Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 95-100 doi:10.4236/jbise.2010.31014 Published Online January 2010 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online January 2010 in SciRes. http://www.scirp.org/journal/jbise Aquaporin 1-expressing MCF-7 mammary carcinoma cells show enhanced migration in vitro Yong Jiang1,2, Zhi-Bin Jiang3 1Key Laboratory of Industrial Microbiology, Ministry of Education, Tianjin University of Science and Technology, Tianjin, China; 2College of Biotechnology, Tianjin University of Science and Technology, Tianjin, China; 3Jilin Combined Traditional Chinese and Western Medicine Hospital in Jilin Province, Jilin, China. Email: jiangyong@tust.edu.cn Received 6 September 2009; revised 19 October 2009; accepted 20 October 2009. ABSTRACT Recent studies have demonstrated that aquaporin (AQP) expression facilitates cell migration and pro- motes angiogenesis and neutrophil motility. Migra- tion of tumor cells is a crucial step in tumor invasion and metastasis. Here we investigated the expression of AQP in MCF-7 human mammary carcinoma cells and characterized its function in cell migration. Re- verse Transcription–Polymerase Chain Reaction, Immunoblot and Immunofluorescence analysis dem- onstrated two populations of MCF-7 cell clones with low (AQP1L) and high (AQP1H) AQP1 expression and the AQP1 protein expression patterns in the plasma membrane of MCF-7 cells. MCF-7 cell clones (AQP1L and AQP1H) with low and about two-fold higher osmotic water permeability were identified by functional assays with corresponding low and high AQP1 expression. Cell migration rate was remarka- bly higher in AQP1H cells as compared to AQP1L cells, assessed by wound healing and transwell mi- gration assays. Adenoviral-mediated mRNA and pro- tein expression of AQP1 in AQP1L cells increased their water permeability and migration rate to the level similar to AQP1H cells. The results provided direct evidence that aquaporin-mediated plasma membrane water permeability played an important role in mammary carcinoma cell migration and may be associated with mammary carcinoma invasion and metastasis. Keywords: Aquaporin; Water Permeability; Cell Migration 1. INTRODUCTION Since Agre laboratory discovered the first aquaporin [1] from red blood cells, thirteen homologous members (AQP0-AQP12) in mammals and hundreds of AQPs in other species have been identified molecularly [2,3]. Aquaporins (AQPs) are membrane water channels that play pivotal roles in physiological and pathophysiologi- cal processes of diverse mammalian organs [4,5,6]. Re- cent studies indicated a novel role of AQPs in cell mi- gration. Mice lacking AQP1, the endothelial water channel, were found to have impaired endothelial cell migration and tumor angiogenesis [7]. In the same study, over-expression of AQP1 or AQP4 (a structurally related water channel) was also found to promote migration of non-endothelial cells. Subsequent studies demonstrated that AQP1 expression promoted the migration of epithe- lial cells of kidney proximal tubules and facilitated wound healing of injured proximal tubules [8]. In the brain, AQP4 deletion in astroglial cells was also found to result in impaired migration and remarkably reduced glial scar formation after stab injury [9]. In addition, some other studies provided evidence that aquaglycero- porin AQP9 may be involved in neutrophil cell motility [10,11]. These studies suggested that aquaporin-Mediat- ed plasma membrane water permeability may be a fun- damental determinant of cell migration and associated physiological and pathological processes. Migration of tumor cells is a crucial step in tumor invasion and metas- tasis [12,13]. Previous studies demonstrated the expres- sion of AQPs in some types of tumor cells [14,15,16]. We hypothesized that AQPs also play a critical role in mammary carcinoma cell migration. In the present study, we provided the first evidence for the involve- ment of AQP1 in mammary carcinoma cell migration. 2. MATERIALS AND METHODS 2.1. Cell Culture MCF-7 human mammary carcinoma cells (from Cell Bank of Shanghai Institute of Biochemistry and Cell Biology) were maintained in DMEM supplemented with 10% FBS, 100 μg/mL penicillin and 100 μg/mL streptomycin. The cells were plated in 6-well plates  96 Y. Jiang et al. / J. Biomedical Science and Engineering 3 (2010) 95-100 SciRes Copyright © 2010 JBiSE and maintained at 37°C and 5% CO2. The monoclonal MCF-7 mammary carcinoma cell line was isolated and expanded by limited dilution to investigate the expression and function of AQP1. 2.2. Reverse Transcription-Polymerase Chain Reaction Total RNA was extracted from the cultured MCF-7 human mammary carcinoma cells with RNeasy micro kit (QIAGEN) and cDNA was reversely transcribed (RT) from the total RNA using a superscript first- strand synthesis system (invitrogen). The cDNA was used as a template for polymerase chain reaction (PCR) amplification (94°C, 5 min and then 30 cycles of 94°C for 30s; 60°C for 30s; 72°C for 60s) using primers flanking a 252 bp fragment of AQP1 (sense, 5’-CTCTCTgTAgCCCTTggACACCTC-3’; antisense, 5’-ggCATCCAggTCATACTCCTCCAC-3’) and flan- king a 213 bp fragment of GAPDH (sense, 5’-ATT- CAACGGCACAGTCAAGG-3’; antisense, 5’- GCA- GAAGGGGCGGAGATGA-3’). The PCR products were analyzed by agarose gel electrophoresis. 2.3. Immunoblotting MCF-7 cells were washed with phosphate-buffered saline (PBS), incubated with 2mL 50 mM Na2-EDTA solution for 5 to 10 min at room temperature and solu- bilized at 60°C for 15 min in Laemmli sample buffer. Equal amounts of protein (10μg) were resolved by SDS-PAGE on 12% polyacrylamide gel, blotted to polyvinylidene difluoride (PVDF) membranes, blocked with 5%(w/v) nonfat milk and washed in Tris-buffered saline (TBS)-Tween solution. Primary antibodies (anti-AQP1, anti-Tubulin) (Chemicon, 1:1000) and secondary antibody conjugated to horse- radish peroxidase (Sigma, 1:3000) were applied suc- cessively and detected by enhanced chemolumines- cence (Amersham). 2.4. Immunofluorescence MCF-7 cells were seeded on coverglasses 24 h before fixation in PBS supplemented with 4% paraformal- dehyde (PFA) for 15 min. Cells were washed with PBS, blocked and permeabilized by incubation in PBS containing 2% (w/v) bovine serum albumin (Sigma), and 0.1% (v/v) Triton X-100 for 30 mins. Cells were then exposed to a polyclonal rabbit anti-rat AQP1 antibody (Chemicon, 1:1000) overnight at 4°C. After washing, a Cy-3-conjugated anti-rabbit IgG secondary antibody (Sigma) was applied at 1:500 for 1 h at room temperature. Cells were washed with PBS and visualized by fluorescence microscopy (Olym- pus). 2.5. Adenovirus-Mediated AQP1 Expression The recombinant adenovirus expressing human AQP1 (Ad-AQP1) and control β-galactosidase adenovirus (Ad-lacZ) were generated using the ViraPower ade- noviral expression system (Invitrogen) according to the manufacturer’s instruction and purified/ concen- trated with an Adeno-X virus purification kit (BD Biosciences-Clontech). The MCF-7l cells were in- fected with Ad-AQP1 or Ad-lacZ (control adenovirus) at a concentration of 150 PFU/cell respectively. AQP1 expression and function were analyzed at 48 h after infection. 2.6. Water Permeability Measurements The osmotic water permeability of MCF-7 cell plasma membrane was measured by a calcein fluorescence quenching method described previously [17], with modifications. Briefly, MCF-7 cells were grown on round glass coverslips pre-coated with polylysine for 24 h. The cells were incubated with 10μmol/L cal- cein-AM (Molecular probes) for 10 min and then mounted in a perfusion chamber designed for rapid solution exchange. The time-course of cytoplasmic calcein fluorescence in response to an osmotic gradi- ent was monitored by exchanging perfusate osmo- lality between 300 mOsmol (PBS) and 150 mOsmol (diluted with distilled water). The rate of change of cell volume was presented as reciprocal exponential time constant (1/τ), which is proportional to osmotic water permeability, where τ is the time needed from the beginning of osmotic switch to the point when the cytoplasmic calcein fluorescence reaches its maxi- mum. 2.7. In Vitro Wound Healing Assay Assays were performed as described previously with modification [7]. Briefly, MCF-7 cells were cultured as confluence monolayers on 6-well plates and syn- chronized in 1% fetal bovine serum for 24 h. The monolayers were wounded by removing a 300-500 μm strip of cells across the well with a 200 μL pipette tip and then washed twice to remove non-adherent cells. Wound healing was quantified as the average linear speed of the wound edges over 24 h. 2.8. Transwell Migration Assays Invasive cell migration was measured using a modi- fied Boyden chamber chemotaxis assay. MCF-7 cells were seeded on top of a culture plate insert (Corning Costar) containing a polycarbonate filter (6.5 mm diameter, 8μm pores) pre-coated with fibronectin (0.5mg/mL). The upper chamber contained cells in 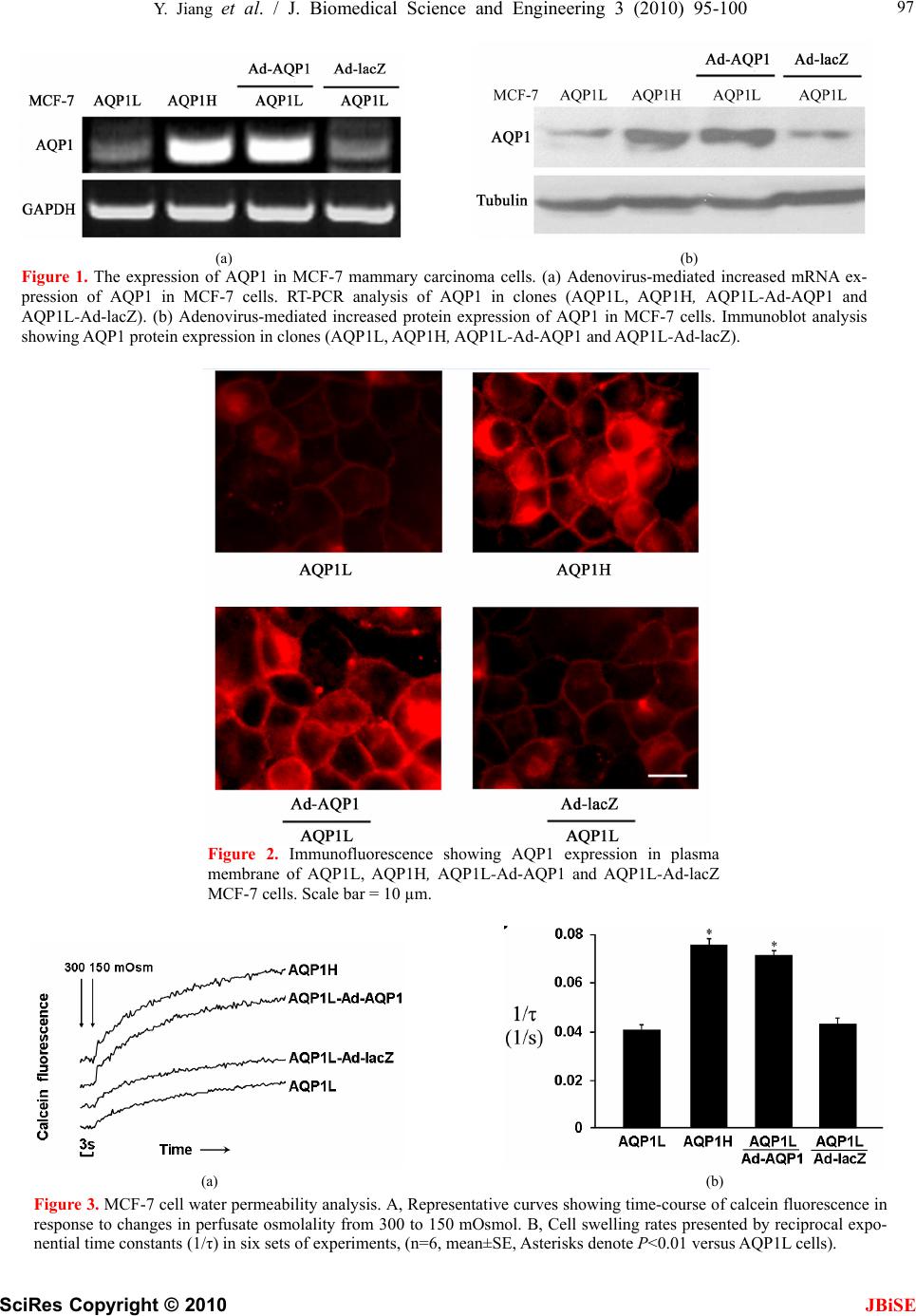 Y. Jiang et al. / J. Biomedical Science and Engineering 3 (2010) 95-100 SciRes Copyright © 2010 JBiSE 97 (a) (b) Figure 1. The expression of AQP1 in MCF-7 mammary carcinoma cells. (a) Adenovirus-mediated increased mRNA ex- pression of AQP1 in MCF-7 cells. RT-PCR analysis of AQP1 in clones (AQP1L, AQP1H, AQP1L-Ad-AQP1 and AQP1L-Ad-lacZ). (b) Adenovirus-mediated increased protein expression of AQP1 in MCF-7 cells. Immunoblot analysis showing AQP1 protein expression in clones (AQP1L, AQP1H, AQP1L-Ad-AQP1 and AQP1L-Ad-lacZ). Figure 2. Immunofluorescence showing AQP1 expression in plasma membrane of AQP1L, AQP1H, AQP1L-Ad-AQP1 and AQP1L-Ad-lacZ MCF-7 cells. Scale bar = 10 µm. (a) (b) Figure 3. MCF-7 cell water permeability analysis. A, Representative curves showing time-course of calcein fluorescence in response to changes in perfusate osmolality from 300 to 150 mOsmol. B, Cell swelling rates presented by reciprocal expo- nential time constants (1/τ) in six sets of experiments, (n=6, mean±SE, Asterisks denote P<0.01 versus AQP1L cells). 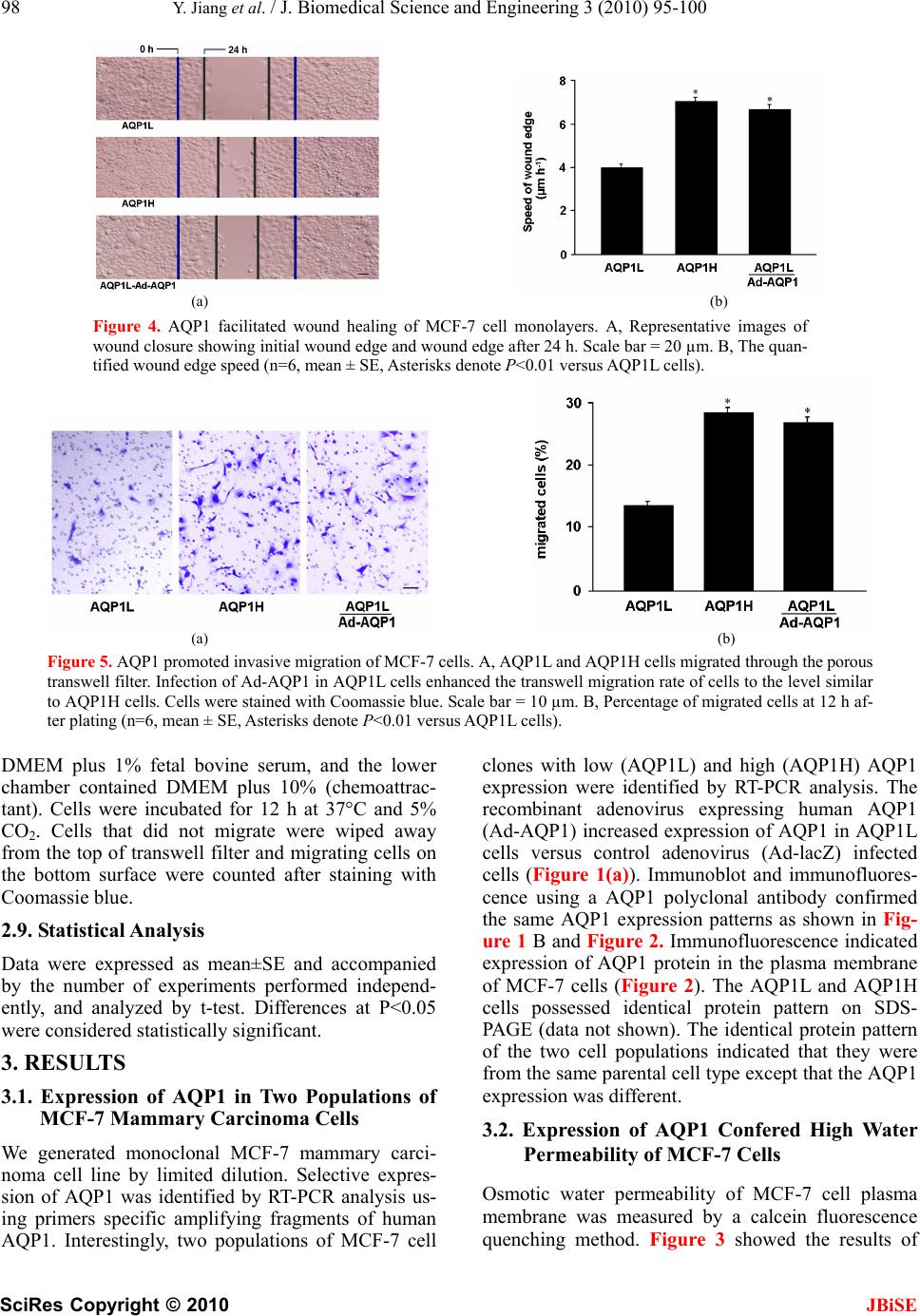 98 Y. Jiang et al. / J. Biomedical Science and Engineering 3 (2010) 95-100 SciRes Copyright © 2010 (a) (b) Figure 4. AQP1 facilitated wound healing of MCF-7 cell monolayers. A, Representative images of wound closure showing initial wound edge and wound edge after 24 h. Scale bar = 20 µm. B, The quan- tified wound edge speed (n=6, mean ± SE, Asterisks denote P<0.01 versus AQP1L cells). (a) (b) Figure 5. AQP1 promoted invasive migration of MCF-7 cells. A, AQP1L and AQP1H cells migrated through the porous transwell filter. Infection of Ad-AQP1 in AQP1L cells enhanced the transwell migration rate of cells to the level similar to AQP1H cells. Cells were stained with Coomassie blue. Scale bar = 10 µm. B, Percentage of migrated cells at 12 h af- ter plating (n=6, mean ± SE, Asterisks denote P<0.01 versus AQP1L cells). DMEM plus 1% fetal bovine serum, and the lower chamber contained DMEM plus 10% (chemoattrac- tant). Cells were incubated for 12 h at 37°C and 5% CO2. Cells that did not migrate were wiped away from the top of transwell filter and migrating cells on the bottom surface were counted after staining with Coomassie blue. 2.9. Statistical Analysis Data were expressed as mean±SE and accompanied by the number of experiments performed independ- ently, and analyzed by t-test. Differences at P<0.05 were considered statistically significant. 3. RESULTS 3.1. Expression of AQP1 in Two Populations of MCF-7 Mammary Carcinoma Cells We generated monoclonal MCF-7 mammary carci- noma cell line by limited dilution. Selective expres- sion of AQP1 was identified by RT-PCR analysis us- ing primers specific amplifying fragments of human AQP1. Interestingly, two populations of MCF-7 cell clones with low (AQP1L) and high (AQP1H) AQP1 expression were identified by RT-PCR analysis. The recombinant adenovirus expressing human AQP1 (Ad-AQP1) increased expression of AQP1 in AQP1L cells versus control adenovirus (Ad-lacZ) infected cells (Figure 1(a)). Immunoblot and immunofluores- cence using a AQP1 polyclonal antibody confirmed the same AQP1 expression patterns as shown in Fig- ure 1 B and Figure 2. Immunofluorescence indicated expression of AQP1 protein in the plasma membrane of MCF-7 cells (Figure 2). The AQP1L and AQP1H cells possessed identical protein pattern on SDS- PAGE (data not shown). The identical protein pattern of the two cell populations indicated that they were from the same parental cell type except that the AQP1 expression was different. 3.2. Expression of AQP1 Confered High Water Permeability of MCF-7 Cells Osmotic water permeability of MCF-7 cell plasma membrane was measured by a calcein fluorescence quenching method. Figure 3 showed the results of JBiSE  Y. Jiang et al. / J. Biomedical Science and Engineering 3 (2010) 95-100 SciRes Copyright © 2010 JBiSE 99 water permeability measurements of MCF-7 cell lines. The two populations of MCF-7 cell clones (AQP1L and AQP1H) with low and about two-fold higher os- motic water permeability with corresponding low and high AQP1 expression were identified by functional assay (Figure 3(a), 3(b)). To determine whether AQP1 expression accounted for the increased water permeability of AQP1L cells, we overexpressed AQP1 in AQP1L cells. Accordingly, osmotic water permeability was increased by about 1.7 fold in AQP1L cells infected with Ad-AQP1. In the same experimental conditions, infection with Ad-lacZ did not increase plasma membrane water permeability of AQP1L cells. 3.3. Expression of AQP1 Facilitated Wound Healing of MCF-7 Cell Monolayers Wound closure experiments were performed to con- firm the role of AQP1 in MCF-7 mammary carcinoma cell migration. Figure 4 showed significantly acceler- ated wound closure in AQP1H cells and AQP1L cells infected with Ad-AQP1 at concentration of 150 PFU/cell as compared to AQP1L cells. 3.4. Expression of AQP1 Promoted Invasive Mi- gration of MCF-7 Cells Transwell migration assay using a modified Boyden chamber was performed as an in vitro model of inva- sive migration of AQP1L and AQP1H cells, and AQP1L cells infected with Ad-AQP1. As shown in Figure 5, migration rate of AQP1H cells towards 10% FBS through the 8 μm pores in the transwell filter was significantly higher (~2.1 fold) than that of AQP1L cells. Infection of Ad-AQP1 at concentration of 150 PFU/cell enhanced the transwell migration rate of AQP1L cells to the level similar to AQP1H cells, suggesting an important role of AQP1 in facilitating cell migration. 4. DISCUSSION Migration of tumor cells through narrow extracellular spaces requires cell volume regulation and shape change. Previous studies revealed that ion channels and trans- porters were essential for cell volume regulation and invasive migration of some human tumors [18,19]. Our present study demonstrated for the first time that aq- uaporin-mediated transmembrane water flux was an im- portant determinant of MCF-7 mammary carcinoma cell migration and may be associated with mammary carci- noma invasion and metastasis. We proposed that trans- membrane water efflux through aquaporin water chan- nels facilitated cell volume change mediated by net salt efflux through ion channels and transporters in the mi- grating mammary carcinoma cells, leading to enhanced motility and invasion. There were evidence suggesting a role of AQPs in tumor cell migration and metastasis. Expression of several AQPs was found in a wide range of tumor cells in addition to the ubiquitous expression of AQP1 in the microvessels of solid tumors [14,15,16]. Warth et al. [16] found that AQP4 expression at the end- feet membranes was redistributed in high-grade astrocy- tomas. Our experiments provided direct evidence that aq- uaporin-mediated high plasma membrane water per- meability contributed to cell shape-volume change required for mammary carcinoma cell migration. Cell migration rate was remarkably higher in AQP1H cells as compared to AQP1L cells in both wound healing and invasive transwell migration assays. Adenoviral- mediated AQP1 expression in AQP1L cells increased their plasma membrane water permeability and mi- gration rate, further supporting the role of AQP1 in facilitating migration of the mammary carcinoma cells. Future studies are needed to uncover the role of aquaporin water channels in vivo tumor cell migration and metastasis. 5. ACKNOWLEDGEMENT This work was supported by the National Natural Science Foundation of China (Grant No.30800561), Tianjin Natural Science Foundation (GrantNo.07JCYBJC16400), and Scientific Research Foundation of Tianjin University of Science and Technology (GrantNo.20090402). REFERENCES [1] Preston, G.M., Carroll, T.P., Guggino, W.B. and Agre P. (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP 28 protein. Science, 256, 385-387. [2] Agre P. (2006) The aquaporin water channels. Proc Am Thorac Soc, 3, 5–13. [3] Takata, K., Matsuzaki, T. and Tajika, Y. (2004) Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem, 39, 1-83. [4] Agre, P., King, L.S., Yasui, M., Guggino, W.B. and Ottersen, O.P. (2002) Aquaporin water channels–from atomic structure to clinical medicine. J Physiol, 542, 3-16. [5] Verkman, A.S. (2002) Physiological importance of aquaporin water channels. Ann Med, 34, 192-200. [6] Verkman, A.S. (2005) More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci, 118, 3225-3232. [7] Saadoun, S., Papadopoulos, M.C., Chikuma, M.H. and Verkman, A.S. (2005) Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption Nature, 434, 786-792. [8] Chikuma, M.H. and Verkman, A.S. (2006) Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol, 17, 39-45. [9] Saadoun, S., Papadopoulos, M.C., Watanabe ,H., Yan, D. and Manley G.T. (2005) Involvement of aquaporin-4 in  100 Y. Jiang et al. / J. Biomedical Science and Engineering 3 (2010) 95-100 SciRes Copyright © 2010 JBiSE astroglial cell migration and glial scar formation. J Cell Sci, 118, 5691-5698. [10] Loitto, V.M., Forslund, T., Sundqvist, T., Magnusson, K. E. and Gustafsson, M. (2002) Neutrophil leukocyte motility requires directed water influx, J Leukoc Biol, 71, 212-222. [11] Loitto, V.M. and Magnusson, K.E. (2004) Hg2+ and small-sized polyethylene glycols have inverse effects on membrane permeability, while both impair neutrophil cell motility. Biochem Biophys Res Commun, 316, 370- 378. [12] Bogenrieder, T. and Herlyn, M. (2003) Axis of evil: molecular mechanisms of cancer metastasis. Oncogene, 22, 6524-6536. [13] Harlozinska, A. (2005) Progress in molecular mecha- nisms of tumor metastasis and angiogenesis. Anticancer Res, 25, 3327–3333. [14] Yang, J.H., Shi, Y.F., Cheng, Q. and Deng, L. (2006) Expression and localization of aquaporin-5 in the epithelial ovarian tumors. Gynecol Oncol, 100, 294-299. [15] Mazal, P.R., Susani, M., Wrba, F. and Haitel, A. (2005) Diagnostic significance of aquaporin-1 in liver tumors. Hum Pathol, 36, 1226-1231. [16] Warth, A., Mittelbronn, M. and Wolburg, H. (2005) Redistribution of the water channel protein aquaporin-4 and the K+ channel protein Kir4.1 differs in low-and high-grade human brain tumors. Acta Neuropathol, 109, 418-426. [17] Solenov, E., Watanabe, H., Manley, G.T. and Verkman, A. S. (2004) Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol Cell Physiol, 286, 426-432. [18] Ransom, C.B., O'Neal, J.T. and Sontheimer, H. (2001) Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci, 21, 7674-7683. [19] Soroceanu, L., Manning, T.J. and Sontheimer, H. (1999) Modulation of glioma cell migration and invasion using Cl- and K+ ion channel blockers. J Neurosci, 19, 5942-5954. |

