosphoinositide 3-Kinase/Akt Pathway. Gastric Cancer, 17, 423-430.
NOTES

*Corresponding author.