Journal of Surface Engineered Materials and Advanced Technology
Vol.3 No.3(2013), Article ID:34492,7 pages DOI:10.4236/jsemat.2013.33032
Synthesis and Characterization of Some New Cationic Derivatives of Biological Interest
![]()
1Applied Surfactant Laboratory, Petrochemicals Department, Egyptian Petroleum Research Institute, Cairo, Egypt; 2Chemistry Department, College of Science, University of Dammam, Dammam, KSA.
Email: *abubshait.s8@gmail.com, *sabubshait@ud.edu.sa
Copyright © 2013 Moshera Z. Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 24th, 2013; revised May 26th, 2013; accepted June 17th, 2013
Keywords: Quaternary Ammonium Amphiphiles; Micellization; Adsorption; Surface Properties; Biological Activity of Quaternized
ABSTRACT
A homologous series of cationic surfactants were synthesized and characterized by spectral studies, mass, IR, 1H NMR, 13C NMR, 2D NMR and elemental analysis. The surface activities of these amphiphiles were measured, including surface tension (γ), critical micelle concentration (CMC), effectiveness (∏cmc), efficiency (PC20), maximum surface excess (Γmax) and minimum surface area (Amin) at 25˚C. Adsorption and micellization free energies of these amphiphiles in their solutions showed a good tendency towards adsorption at the interfaces. The synthesized amphiphiles showed good antimicrobial activity.
1. Introduction
A clear understanding of the process of micellizations is necessary for rational interpretation of the effect of structural and environmental factors on the value of the critical micelle concentration. Quaternary ammonium compounds have scores of uses because of their affinity for negatively charged surfaces. Their surface active properties also help in removing oil from the sand stone formation. They also exhibit excellent germicidal activity in the bactericidal market [1,2]. These compounds are most effective against anaerobic bacteria (e.g. those that occur in oil wells). These bacteria are mainly sulfate reducers, and their growth frequently causes severe corrosion problems in oil well pipes. Due to the economic losses as well as environmental health and safety hazards caused by the activity of stabilized mixed culture containing sulfate reducing bacteria (SMC-SRB) in many industrial sectors such as the oil and gas industry, it was important to minimize the risks resulting from SRB activity. Chemical control by the use of the biocides was probably the most common method of controlling of biocorrosion [3]. Quaternary ammonium compounds (quats) are frequently used. However, it ought to be emphasized that SRB varied in their susceptibility to biocides [4]. Several studies indicated that some quats act as corrosion inhibitors and decrease sulfide production by SRB at low concentration than some biocides of commercial source [5]. This meant that quats had double purposes. Furthermore, it was found that quats were safe to handle [6].
2. Experimental Procedures
2.1. Methods of Analysis and Instruments
Firs Infrared spectra for prepared surfactants were measured using Perkin Elmer Infrared spectrophotometer FTIR- 16FPC. The elemental analysis for the obtained surfactants was carried out using Perkin Elmer Elemental Analyzer (series Π 2400). Proton nuclear magnetic resonance measurements H1 NMR and 13C NMR Spectroscopy were performed on a Bruker 400 MHZ advance 3 and the samples were run in DMSO-d6. The 2D NMR (COSY and HMBC) spectra were measured using Jeol 500 MHZ JNM-LA 500.
2.2. Synthesis of Quaternary Ammonium Surfactants
Synthesis of quaternary ammonium surfactants (Quats) was performed on two steps as shown in Scheme 1.
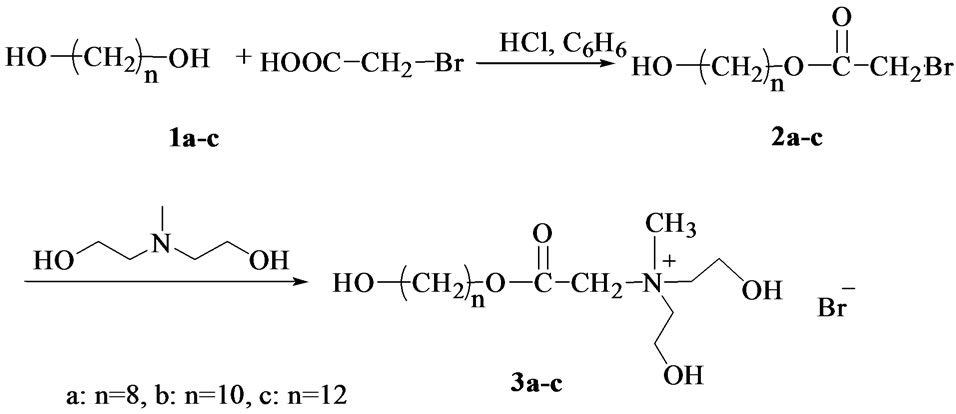
Scheme 1. Synthesized of quaternary ammonium surfactant (3a-c).
2.2.1. Synthesis of Alkyldiols Monobromoacetate (Halo-Compounds) (2a-c)
1,8-Octanediol (1a) or 1,10-decanediol (1b) or 1,12-dodecondiol (1c) (0.1 mol) was esterified by bromoacetic acid (0.1 mol) in benzene as a solvent in presence of concentrated hydrochloric acid as a catalyst till the desired amount of water (1.8 ml, 0.1 mol) was removed. The product was washed with 100 ml of alkaline solution of NaHCO3 at 40˚C (1N) to neutralize the catalyst and allowed to separate in a 250 ml separating funnel. Then, halo compounds were extracted three times by petroleum ether (50 ml). Solvent was stripped off by rotary evaporator followed by complete drying using dry over at 30˚C under vacuum [7].
2.2.2. Preparation of Quaternary Ammonium Surfactant (3a-c)
Cationic surfactants were synthesized by quaternization reaction between the synthesized halo-compounds (2a-c) and N-methyldiethanol amine in ethanol. A mixture of equimolecular amounts of N-methyldiethanol amine (0.1 mol) and each of alkyl diols monobromo acetate (2a-c) (0.1 mol) was refluxed for 23 hour, then left overnight for complete precipitation of quaternary ammonium compounds. The products was filtered and recrystallized three times from ethanol to produce the following quaternary ammonium salts (3a-c) [8,9].
2.3. Surface Tension Measurements
Surface tension of the prepared surfactants (g) were made at 25˚C with Du Nouy tensiometer (Kruss K12) with a platinum ring for various concentrations of the synthesized surfactants 3a-c (from 1 × 10−2 to 5 × 10−8 mol/L). Doubly distilled water having a surface tension of 72.0 dyne/cm at 25˚C was used to prepare all solutions. Before each measure, the glass plate was throughly washed by immersion in hot chromic acid followed by washing with doubly distilled water. The surfactant solution was placed in double walled vessel through which water was circulated from a thermostat bath (accurate to ± 0.1˚C). The establishment of equilibrium was checked repeated measurements at 5 min. intervals until the surface tension readings stabilized [10]. The accuracy of the surface tension was in most cases better than ±0.2 mNm−1.
2.3.1. Efficiency (PC20) and Surface Pressure (Effectiveness)∏cmc
The efficiency (PC20) was determined as the concentration (mol/L) capable to suppress the surface tension by 20 dyne/cm. The efficiency have been determined by extrapolating from γ = 52 dyne/cm to the linear portion before CMC of the γ-versus—log c plot at 25˚C. The surface tension γcmc values at CMC were used to calculate values of surface pressure (effectiveness) from the following expression Equation (1):
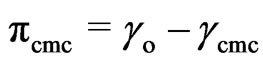 (1)
(1)
where γo is the surface tension measured for the pure water at the appropriate temperature and γcmc is the surface tension at CMC.
2.3.2. Determination of Critical Micelle Concentration (CMC)
Critical micelle concentration (CMC) of the prepared surfactant was determined by the surface tension method [11]. In this method, values of the surface tension obtained for various concentrations of aqueous solutions of the prepared surfactants were plotted versus the corresponding concentrations.
2.3.3. Determination of Maximum Surface Excess Concentration (Γmax) and Minimum Surface Area (Amin)
The maximum surface excess is defined as the surface concentration at surface saturation, Γmax is a useful measure of the effectiveness of adsorption of the surfactant at the water-air interface, since it is the maximum value to which adsorption can be attained by Gibbs Equation (2):
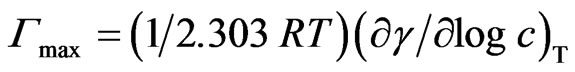 (2)
(2)
where R = 8.31 × 107 erg·mol−1·K−1, γ : mNm−1, Γ (is in mol/cm2), T is absolute temperature, (∂γ/ ∂logc)T is the slope of the γ-versus—log c plot at 25˚C [12]. Amin is the minimum area per molecule of the prepared compounds at interface and was calculated from the following Equation (3) [13].
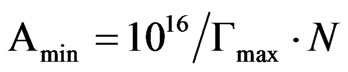 (3)
(3)
where N is Avogadro’s number and Γmax is the maximum surface excess in mol/cm2, Amin is in square angstrom.
2.3.4. The Standard Free Energy Change of
Micellization Δ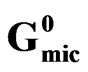 and Adsorption Δ
and Adsorption Δ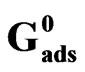
The standard free energy of micellization and adsorption are given by Equations (4, 5):
 (4)
(4)
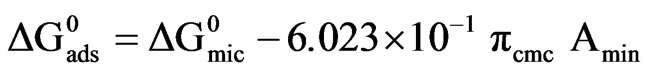 (5)
(5)
2.4. Biological Activity for Quaternary Ammonium Compounds
2.4.1. Determination of Minimal Inhibitory Concentration (mic) against the Stabilized Mixed Culture of Sulfate Reducing Bacteria (SMC-SRB)
The stabilized mixed culture of sulfate reducing bacteria (SMC-SRB) was isolated from the National Research Center (NRC) garden soil. Postage medium C [3] was expressed in g/L was used for the following purposes isolation, enrichment of SRB and biological assay using most probable number (MPN) technique [14,15]. Cetyl trimethyl ammonium bromide (CTAB) was the reference biocide from sigma chemical company. The mic of the reference and prepared compounds against SMC-SRB activity were determined by preparing postage medium C containing the following concentrations (10, 30, 60, 90, 100, 120 ppm). The inoculation was done by inoculating one ml of a four days enriched old culture of SMCSRB into sterile capped bottles containing each biocide amended medium. Incubation was done at 25˚C for 7 days. The activity of SMC-SRB was detected using MPN technique.
2.4.2. Determination of Minimal Inhibitory Concentration (mic) against Representative Pathogenic Microorganism by Using Well Diffusion Assay Method [16]
Eight pure strains used to detect the bioassay activity of the prepared compounds: Gram-negative as Escherichia coli, Pseudomonas spp. The Gram-positive as Bacillus subtilis and Staphylococcus aureus, the fungi as Aspergillus niger and Penicillium notatum, yeast as Saccharomyces cerevisiae and Candida albicans. A Nutrient agar medium [17] was used for enrichment bacterial growth and yeast and in the analytical biological test for bacteria and yeast. Molt extract agar [17] was used for enrichment fungal growth and in the biocidal assay test for fungi. Different concentrations of the prepared compounds were prepared 10−2 - 10−5 ppm. These concentrations screened against the previously mentioned pathogenic microorganisms. All isolated enriched for 24 hour and streaked on solid agar medium. The well diffusion method was applied as previously mentioned. Incubation was done at 37˚C for 48 hour. The inhibition zones were measured.
3. Results and Discussion
3.1. Characterization of the Synthesized Compounds
The 8-hydroxy-octylmonobromo acetate (2a) as Yellow semisolid, yield 89.69% from ethanol. IR (KBr, n in cm−1): 3327.0 - 3393.2 OH), 2920.9 - 2848.9 (CH2) Aliphatic, 1740.5 (C = O) ester, 1461.6 (CH2), 728.4 (CH2)n chain, 1295.8 - 1017.7 (C-O) ester, 1054.4 (C-OH), 619.3 (C - Br). 1H NMR (DMSO-d6): δ in ppm : 1.32 (m, 8H, -CH2-(CH2)4-CH2-), 1.56 (m, 2H, -CH2 -CH2-OH), 1.66 (m, 2H, -CH2-CH2-O-), 3.64 (t, 2H, -CH2 -OH), 4.06 (s, 2H, -CH2-Br), 4.18 (t, 2H, -CH2-O-CO-), 7.27 (br, 1H, -OH). 13C NMR (DMSO-d6): δ in ppm: 27.19, 28.48, (5C, -(CH2)5-CH2-O-), 29.05 (1C, -CH2CH2-OH), 32.57 (1C, -CO-CH2-Br), 60.77 (1C, -CH2-O-), 65.57 (1C, -CH2-OH), 172.2 (1C, -CO-), and the 2D NMR (COSY, HMBC) Figure 1. MS: m/z(%), M+, 267.16 [C10H19BrO3] (50%), Bp+, 55.1 [C3H3O]+ (100%). Anal. of C10H19BrO3. Calcd: C, 44.96; H, 7.17; Br, 29.91. Found: C, 45.31; H, 7.38; Br, 30.16.
The 10-hydroxy-decylmonobromo acetate (2b) as Yellow semisolid, yield 90.83% from ethanol. IR (KBr, n in cm−1): 3320.0 - 3350.3 (OH), 2924.5 - 2849.8 (CH2) Aliphatic, 1728.7 (C = O)ester, 1485.8 (CH2), 730.5 (CH2)n chain, 1018.8 - 1193.4 (C-O) ester, 1054.1 (COH), 622.0 (C-Br). 1H NMR (DMSO-d6): δ in ppm: 1.22 (m, 12H, -CH2-), 1.37 (m, 2H, -CH2 -CH2-OH), 1.56 (m, 2H, -CH2-CH2-O-), 3.49 (t, 2H, -CH2 -OH), 4.07 (s, 2H, -CH2-Br), 4.34 (t, 2H, -CH2-O-CO-), 6.40 (br, 1H, -OH). 13C NMR (DMSO-d6): δ in ppm: 25.56 (4C ,-CH2-CH2CH2CH2-O-), 28.68, (2C, -CH2-CH2CH2- O-), 29.15 (1C, -CH2CH2-OH), 32.58 (1C, -CH2- CH2OH), 41.15 (1C, -CH2-Br), 60.77(1C, -CH2-O-), 65.57 (1C, -CH2-O-CO-), 172.67 (1C, -CO-), and the 2D NMR (COSY, HMBC) Figure 1. MS: m/z (%), M+, 295.21 [C12H23BrO3] (40%) , Bp+, 55.1 [C3H3O]+ (100%). Anal. of C12H23BrO3. Calcd: C, 48.82; H, 7.85; Br, 27.07. Found: C, 49.15; H, 8.07; Br, 27.33.
The 12-hydroxy-dodecyl monobromo acetate (2c) as White semisolid, yield 97.59% from ethanol. IR (KBr, n in cm−1): 3329.4 - 3400.0 (OH), 2919.9 - 2848.5 (CH2)
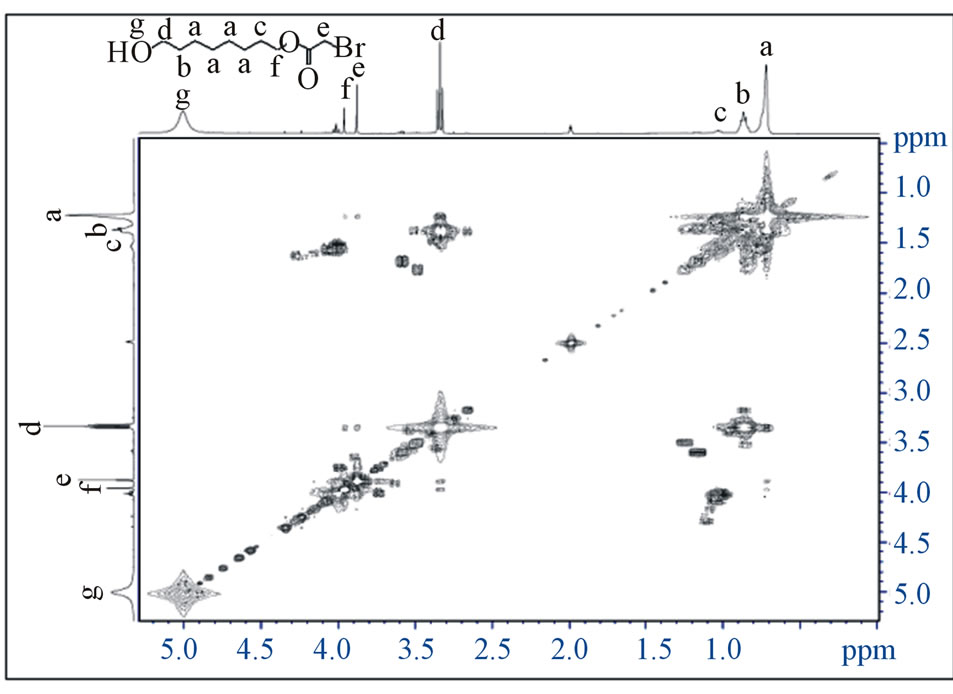
Figure 1. The 2D NMR spectrum the 1H-1H COSY of compound (2a).
Aliphatic, 1740.5 (C=O) ester, 1461.8 (CH2), 728.1 (CH2)n chain, 1331.6 - 1017.3 (C-O) ester, 1054.2 (C - OH), 532.2 (C-Br). 1H NMR (DMSO-d6): δ in ppm: 1.33 (m, 16H, -CH2-), 1.59 (m, 2H, -CH2 -CH2-OH), 1.66 (m, 2H, -CH2-CH2-O-), 3.65 (t, 2H, -CH2-OH), 4.06 (s, 2H, -CH2-Br), 4.18 (t, 2H, -CH2-OCO), 7.26 (br, 1H, -OH). 13C NMR (DMSO-d6): δ in ppm: 27.19 (6C ,-CH2-), 28.60 (2C ,-CH2 -CH2CH2-O-), 28.99 (1C ,-CH2-CH2-O-), 32.56 (1C, -CH2-CH2OH), 60.57 (1C, -CH2-Br-), 63.87 (1C, -CH2-OH), 65.57 (1C, -CH2-O-CO-), 175.2 (1C, -CO-), and the 2D NMR (COSY and HMBC) (Figures 1 and 2). MS: m/z (%), M+, 323.27 [C14H27BrO3] (50%), Bp+, 55.1 [C3H3O]+ (100%). Anal. of C14H27BrO3. Calcd: C, 52.02; H, 8.42; Br, 24.72. Found: C, 53.02; H, 8.62; Br, 25.04.
The N-methyl diethanol amine N-(carboxy methyl) octanol bromide (3a) as Paige semisolid, yield 70.46% from ethanol. IR (KBr, n in cm−1): 3329.2 - 3397.3 (OH), 2920.0 (CH3) Aliphatic, 2848.6 (CH2) Aliphatic, 1745.3 (C=O) ester, 1462.4 (CH2), 1359.9 (CH3), 728.2 (CH2)n chain, 1332.0 - 1017.1(C-O) ester, 1054.1 (C-OH). 1H NMR (DMSO-d6): δ in ppm: 1.21 (m, 8H, -CH2-(CH2)4- CH2-), 1.36 (m, 2H, -CH2 -CH2-OH), 1.37 (m, 2H, -CH2- CH2-O-), 3.32 (s, 3H, -N+-CH3), 3.34 (t, 4H, -N+-CH2- CH2OH), 3.80 (t, 2H, -CH2 -OH), 3.88 (t, 4H, -N+-CH2- CH2-OH), 4.03 (t, 2H, -CH2-O-), 4.63 (s, 2H, -CH2- (CO)-), 7.27 (br, 3H, -OH). 13C NMR (DMSO-d6): δ in ppm: 25.55 (2C , HO-(CH2)2-CH2-, HO-(CH2)5-CH2-), 29.07 (3C, HO-(CH2)3-(CH2)2-CH2-CH2-), 32.57 (1C, -CH2-CH2-OH), 49.77 (1C, -CH3-), 54.72 (2C, -N+-CH2- CH2 OH), 60.80 (1C, -CH2OH), 63.83 (2C, -N+- CH2CH2OH), 66.57 (1C, -CH2-O-CO-), 162 (1C, -CH2-CO-), 167 (1C, -CO-). MS: m/z(%), M+, 386.32 [C15H32O5NBr] (2%) , Bp+, 55.1 [C3H3O]+ (100%). Anal. of C15H32O5NBr. Calcd: C, 47.63; H, 8.35; N, 3.63; Br, 20.68. Found: C, 46.85; H, 8.63; N, 3.98; Br, 20.99.
N-Methy diethanol amine (N-Carboxy methyl) decanol
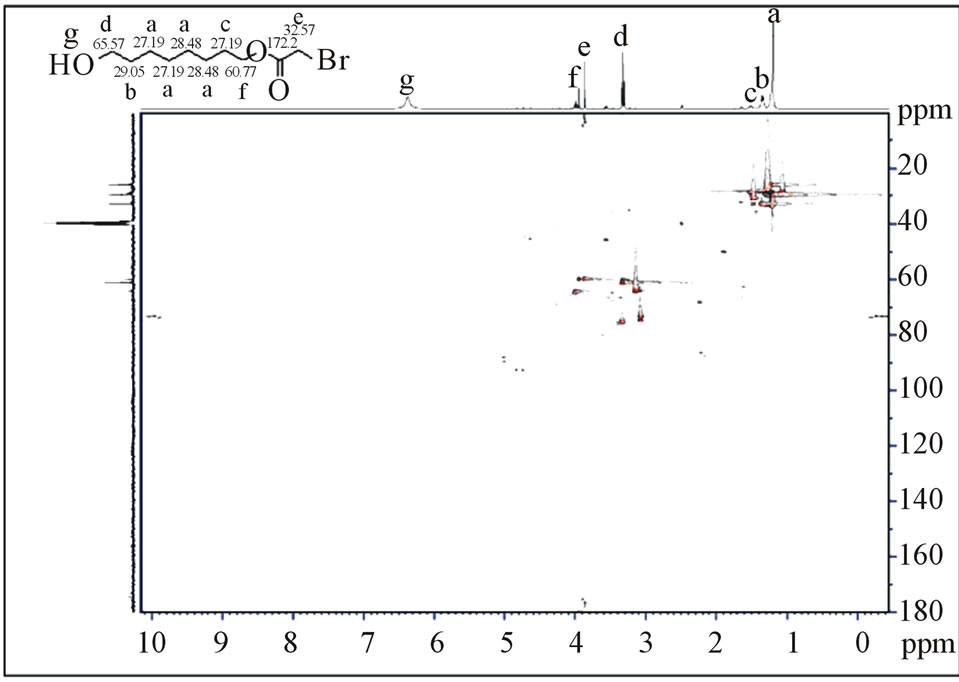
Figure 2. The 2D NMR spectrum the 1H-13C HMBC of compound (2a).
bromide (3b) as Paige semisolid, yield 82.20%, from ethanol. IR (KBr, n in cm−1): 3360 - 3312.7 (OH), 2922.9 (CH3) Aliphatic, 2849.2 (CH2) Aliphatic, 1729.1 (C=O), 1462.1 (CH2), 1359.7 (CH3), 1054.4 (C - OH), 1017.1 - 1195.8 (C-O) ester, 720.0 (CH2)n chain. 1H NMR (DMSO-d6): δ in ppm: 7.27 (br, 3H, -OH) 1.21 (m, 12H, -CH2-(CH2)6-CH2-), 1.36 (t, 2H, -CH2-CH2-O-), 2.49 (m, 2H, -CH2-CH2-O-), 3.32 (s, 3H, -N+-CH3), 3.35 (t, 4H, -N+-CH2 -CH2OH), 3.66 (t, 2H, -CH2-OH), 3.86 (t, 4H, -N+-CH2-CH2-OH), 4.47 (t, 2H, -CH2-O-), 4.63 (s, 2H, -CH2- CO-). 13C NMR(DMSO-d6): δ in ppm: 25.58 (5C, HO-(CH2)3-(CH2)4-CH2-CH2-), 29.15 (2C, HO-(CH2)2- CH2-, -CH2-(CH2)2-O-), 32.57 (1C,-CH2-CH2-OH), 49.71 (1C, -CH3-), 55.03 (2C, -N+-CH2-CH2 OH), 57.32 (1C,- CH2OH), 60.80 (2C, -N+-CH2CH2OH), 63.57 (1C, -CH2OCO), 66.60 (1C, -CH2-CO-), 165.97 (1C, -CO-). MS: m/z(%): M+, 414.38 [C17H36O5NBr.] (2%), Bp+, 55.1 [C3H3O]+ (100%). Anal. C17H36O5NBr. Calcd: C, 49.27; H, 8.76; N, 3.38; Br, 19.28. Found: C, 49.53; H, 9.07; N, 3.72; Br, 19.52.
N-Methyl diethanol amine (N-Carboxy methyl) dodecanol bromide (3c) as White semisolid, yield 79.00%, from ethanol. IR (KBr, n in cm−1): 3373.4 - 3420 (OH), 2922.7 (CH3) Aliphatic, 2852.9 (CH2) Aliphatic, 1729.1 (C=O), 1400 (CH2), 1399.7 (CH3), 1100 - 1300 (C-O) ester, 1027.7 (C-OH), 740.0(CH2)n chain. 1H NMR (DMSO-d6): δ in ppm: 1.22 (m, 16H, -(CH2 )8-CH2- CH2-O-), 1.37 (m, 2H, -CH2-CH2-OH), 1.96 (m, 2H, -CH2-CH2-O-), 3.29 (s, 3H, -N+-CH3), 3.34 (t, 4H, -N+- CH2-CH2OH), 3.35(t, 2H, -CH2-OH), 3.96 (t, 4H, -N+-CH2-CH2-OH), 4.01 (t, 2H, -CH2-O-), 4.60 (s, 2H, -CH2 -CO-), 8.82 (br, 3H, -OH). 13C NMR (DMSO-d6): δ in ppm: 25.53 (6C, HO-(CH2)3-(CH2)6-), 29.05 (1C, -OCH2-CH2-), 29.10 (2C, -CH2-(CH2 )2-O-, HO-(CH2)2- CH2-), 32.56 (1C, CH2-CH2-OH), 54.74 (1C, -CH3), 55.16 (2C, -N+-CH2-CH2-OH), 59.61 (1C, -CH2OH), 60.76 (2C, -N+-CH2CH2OH), 63.88 (1C, -CH2-O-CO-), 162.03 (1C, -CH2-CO-), 172.69 (1C, -CO-). MS: MS: m/z(%): M+, 442.39 [C19H40O5NBr] (2%), Bp+, 55.1 [C3H3O]+ (100%). Anal. C19H40O5NBr. Calcd: C, 51.53; H, 9.04; N, 3.16; Br, 18.06. Found: C, 51.85; H, 9.24; N, 3.52; Br, 18.43. Mass, IR, 1H NMR, 13C NMR, 2D NMR and elemental analysis were consistent with the structure of products.
3.2. Surface Activity
3.2.1. Surface Tension (γ) and Critical Micelle Concentration
Figure 2 represents the variation of surface tension against—log concentration of the synthesized cationic quaternary ammonium surfactants at 25˚C. The hydrophobic chain length of the various surfactants under consideration plays an effective role on the surface tension reduction of the surfactants at the identical concentration. A cationic surfactant contains a short hydrophobic chain 3a and exhibits a lower depression in the surface tension, while increasing the hydrophobic chain to 10 or 12 methylene groups. This can be attributed to the higher tendency of the longer hydrophobic chains towards adsorption at the air/water interface. The repulsion between the aqueous medium and the hydrophobic chain is increased by increasing the number of repeated methylene groups due to the differences between the later and the former in their polar nature. At higher surfactant concentrations, the values of the surface tension curve stay almost constant which indicates the critical micelle concentration values of the different surfactant, Tables 1 and 2. It is clear that the CMC values of the synthesized surfactants decreased by increasing the hydrophobic chain length. This is explained by increasing hydrophobicity of the molecules and consequently decreases their CMCs.
3.2.2. The Efficiency (PC20) and the Effectiveness (∏cmc)
Efficiency values of the prepared surfactants were shown in Table 1. The efficiency increases with increasing molar ratio of methylene units. This is because the efficiency of adsorption at interfaces increases linearly with increase in the carbon atoms in hydrophobic group [18]. The surface tension of the surfactant solution at critical micelle concentration (γcmc) determines the effectiveness (∏cmc). Increasing the hydrophobic chain lengths of the prepared surfactants increases the depression of the surface tension at the interface. The most efficient one that gives the greatest lowering in surface tension at CMC. 3c
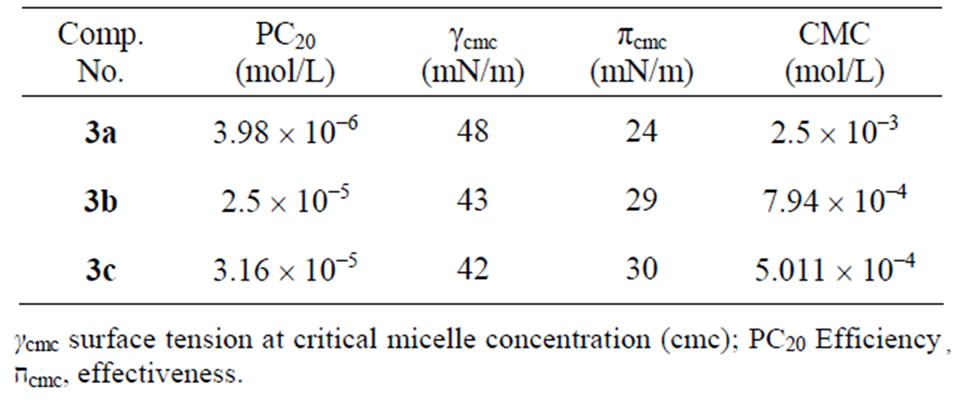
Table 1. Critical Micelle Concentration (CMC) of the prepared compounds 3a-c at 25˚C.
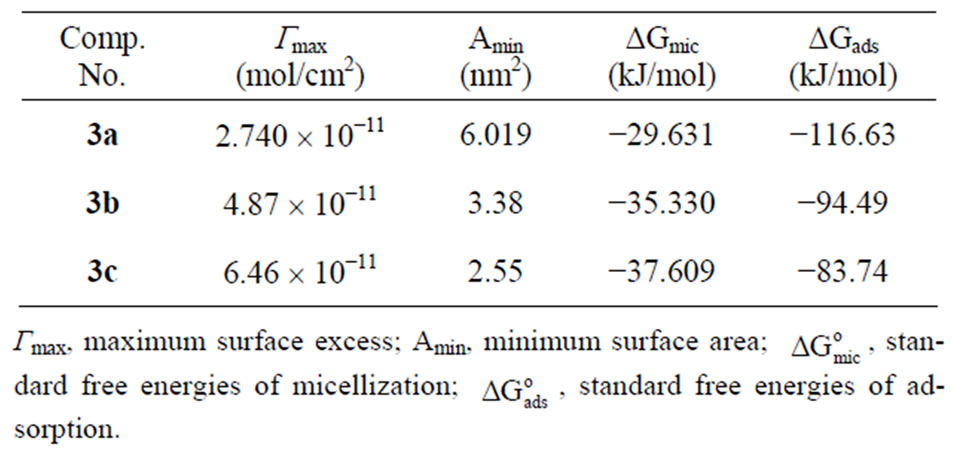
Table 2. Surface Parameters of the prepared compounds 3a-c at 25˚C.
is the most efficient (Table 1) because it achieved the maximum reduction of the surface tension at CMC (Figure 3). The effectiveness of adsorption is an important factor to determine properties of surfactant such as foaming, wetting, and emulsification, since tightly packed coherent interfacial films have very different interfacial properties than loosely packed, non coherent films [19].
3.2.3. Maximum Surface Excess (Γmax) and Minimum Area per Molecule (Amin)
The values of Γmax are shown in Table 2. As the surface tension decreases with increasing activity of surfactant, Γmax is positive. It is evident from Table 2 that, by increasing the number of methylene units Γmax increases. The results given in Table 2 indicate that the consequence increase of Γmax leads to crowdedness occurred at the interface which causing decrease in Amin values. The low Amin values of the synthesized compounds (2.55 nm2) indicate highly packed molecules at the interface. The high packing of the surfactant molecules at the interface is due to the overlapping between their hydrophobic chains. The attraction between the positively charged adsorbed head groups also increases the packing effect of these molecules.
3.2.4. Thermodynamics of Interfacial Adsorption and Micelle Formation
It is clear from Tables 1 and 2 that the standard free energies of adsorption and micellization of the synthesized cationic surfactants are always negative. This indicates that both adsorption and micellization processes occurred spontaneously. Moreover, the standard free energies of adsorption of the different surfactant molecules at the air/water interface are more negative than those of the micellization, which reveals that the adsorption process is more favorable than micellization. The high negativity of ∆Gads of the synthesized cationic surfactants, one can conclude that these surfactants have a surface activity
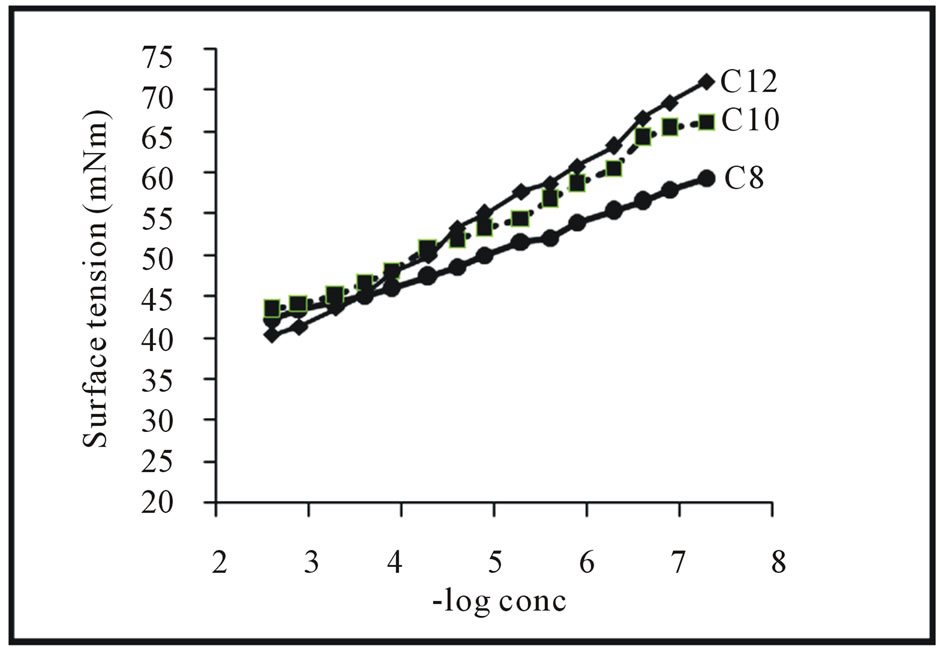
Figure 3. Variation in surface tension of surfactants 3a-c vs. concentration at 25˚C.
which may result in good applicability in some applications including antimicrobial utility and corrosion inhibition.
3.3. Biocidal Activity of Quaternary Ammonium Compounds
The reproducible stabilized mixed culture was called SMC-SRB indicating that it contained the sulfate reducing bacteria, in addition to the most biofilm aerobic coexisting bacteria which caused pitting corrosion of mild steel. The results revealed that the SRB activity not inhibited by using compound 3a as shown in Table 3.
CTAB recorded its mic value at 120 ppm against SRB. On the other hand the compounds 3b and 3c showed a good response against SRB look like CTAB. This is may be due to that the biocidal activity of quats depends on the length of alkyl chain [20]. Some of the most striking properties of quats are their biocidal activities against a full range of pathogens, including Gram-positive, Gramnegative bacteria, yeast and fungi.
Table 4 showed that 3a has no effect against Es-
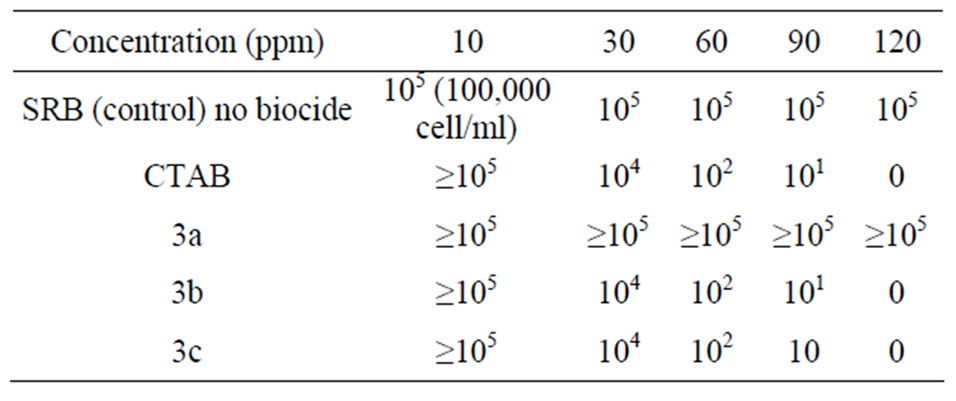
Table 3. MPN for SRB Treated with Different Concentration (10, 30, 60, 90 and 120 ppm) of Prepared Compounds and CTAB as Pure Reference Compound.
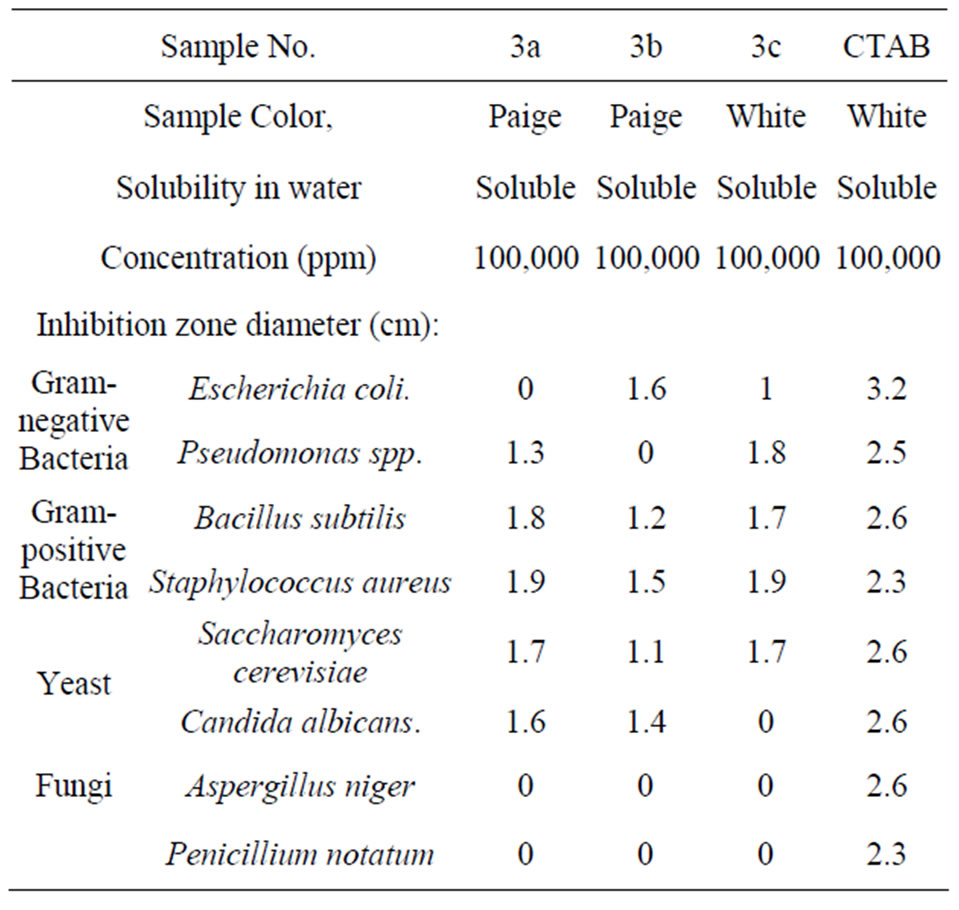
Table 4. Microbial Activity Against Pathogenic Microorganisms.
cherichia coli, Aspergillus niger and Penicillium notatum, at 105 ppm but showed inhibition zones ranged between 1.3 - 1.8 cm against the rest microorganism. 3b shows inhibition zones ranged between 1.1 - 1.6 cm and it has no effect against Pseudomonas spp., Aspergillus niger and Penicillium notatum. On the other hand, 3c shows good biocidical activity against all organisms except Aspergillus niger at 105 ppm. The higher biocidal activity could be explained due to electrostatic attraction of positively charged (N+) of quats and negatively charged of phospholipids present in the cell wall. Finally, the biocidal activity of quats not only depends on the chain length, and concentration but also the organism under test.
REFERENCES
- W. A. Jacobs and M. Heidelberger, “The Quaternary Salts of Hexamthylenetetramine: I. Substituted Benzyl Halides and the Hexamthylenetetramine Salts Derived Thereform,” The Journal of Biological Chemistry, Vol. 20, No. 4, 1915, pp. 659-683.
- W. A. Jacobs, M. Heidelberger and H. L. Amoss, “The Bactericidal Properties of the Quaternary Salts of Hexamthylenetetramine,” The Journal of Experimental Medicine, Vol. 23, No. 5, 1916, pp. 569-576. doi:10.1084/jem.23.5.569
- E. Bessems, “Biological Aspects of the Assessment of Biocides,” Proceedings of the Metals Society Conference, London, 8-10 March 1983, pp. 84-89.
- J. R. Postage, “The Sulphate Reducing Bacteria,” 2nd Edition, Cambridge University Press, Cambridge, 1984.
- B. G. Ateya, S. M. El-Raghy, M. E. Abdel Samie, M. N. Mahmoud and R. S. Bayounie, “Effect of Some Biocides and Corrosion Inhibitors on Stablished Mixed Culture Containing Sulfate Reducing Bacteria,” 17th Annual Conferences of Corrosion Problems in Industry, Ismailia, December 1998, pp. 1-3.
- M. A. Lewis, “Chronic and Sublethal Toxicities of Surfactants to Aquatic Animals: A Review and Risk Assessment,” Water Research, Vol. 25, No. 1, 1991, pp. 101-113. doi:10.1016/0043-1354(91)90105-Y
- P. Tundo, D. J. Kippenberg, M. J. Politi, P. Klahn and J. H. Fendler, “Redox Active Functionally Polymerized Surfactant Vesicles. Syntheses and Characterization,” Journal of the American Chemical Society, Vol. 104, No. 20, 1982, pp.5352-5358. doi:10.1021/ja00384a018
- R. C. Bazito and O. A. El Seoud, “Suger-Based Cationic Surfactants: Synthesis and Aggregation of Methyl-2-Acylamido-6-trimethylammonio-2,6-dideoxy-D-glucopyranoside Chlorides,” Journal of Surfactants and Detergents, Vol. 4, No. 4, 2001, pp. 395-400. doi:10.1007/s11743-001-0193-1
- N. A. Negm and A. S. Mohamed, “Synthesis, Characterization and Biological Activity of Sugar-Based Gemini Cationic Amphiphiles,” Journal of Surfactants and Detergents, Vol. 11, No. 3, 2008, pp. 215-221. doi:10.1007/s11743-008-1071-9
- J. W. Hampson and D. G. Consell, “Surface-Tension Properties of Some Poly dispersed Alkyl-Substituted Polyoxy-Ethylated Phenyl Sulfonamides,” Journal of the American Oil Chemists’ Society, Vol. 73, No. 7, 1996, pp. 891- 895. doi:10.1007/BF02517991
- T. Hikota and K. J. Merguro, The Chemical Society of Japan, Vol. 47, 1970, p. 158.
- A. S. Mohamed and M. M. Awad, Material Science Research India, Vol. 1, 2003, p. 59.
- M. J. Rosen, “Surfactant and Interfacial Phenomena,” 3rd Edition, John Willey and Sons, New York, 2004, p. 63. doi:10.1002/0471670561
- Apha, American Public Health Association (APHA), Stander Methods for the Examination of Water and Waste Water, 17th Edition, New York, 1989.
- Apha, American Public Health Association (APHA), “Identification of Iron and Sulfur Bacteria, Standard Methods for the Examination of Water and Waste Water, 14th Edition, New York, 1975.
- C. O. Akujobi and H. O. Njoku, “Bioassay for Determination of Microbial Sensitivity to Nigerian Honey,” Global Journal of Pharmacology, Vol. 4, No. 1, 2010, pp. 36-40.
- P. Gehardt, Q. G. Murray, R. N. Costillow and E. W. Nesler, “Wood WA, Kreig NR, Phillips GB,” Manual of Methods for General Microbiology, Washington DC, 1981.
- S. Przestalski, J. Sarapuk, H. Kleszczyñska, J. Gabrielska, J. Hladyszowski, Z. Trela and J. Kuczera, “Influence of Amphiphilic Compounds on Membranes,” Acta Biochimica Polonica, Vol. 47, No. 3, 2000, pp. 627-638.
- M. J. Rosen, “Surfactants and Interfacial Phenomena,” John Willey and Sons, New York, 1978.
- R. M. E. Richard and R. H. Cavil, Microbios, Vol. 29, 1980, p. 23.
NOTES
*Corresponding author.

