International Journal of Clinical Medicine
Vol.3 No.2(2012), Article ID:17885,2 pages DOI:10.4236/ijcm.2012.32028
Reactivation of Hepatitis B after Administration of Anti-TNFα in a Patient with Psoriasis
![]()
Hospital Universitario Doctor Peset, Valencia, Spain.
Email: *ainatdc@hotmail.com, diaz_tan@gva.es
Received December 4th, 2011; revised January 26th, 2012; accepted February 4th, 2012
Keywords: Hepatitis B Virus Infection; Anti-TNFα Agent; Psoriasis; Viral Reactivation; Adalimumab
ABSTRACT
In patients with severe psoriasis that at the same time have multiple comorbidities and chronic infections, is difficult to establish safer and more effective therapy. There are contradictions between the indications of the international protocols of systemic treatments, and the most recent publications which show that anti-TNFα agents could be safe therapeutic options in patients with chronic hepatitis. In the case of chronic viral infections, specifically hepatitis B, there are several conflicts in reference to the biological treatments that can block tumoral necrosis factor-α (TNFα) because there is evidence of a risk of reactivation of hepatitis. Most viral reactivations with the administration of an anti-TNFα agent have been reported in patients with inflammatory diseases. However, there are a few cases described in psoriasis. We’ll present the first case reported in literature of hepatisis B viral (HBV) reactivation in a patient with severe psoriasis secondary to the administration of adalimumab.
1. Introduction
In our daily clinical practice we often find patients with severe psoriasis that at the same time have multiple comorbidities and chronic infections. In these patients, is difficult to establish safer and more effective therapy because of the contradictions between the indications of the international protocols of systemic treatments [1,2] and the most recent publications [3,4]. In the case of chronic viral infections, specifically hepatitis B, there are several conflicts in reference to the biological treatments that can block tumoral necrosis factor-α (TNFα).
2. Case Report
We present the case of a 48-year-old woman with an history of 18 years of psoriasis in plaques who had a chronic hepatitis B virus (HBV) infection with hepatitis B surface antigen positive (HBsAg). She received multiple topical treatments, narrow band UVB (nb-UVB) therapy and cyclosporine. During a period of two and a half years she was given a standard dose of etanercept for plaque-type psoriasis, in addition to an adequate antiviral medication (lamivudine) administered by her hepatologist, with no evidence of viral reactivation in serum aminotransferase levels and the viral load. In the last months the patient didn’t comply with her treatment regimen, so the biological drug was decided to withdrawn. The patient showed progressive worsening of her psoriasis with a Psoriasis Area Severity Index (PASI) of 13.5 (Figure 1). Two months later, she began treatment with adalimumab 40 mg s.c. every two weeks. After a very good initial response, with almost complete bleaching of the lesions, the patient presented at six weeks of treatment a mild increase in the viral load (less than 2000 UI/ml). Therefore, the hepatologist introduced a second antiviral drug (tenofovir). Four months after starting adalimumab, the patient showed a rapid worsening of her psoriasis with widespread and infiltrated plaques, presenting a PASI of 9 despite of the treatment with the biological drug, whereas her number of HBV copies increased until 43,880 UI/ml, so we decided to withdrawn the drug. She continued the treatment with lamivudine and tenofovir, and two months after removing adalimumab, viral load became negative. Despite treatment with nb-UVB therapy, her psoriasis became worse (PASI 22) (Figure 2), so we decided to add acitretin 25 mg/day with a good clinical response and undetectable viral load to date.
3. Discussion
There is not much information about the safety of administering anti-TNF agents for the treatment of severe psoriasis in patients with chronic viral infections. In the case of chronic hepatitis, some recent publications show
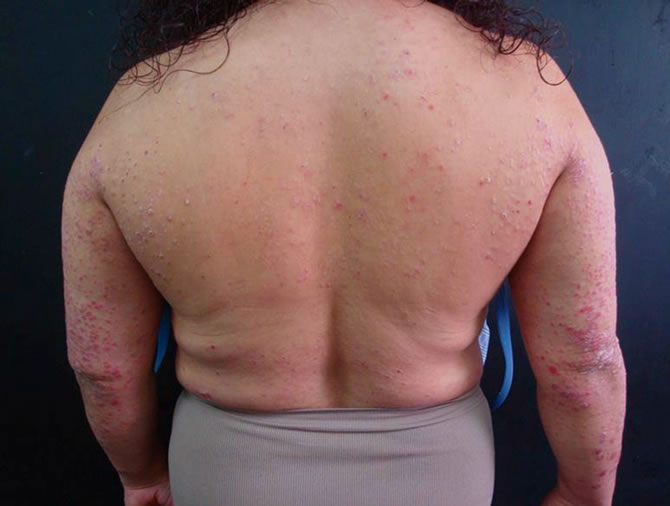
Figure 1. Severity of psoriasis (PASI 13.5) at the beginning of treatment with adalimumab.
 (a)
(a)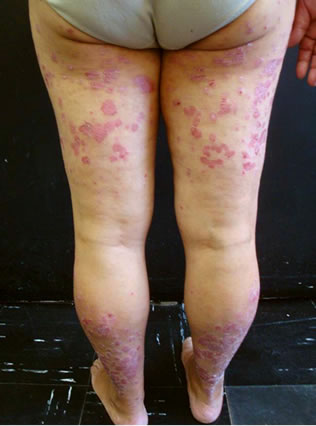 (b)
(b)
Figure 2. Severity of psoriasis (PASI 22) two months after removing adalimumab, upper (a) and lower (b) extremities.
that both etanercept and adalimumab could be safe therapeutic options under strict clinical and laboratory control of the patient with concomitant administration of antiretrovirals [3,4]. It is known that the administration of an anti-TNF has been associated with a probable beneficial effect in the case of chronic HCV infection. However, in the case of chronic HBV infection there is evidence of a risk of reactivation of hepatitis by using an anti-TNFα agent. This is because the TNFα has a protective role, controlling viral replication through the Th2 lymphocytes [5,6].
4. Conclusion
Most viral reactivations with the administration of an anti-TNFα agent have been reported in patients with inflammatory diseases. However, there are a few cases described in psoriasis [7]. We have presented the first case reported in the literature of reactivation of HBV in a patient with severe plaque-type psoriasis secondary to the administration of adalimumab. For this reason, we consider that it’s necessary to confirm with larger randomized and controlled studies that anti-TNFα agents could be safely administered in patients with chronic HBV infection since there are cases which could lead to the reactivation of viral infection, endangering the patient’s life. Because this kind of reactivation may happen, physicians and pharmaceutical companies should consider it in every individual case, and these cases must be reported to the government authorities.
5. Conflict of Interest
Dr. Díaz received financial support from Pfizer Spain in connection with the development of this manuscript.
REFERENCES
- D. Pathirana, A. D. Ormerod, P. Saiag, et al., “European S3-Guidelines on the Systemic Treatment of Psoriasis Vulgaris,” Journal of the European Academy of Dermatology and Venereology, Vol. 23, Suppl. 2, 2009, pp. 1-70. doi:10.1111/j.1468-3083.2009.03389.x
- B. Strober, E. Berger, J. Cather, et al., “A Series of Critically Challenging Case Scenarios in Moderate to Severe Psoriasis: A Delphi Consensus Approach,” Journal of the American Academy of Dermatology, Vol. 61, Suppl. 1, 2009, pp. S1-S46. doi:10.1016/j.jaad.2009.03.017
- C. Fotiadou, E. Lazaridou and D. Loannides, “Safety of Anti-Tumour Necrosis Factor-α Agents in Psoriasis Patients Who Were Chronic Hepatitis B Carriers: A Retrospective Report of Seven Patients and Brief Review of the Literature,” Journal of the European Academy of Dermatology and Venereology, Vol. 25, No. 4, 2011, pp. 471- 474. doi:10.1111/j.1468-3083.2010.03754.x
- F. Prignano, F. Ricceri, L. Pescitelli, F. Zanieri and T. Lotti, “Tumour Necrosis Factor-α Antagonists in Patients with Concurrent Psoriasis and Hepatitis B or Hepatitis C: A Retrospective Analysis of 17 Patients,” British Journal of Dermatology, Vol. 164, No. 3, 2011, pp. 645-657.
- S. Domm, J. Cinatl and U. Mrowietz, “The Impact of Treatment with Tumour Necrosis Factor-Alpha Antagonists on the Course of Chronic Viral Infections: A Review of the Literature,” British Journal of Dermatology, Vol. 159, No. 6, 2008, pp. 1217-1228. doi:10.1111/j.1365-2133.2008.08851.x
- M. B. Carroll and M. A. Forgione, “Use of Tumor Necrosis Factor Alpha Inhibitors in Hepatitis B Surface Antigen-Positive Patients: A Literature Review and Potential Mechanisms of Action,” Clinical Rheumatology, Vol. 29, 2010, pp. 1021-1029. doi:10.1007/s10067-010-1523-2
- R. Navarro, S. Ibanes, M. Llamas, E. Sotomayor, P. Garcia-Martin and E. Dauden, “Awareness about Possible Reactivation of HBsAg-Negative Patients When Prescribing Biological Therapy,” Journal of the European Academy of Dermatology and Venereology, Vol. 25, No. 10, 2011, pp. 1232-1233. doi:10.1111/j.1468-3083.2010.03833.x
NOTES
*Corresponding author.

