Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2008, 1, 147-151 Published Online November 2008 in SciRes. http://www.srpublishing.org/journal/jbise JBiSE Recent developments in biomedicine fields for laser induced breakdown spectroscopy Xian-Yun Liu, Wei-Jun Zhang Laboratory of Environmental Spectroscopy, Anhui Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, P.O. Box 1125, Hefei, Anhui 230031, P.R. China. Correspondence should be addressed to Wei-Jun Zhang (wjzhang@aiofm.ac.cn), Tel.: +86 551 5591961. Received on March 13, 2008; revised and accepted on September 9, 2008 ABSTRACT Laser induced breakdown spectroscopy (LIBS) can be used to determine solid, liquid, colloi- dal, and biological samples. It is a promising technique for analysis and characterization of the composition of a broad variety of objects. This review describes in brief the basic prin- ciples and technological aspects of LIBS, and the most recent progress of the various ap- plications of this technique in biomedicine fields will be reviewed in detail, including bio-aerosols detection and identification, tis- sue analysis, mineral analysis in human body, and detection of zinc in human skin. Finally new approaches and the prospects in bio- medicine fields of LIBS technique are de- scribed. Keywords: Biomedicine, LIBS, Elemental analysis, Atomic emission spectroscopy. 1. INTRODUCTION There are at least about 40 chemical elements in the liv- ing organisms of a human body. These elements can be grouped into three groups: The major group comprising H, C, N, O (~96.6%); The trace elements group com- prising Na, Mg, P, S, Cl, K, Ca , Fe, Mn, Co, Zn and Ni (< 5%); The minor group of trace elements including V, Mo, Li, F, Si, As, Br, Sn, I and Ba (0.001%) . Studies about the possible correlation between some elements and disease are often among the medicine experts’ and biologists’ interesting. Different techniques are practiced to investigate the correlation between the consumption of certain elements and certain types of disease, including complementary DNA microarrays and serial analysis of gene expression [1]; matrix-assisted laser desorption ionization mass spectroscopy [2, 3] and surface enhanced laser desorption ionization mass spectroscopy [4]; x-ray fluorescence and proton-induced x-ray fluorescence [5, 6]. All these techniques have the common disadvantages of being time-consuming, expensive, and requiring a relatively complicated sample preparation. This review describes a modern analytical technique based on emission of electromagnetic radiation produced after excitation of atoms, ions or molecules, which has been named Laser Induced Breakdown Spectroscopy (LIBS). LIBS technique is a useful method for determining the elemental composition of various solids, liquids and gases. With many advantages as described in Table 1, numerous experimental as well as theoretical investiga- tion results are found in literature as well as several re- view papers [7, 8, 9, 10] and three recent text books [11, 12, 13] have been published. However, only a few works related to analysis of biomedicine samples by LIBS have been reported so far. Detection of biomedical samples has become urgent because of the threats of biological warfare and epidemic spread. Table 1. Advantages of LIBS. Advantages 1 The need for little or no sample preparation. The result is increased throughput, greater convenience and fewer opportunities for contamination to occur. 2 Versatile sampling for all media, including solids, gases or liquids (also conducting and non-conducting materials). 3 Very small amounts of sample (0.1 µg to 1 mg) are vaporized, therefore LIBS can be considered as quasi non-destructive. 4 Permits analysis of extremely hard materials that are difficult to digest or dissolve (e.g., ceramics, glasses and superconductors). 5 Analysis in micro-regions offers a spatial resolving power of about 1-100 µm. 6 Multiple elements can be analyzed simultaneously. 7 Potential for direct detection of aerosols (a solid or liquid particle in a gaseous medium) or ambient air. 8 The analysis is simple and rapid (ablation and excitation processes are carried out in a single step). SciRes Copyright © 2008 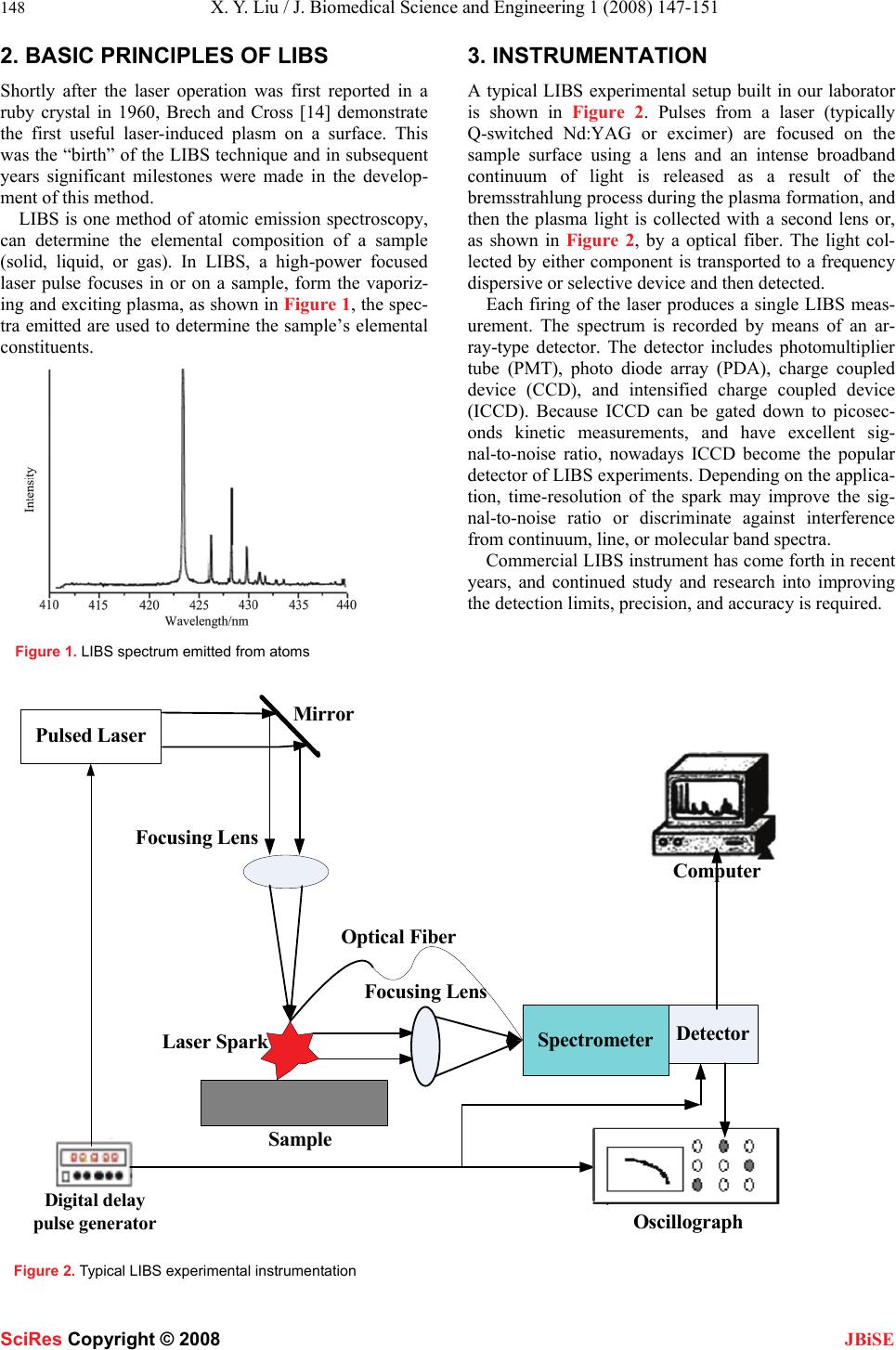 148 X. Y. Liu / J. Biomedical Science and Engineering 1 (2008) 147-151 SciRes Copyright © 2008 JBiSE 2. BASIC PRINCIPLES OF LIBS Shortly after the laser operation was first reported in a ruby crystal in 1960, Brech and Cross [14] demonstrate the first useful laser-induced plasm on a surface. This was the “birth” of the LIBS technique and in subsequent years significant milestones were made in the develop- ment of this method. LIBS is one method of atomic emission spectroscopy, can determine the elemental composition of a sample (solid, liquid, or gas). In LIBS, a high-power focused laser pulse focuses in or on a sample, form the vaporiz- ing and exciting plasma, as shown in Figure 1, the spec- tra emitted are used to determine the sample’s elemental constituents. Figure 1. LIBS spectrum emitted from atoms 3. INSTRUMENTATION A typical LIBS experimental setup built in our laborator is shown in Figure 2. Pulses from a laser (typically Q-switched Nd:YAG or excimer) are focused on the sample surface using a lens and an intense broadband continuum of light is released as a result of the bremsstrahlung process during the plasma formation, and then the plasma light is collected with a second lens or, as shown in Figure 2, by a optical fiber. The light col- lected by either component is transported to a frequency dispersive or selective device and then detected. Each firing of the laser produces a single LIBS meas- urement. The spectrum is recorded by means of an ar- ray-type detector. The detector includes photomultiplier tube (PMT), photo diode array (PDA), charge coupled device (CCD), and intensified charge coupled device (ICCD). Because ICCD can be gated down to picosec- onds kinetic measurements, and have excellent sig- nal-to-noise ratio, nowadays ICCD become the popular detector of LIBS experiments. Depending on the applica- tion, time-resolution of the spark may improve the sig- nal-to-noise ratio or discriminate against interference from continuum, line, or molecular band spectra. Commercial LIBS instrument has come forth in recent years, and continued study and research into improving the detection limits, precision, and accuracy is required. Detector Spectrometer Oscillograph Pulsed Laser Focusing Lens Sample Digital delay pulse generator Computer Focusing Lens Laser Spark Mir r o r Optical Fiber Figure 2. Typical LIBS experimental instrumentation  X. Y. Liu / J. Biomedical Science and Engineering 1 (2008) 147-151 149 SciRes Copyright © 2008 JBiSE 4. LIBS IN BIOMEDICINE SCIENCE 4.1. Analysis of Biological Aerosols The analysis of microscopic particles, aerosols, and cells has received increased interest in recent years, especially bio-aerosols (bacteria, fungi, viruses, pollen) have at- tracted wide attention because they are found nearly everywhere and of the threats of biological warfare and epidemic spreads. Inhaled minute amounts of bio-aerosols can cause disease or toxic or allergic reac- tions. Thus determination and monitor the presence of airborne particulates and their actual concentration is of high interest. LIBS is found to be the most convenient technique for in-situ and real-time measurement of metal species in the gaseous and aerosol phases, thus it is suit- able for analysis and characterization of biological aero- sols. Time-resolved laser-induced breakdown spectroscopy (TRELIBS) is a method that has the advantages of rapid, reliable, and highly selective. Stéphane Morel et al. [15] use TRELIBS to detection and sort species. They choose six bacteria (including Bacillus globigii as a surrogate for Bacillus anthracis) and two pollens in pellet form for detection, and get the conclusion that TRELIBS exhibits a good ability to differentiate among all investigated species, whatever the culture medium, the species or the strain. Samuels et al.[16] analyzed bacterial spores, molds , and pollens using LIBS technique with a broadband spectrometer (200-900nm). The authors analyzed each LIBS spectrum using principal-components analysis method, and found to contain adequate information to discrimination among the biomaterials. They got the re- sult that it was possible to discriminate between the bac- terial spores and the molds and pollens. Kim and coworkers [17] examined five bacterial strains (Bacillus thuringiensis T34, Escherichia coli IHII/pHT315, Bacillus subtilis 168, Bacillus megaterium QM B1551, and Bacillus megaterium PV361.) using LIBS, performing measurements directly on the bacterial culture plates. The difference in bacterial strains was clearly distinguished by two-dimensional charts of the bacterial components, calcium versus phosphate. The authors noted that their experimental results demonstrate the potential of the LIBS method for rapid and precise classification of bacteria with minimum sample prepara- tion. Hybl et al. [18] examined some common biological agent simulants (Bacterial spore, Media/protein, Fun- gal/mold spores, and pollen) using spectrally broadband LIBS system. Instead of using pellets or sub- strate–deposited layers, homogeneous samples were aerosolized in a micro-centrifuge tube by two ways: by making use of the laser-induced shock wave or aerosol- ized acoustically by dispersing a dry power suspension above a loudspeaker. From the experiments they demon- strated that LIBS has significant potential as a bio-aerosol classifier and that LIBS technique is able to resolve differing elemental ratios in biowarfare-agent simulants and in common biological and environmental interferants. Boyain-Goitia et al. [19] analyzed single biological microparticles (pollens of a variety of flowers) by the method of LIBS for the first time. Their experimental results demonstrated that single-laser-pulse laser-induced breakdown spectroscopy can be performed on single biological microparticles, and that many more species need to be measured to generate a suitable reference li- brary before detection and identification can be made reliably in real time. In recent years many researchers are focused on the detection and identification of individual bio-aerosols using LIBS. Dixon et al. [20] demonstrated the feasibil- ity of LIBS-based single-shot analysis of mental-rich bioaerosols(Bacillus sporses). Beddows and Telle [21] discussed the prospects of real-time, in situ laser-induced breakdown spectroscopy applied for the identification and classification of bio-aerosols (including species of potential bio-hazard) within common urban aerosol mix- tures. Compared laser-induced breakdown spectroscopy measurements with data from a mobile single-particle aerosol mass spectrometer (ATOFMS), they got results that data from the ATOFMS provide statistical data over an extended period of time, highlighting the variation of the background composition. Baudelet and coworkers analyzed Escherichia coli using femtosecond pulses LIBS system [22], they also compared it with the nano- second regime [23]. Gibb et al. [24] realized size-selective sampling of Bacillus anthracis surrogate spores from realistic, common aerosol mixtures by LIBS. Diedrich and coworkers [25] analyzed four strains of Escherichia coli bacteria using LIBS with nanosecond pulses. The experimental results show that LIBS has the ability to discriminate an environmental strain from a pathogenic strain, which suggests the possibility of using LIBS as a practical diagnostic test to identify strains ob- tained from environmental assays. 4.2. Tissue Analysis Cancer diagnosis and classification is extremely compli- cated and, for the most part, relies on subjective inter- pretation of biopsy material. Automated, real-time diag- nostic procedures would greatly facilitate cancer diagno- sis and classification. LIBS can detect the elemental con- stituent in both low and high atomic number elements, and can provide rapid, non-destructive tissue analysis. For the first time Kumar et al.[26, 27] demonstrate in principle that LIBS can be used for tissue analysis, spe- cially the ability to differentiate between malignant and normal tissue. By analysis of malignant and normal tis- sue from a canine hemangiosarcoma, they found distinct differences in elemental composition in two type of sam- ple. Figure 3 is the LIBS spectrum they got from the malignant and normal tissue cells of dog liver [27]. They concluded that the line intensity ratios of different ele- ments can be used to determine the concentration ratio of 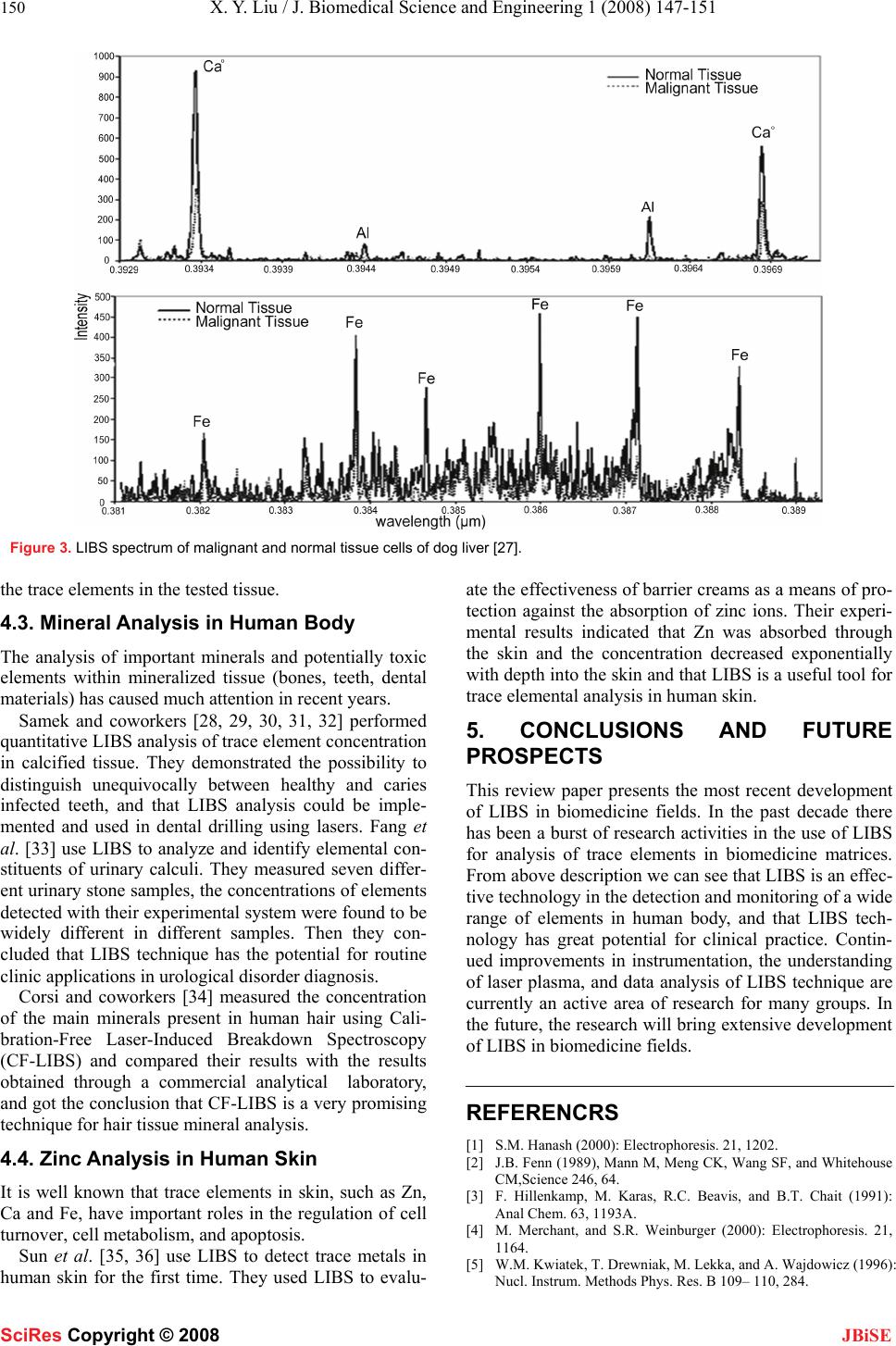 150 X. Y. Liu / J. Biomedical Science and Engineering 1 (2008) 147-151 SciRes Copyright © 2008 JBiSE Figure 3. LIBS spectrum of malignant and normal tissue cells of dog liver [27]. the trace elements in the tested tissue. 4.3. Mineral Analysis in Human Body The analysis of important minerals and potentially toxic elements within mineralized tissue (bones, teeth, dental materials) has caused much attention in recent years. Samek and coworkers [28, 29, 30, 31, 32] performed quantitative LIBS analysis of trace element concentration in calcified tissue. They demonstrated the possibility to distinguish unequivocally between healthy and caries infected teeth, and that LIBS analysis could be imple- mented and used in dental drilling using lasers. Fang et al. [33] use LIBS to analyze and identify elemental con- stituents of urinary calculi. They measured seven differ- ent urinary stone samples, the concentrations of elements detected with their experimental system were found to be widely different in different samples. Then they con- cluded that LIBS technique has the potential for routine clinic applications in urological disorder diagnosis. Corsi and coworkers [34] measured the concentration of the main minerals present in human hair using Cali- bration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS) and compared their results with the results obtained through a commercial analytical laboratory, and got the conclusion that CF-LIBS is a very promising technique for hair tissue mineral analysis. 4.4. Zinc Analysis in Human Skin It is well known that trace elements in skin, such as Zn, Ca and Fe, have important roles in the regulation of cell turnover, cell metabolism, and apoptosis. Sun et al. [35, 36] use LIBS to detect trace metals in human skin for the first time. They used LIBS to evalu- ate the effectiveness of barrier creams as a means of pro- tection against the absorption of zinc ions. Their experi- mental results indicated that Zn was absorbed through the skin and the concentration decreased exponentially with depth into the skin and that LIBS is a useful tool for trace elemental analysis in human skin. 5. CONCLUSIONS AND FUTURE PROSPECTS This review paper presents the most recent development of LIBS in biomedicine fields. In the past decade there has been a burst of research activities in the use of LIBS for analysis of trace elements in biomedicine matrices. From above description we can see that LIBS is an effec- tive technology in the detection and monitoring of a wide range of elements in human body, and that LIBS tech- nology has great potential for clinical practice. Contin- ued improvements in instrumentation, the understanding of laser plasma, and data analysis of LIBS technique are currently an active area of research for many groups. In the future, the research will bring extensive development of LIBS in biomedicine fields. REFERENCRS [1] S.M. Hanash (2000): Electrophoresis. 21, 1202. [2] J.B. Fenn (1989), Mann M, Meng CK, Wang SF, and Whitehouse CM,Science 246, 64. [3] F. Hillenkamp, M. Karas, R.C. Beavis, and B.T. Chait (1991): Anal Chem. 63, 1193A. [4] M. Merchant, and S.R. Weinburger (2000): Electrophoresis. 21, 1164. [5] W.M. Kwiatek, T. Drewniak, M. Lekka, and A. Wajdowicz (1996): Nucl. Instrum. Methods Phys. Res. B 109– 110, 284.  X. Y. Liu / J. Biomedical Science and Engineering 1 (2008) 147-151 151 SciRes Copyright © 2008 JBiSE [6] J Hewlett, V Nadeau, J Ferguson, H Moseley, S Ibbotson, J.W. Allen, Sibbett W, and Padgett M (2001): Photochemistry and Photobiology. 73, 278. [7] P. Celio, C. Juliana, M.C.S. Lucas, and B.G Fabiano. (2007) J. Braz. Chem. Soc. 18, 463. [8] G. Anastasia, M. Kristalia, and A. Demetrios (2007): Anal. Bioanal. Chem. 387, 749. [9] K. Song, Y.I. Lee and S. Joseph. (2002) Appl. S. Rev. 37 89. [10] D.A. Rusak, B.C. Castle, B.W. Smith, and J.D. Winefordner (1998): Trends in analytical chemistry. 17 453. [11] D.A. Cremers, and L.J. Radziemski (2006): in Handbook of La- ser-Induced Breakdown Spectroscopy. Wiley: New York.. [12] A.W. Misiolek, and V. Palleschi, Schechter (2006): in Laser In- duced Breakdown Spectroscopy (LIBS): Fundamentals and Ap- plications. (Cambridge University Press: Cambridge) I eds. [13] J.P. Singh, and S.N. Thakur (2006): in Laser Induced Breakdown Spectroscopy.(Elsevier Science: Amsterdam). I Eds. [14] F. Brech, and L. Cross (1962): Appl. Spectrosc. 16 59. [15] Stéphane M, Nicolas L, Philippe A, and Jacques A (2003): Appl. Opt. 42, 6184. [16] A.C. Samuels, F.C. DeLucia, K.L. McNesby, and A.W. Miziolek (2003): Appl. Opt. 42 6205. [17] T. Kim, Z.G. Specht, P.S. Vary, and C.T. Lin (2004): J. Phys. Chem. B 108, 5477. [18] J.D. Hybl, G.A. Lithgow, and S.G. Buckley (2003): Appl. Spectro. 57, 1207. [19] A.R. Boyain-Goitia, D.C.S. Beddows, B.G. Griffiths, and H.H. Telle (2003): Appl. Opt. 42, 6119. [20] P.B. Dixon, and D.W. Hahn (2005): Anal. Chem. 77, 631. [21] D.C.S. Beddows, and H.H. Telle (2005): Spectrochimica Acta Part B. 60, 1040. [22] M. Baudelet, L. Guyon, J. Yu, and J.P. Wolf (2006): Appl. Phys. Lett. 88, 063901. [23] M. Baudelet, L. Guyon, J. Yu, and J.P. Wolf (2006): J. Appl. Phys. 99, 084701. [24] E. Gibb, B. Gullett, S. Ryan, L. Oudejans, and A. Touati (2006): Appl. Spectrosc. 60, 860. [25] J. Diedrich, S.J. Rehse, and S. Palchaudhuri (2007): J. Appl. Phys. 102, 014702. [26] A. Kumar, F.Y. Yueh, J. P. Singh, and S. Burgess (2004): Appl. Opt. 43, 5399. [27] A. Kumar, and P.C. Sharma (2006): Use of LIBS technology in biological media. Proc. of SPIE 6377, 637701. [28] O. Samek, M. Liška, J.Kaiser, and V. Krzyžánek (1998): SPIE. 3570, 263. [29] O. Samek, D.C.S. Beddows, H.H. Telle, G.W. Morris, M. Liska, and J. Kaiser (1999): Appl. Phys. A 69 [Suppl], S179. [30] O. Samek, M. Liška, J. Kaiser, D.C.S. Beddows, H.H. Telle and S.V. Kukhlevsky (2000): Journal of Clinical Laser Medicine & Surgery 18, 281. [31] O. Samek, D.C.S. Beddows, H.H. Telle, J. Kaiser, M. Liška, J.O. Cáceres, and A. Ureña G(2001): Spectrochimica Acta Part B 56, 865. [32] O. Samek, H.H. Telle, and D.C.S. Beddows (2001): BMC Oral Health 1, 1. [33] X. Fang, S.R. Ahmad, M. Mayo, and S. Iqbal (2005): Lasers in Medical Science 20, 132. [34] M. Crosi, G. Cristoforetti, M. Hidalgo, S. Legnaioli, V. Palleschi, Salvetti A, Tognoni E, and Vallebona C (2003): Appl. Opt. 42, 6133. [35] Q. Sun, M. Tran, B.W. Smith, and J.D. Winefordner (2000): Ta- lanta 52, 293. [36] Q. Sun, M. Tran, B.W. Smith, and J.D. Winefordner (2000): Con- tact Dermatitis 43, 259. |

