Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 65-77 doi:10.4236/jbise.2010.31010 Published Online January 2010 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online January 2010 in SciRes. http://www.scirp.org/journal/jbise Phosphatidylinositol transfer proteins: sequence motifs in structural and evolutionary analyses Gerald J. Wyckoff1, Ada Solidar2, Marilyn D. Yoder3 1Division of Molecular Biology and Biochemistry, University of Missouri-Kansas City, Kansas City, USA; 2Vassa Informatics, Kansas City, USA; 3Division of Cell Biology and Biophysics, University of Missouri-Kansas City, Kansas City, USA. Email: wyckoffg@umkc.edu Received 30 June 2009; revised 28 September 2009; accepted 30 September 2009. ABSTRACT Phosphatidylinositol transfer proteins (PITP) are a family of monomeric proteins that bind and transfer phosphatidylinositol and phosphatidylcholine be- tween membrane compartments. They are required for production of inositol and diacylglycerol second messengers, and are found in most metazoan organ- isms. While PITPs are known to carry out crucial cell-signaling roles in many organisms, the structure, function and evolution of the majority of family members remains unexplored; primarily because the ubiquity and diversity of the family thwarts tradi- tional methods of global alignment. To surmount this obstacle, we instead took a novel approach, using MEME and a parsimony-based analysis to create a cladogram of conserved sequence motifs in 56 PITP family proteins from 26 species. In keeping with pre- vious functional annotations, three clades were sup- ported within our evolutionary analysis; two classes of soluble proteins and a class of membrane-associat- ed proteins. By, focusing on conserved regions, the analysis allowed for in depth queries regarding pos- sible functional roles of PITP proteins in both intra- and extra- cellular signaling. Keywords: Protein Evolution; Structural Domain; Phylogenetics; Sequence Motif 1. INTRODUCTION Phosphatidylinositol transfer proteins (PITP) are mono- meric, lipid-binding proteins that bind and transfer phosphatidylinositol (PtdIns) and phosphatidylcholine (PtdCho) between membrane compartments (see reviews by: [1,2,3,4,5,6]. Inositol lipids have specialized func- tions in the regulation of eukaryotic cells, providing a source of second messengers and acting as signaling molecules. Monomeric phospholipids have extremely low solubilities and negligible spontaneous transfer rates between membranes, necessitating protein factors to shield them in the aqueous environment of the cell. Al- most all phospholipid exchange activity within the eu- karyotic cytosol is accomplished by three groups of pro- teins: PITP, phosphatidylcholine transfer protein (PCTP), or sterol carrier protein 2. PITP-domain proteins, which are the focus of this study, are found in five classes of proteins: three are soluble and cytosolic, and two are membrane-associated proteins [1,2] (Figure 1). Soluble PITPs are found in virtually all metazoan or- ganisms. These are approximately 77% identical at the amino-acid level. Two isoforms, PITP and PITP, exist in mammals; they are highly conserved with about 98% sequence identity at the amino acid level. The proteins bind one molecule of PtdIns or PtdCho and are typically about 32 kDa in mass. PITP-like domains are also detected in the retinal de- generation B (rdgB) class of proteins (see reviews [6,7]). These 160-170 kDa proteins are membrane-associated proteins first identified in Drosophila melanogaster, as mutations in these genes lead to retinal degeneration. The rdgBs contain an Figure 1. Gene structure of PITP-domain proteins. Grey cyl- inder is PITP-like domain, white cylinder is the FFAT region of ER binding, the grey box is the DDHD region, and the white box is the LSN2 region. The the human pitpnm1 protein, the FFAT region is residues 360-365, the DDHD region is 686-879, and the LSN2 domain is residues 1022-1152 [6]. 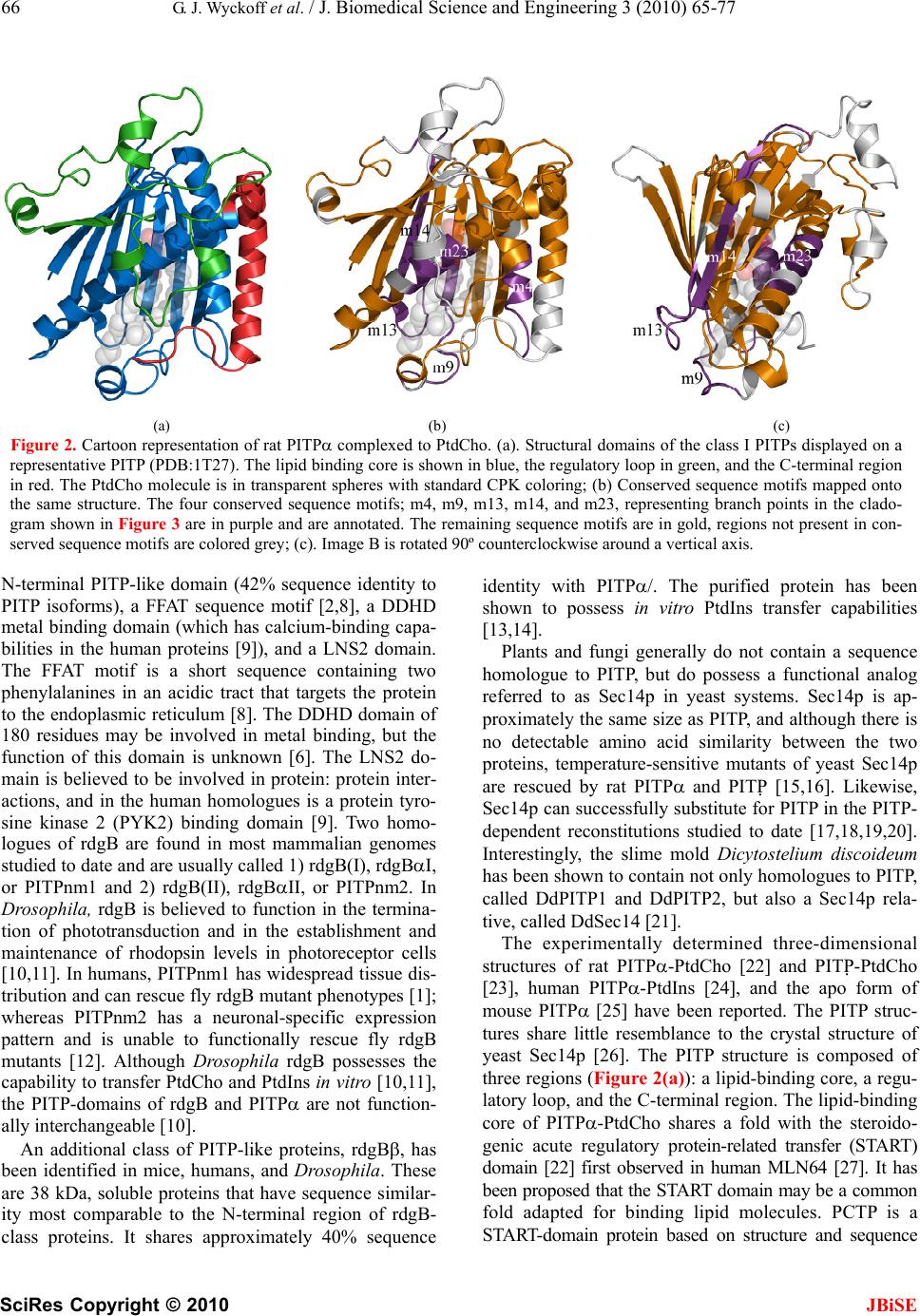 66 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE (a) (b) (c) Figure 2. Cartoon representation of rat PITP complexed to PtdCho. (a). Structural domains of the class I PITPs displayed on a representative PITP (PDB:1T27). The lipid binding core is shown in blue, the regulatory loop in green, and the C-terminal region in red. The PtdCho molecule is in transparent spheres with standard CPK coloring; (b) Conserved sequence motifs mapped onto the same structure. The four conserved sequence motifs; m4, m9, m13, m14, and m23, representing branch points in the clado- gram shown in Figure 3 are in purple and are annotated. The remaining sequence motifs are in gold, regions not present in con- served sequence motifs are colored grey; (c). Image B is rotated 90º counterclockwise around a vertical axis. N-terminal PITP-like domain (42% sequence identity to PITP isoforms), a FFAT sequence motif [2,8], a DDHD metal binding domain (which has calcium-binding capa- bilities in the human proteins [9]), and a LNS2 domain. The FFAT motif is a short sequence containing two phenylalanines in an acidic tract that targets the protein to the endoplasmic reticulum [8]. The DDHD domain of 180 residues may be involved in metal binding, but the function of this domain is unknown [6]. The LNS2 do- main is believed to be involved in protein: protein inter- actions, and in the human homologues is a protein tyro- sine kinase 2 (PYK2) binding domain [9]. Two homo- logues of rdgB are found in most mammalian genomes studied to date and are usually called 1) rdgB(I), rdgBI, or PITPnm1 and 2) rdgB(II), rdgBII, or PITPnm2. In Drosophila, rdgB is believed to function in the termina- tion of phototransduction and in the establishment and maintenance of rhodopsin levels in photoreceptor cells [10,11]. In humans, PITPnm1 has widespread tissue dis- tribution and can rescue fly rdgB mutant phenotypes [1]; whereas PITPnm2 has a neuronal-specific expression pattern and is unable to functionally rescue fly rdgB mutants [12]. Although Drosophila rdgB possesses the capability to transfer PtdCho and PtdIns in vitro [10,11], the PITP-domains of rdgB and PITP are not function- ally interchangeable [10]. An additional class of PITP-like proteins, rdgB, has been identified in mice, humans, and Drosophila. These are 38 kDa, soluble proteins that have sequence similar- ity most comparable to the N-terminal region of rdgB- class proteins. It shares approximately 40% sequence identity with PITP/. The purified protein has been shown to possess in vitro PtdIns transfer capabilities [13,14]. Plants and fungi generally do not contain a sequence homologue to PITP, but do possess a functional analog referred to as Sec14p in yeast systems. Sec14p is ap- proximately the same size as PITP, and although there is no detectable amino acid similarity between the two proteins, temperature-sensitive mutants of yeast Sec14p are rescued by rat PITP and PITP [15,16]. Likewise, Sec14p can successfully substitute for PITP in the PITP- dependent reconstitutions studied to date [17,18,19,20]. Interestingly, the slime mold Dicytostelium discoideum has been shown to contain not only homologues to PITP, called DdPITP1 and DdPITP2, but also a Sec14p rela- tive, called DdSec14 [21]. The experimentally determined three-dimensional structures of rat PITP-PtdCho [22] and PITP-PtdCho [23], human PITP-PtdIns [24], and the apo form of mouse PITP [25] have been reported. The PITP struc- tures share little resemblance to the crystal structure of yeast Sec14p [26]. The PITP structure is composed of three regions (Figure 2(a)): a lipid-binding core, a regu- latory loop, and the C-terminal region. The lipid-binding core of PITP-PtdCho shares a fold with the steroido- genic acute regulatory protein-related transfer (START) domain [22] first observed in human MLN64 [27]. It has been proposed that the START domain may be a common fold adapted for binding lipid molecules. PCTP is a START-domain protein based on structure and sequence 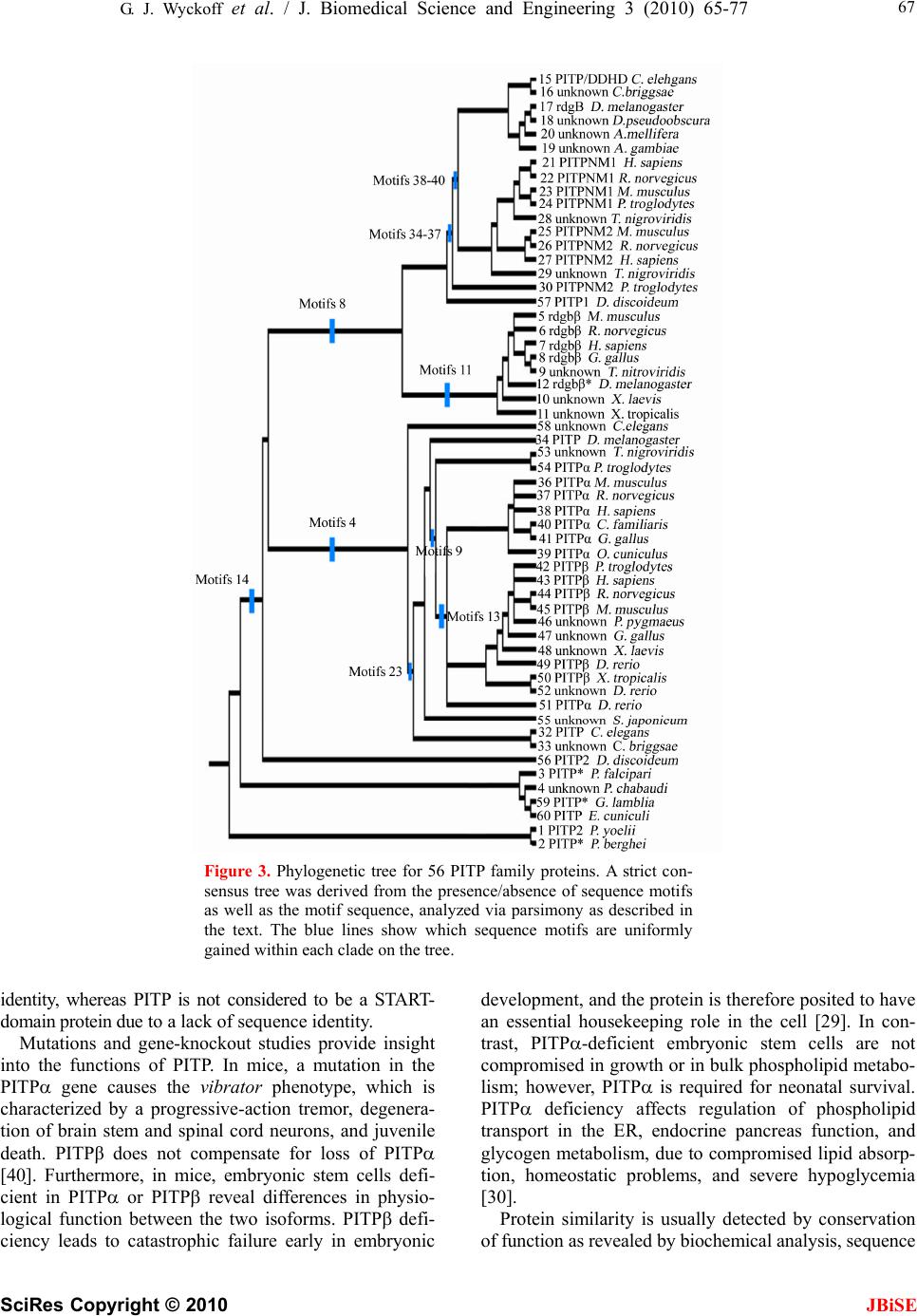 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE 67 Figure 3. Phylogenetic tree for 56 PITP family proteins. A strict con- sensus tree was derived from the presence/absence of sequence motifs as well as the motif sequence, analyzed via parsimony as described in the text. The blue lines show which sequence motifs are uniformly gained within each clade on the tree. identity, whereas PITP is not considered to be a START- domain protein due to a lack of sequence identity. Mutations and gene-knockout studies provide insight into the functions of PITP. In mice, a mutation in the PITP gene causes the vibrator phenotype, which is characterized by a progressive-action tremor, degenera- tion of brain stem and spinal cord neurons, and juvenile death. PITPβ does not compensate for loss of PITP [40]. Furthermore, in mice, embryonic stem cells defi- cient in PITP or PITP reveal differences in physio- logical function between the two isoforms. PITP defi- ciency leads to catastrophic failure early in embryonic development, and the protein is therefore posited to have an essential housekeeping role in the cell [29]. In con- trast, PITP-deficient embryonic stem cells are not compromised in growth or in bulk phospholipid metabo- lism; however, PITP is required for neonatal survival. PITP deficiency affects regulation of phospholipid transport in the ER, endocrine pancreas function, and glycogen metabolism, due to compromised lipid absorp- tion, homeostatic problems, and severe hypoglycemia [30]. Protein similarity is usually detected by conservation of function as revealed by biochemical analysis, sequence 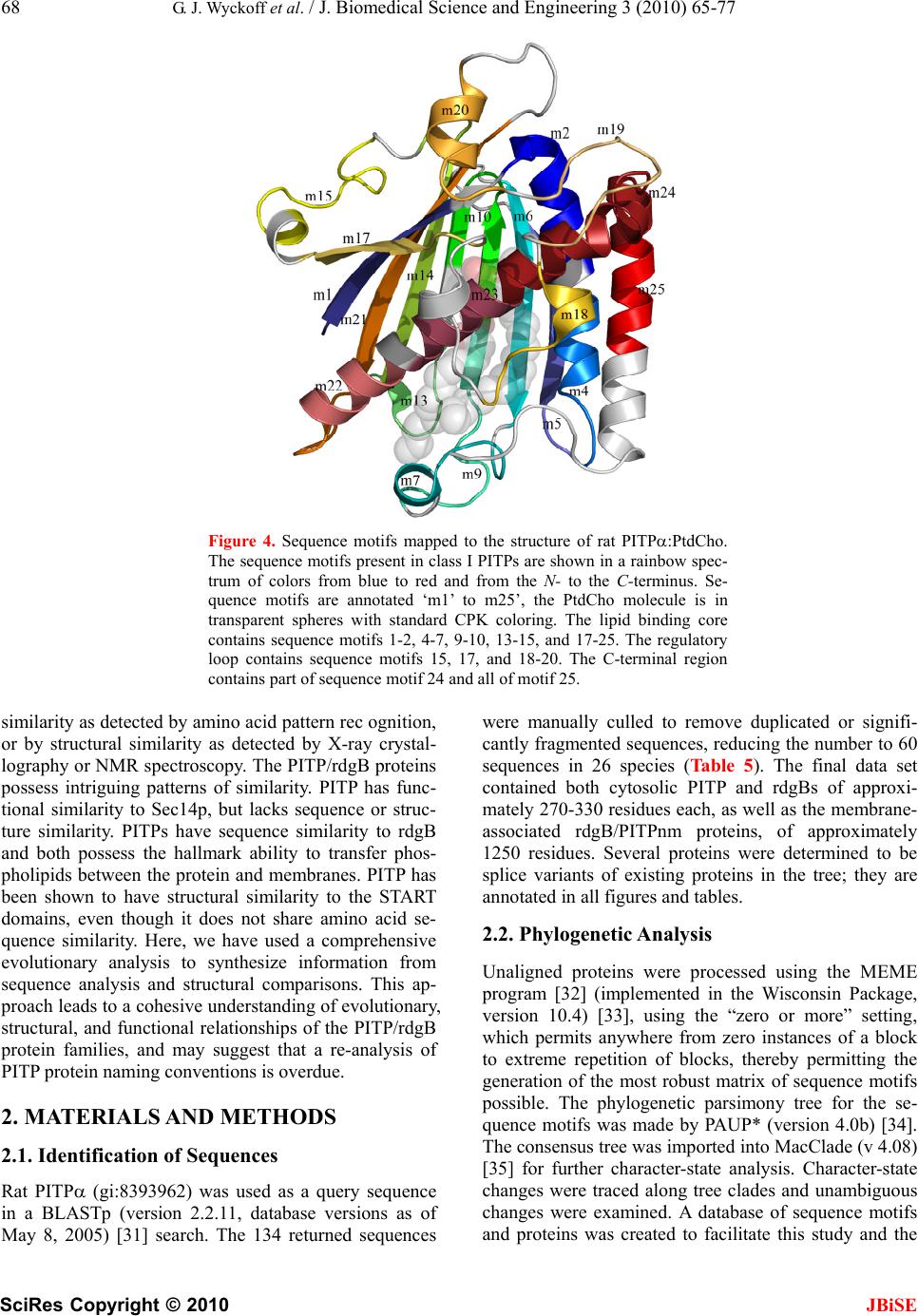 68 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE Figure 4. Sequence motifs mapped to the structure of rat PITP:PtdCho. The sequence motifs present in class I PITPs are shown in a rainbow spec- trum of colors from blue to red and from the N- to the C-terminus. Se- quence motifs are annotated ‘m1’ to m25’, the PtdCho molecule is in transparent spheres with standard CPK coloring. The lipid binding core contains sequence motifs 1-2, 4-7, 9-10, 13-15, and 17-25. The regulatory loop contains sequence motifs 15, 17, and 18-20. The C-terminal region contains part of sequence motif 24 and all of motif 25. similarity as detected by amino acid pattern rec ognition, or by structural similarity as detected by X-ray crystal- lography or NMR spectroscopy. The PITP/rdgB proteins possess intriguing patterns of similarity. PITP has func- tional similarity to Sec14p, but lacks sequence or struc- ture similarity. PITPs have sequence similarity to rdgB and both possess the hallmark ability to transfer phos- pholipids between the protein and membranes. PITP has been shown to have structural similarity to the START domains, even though it does not share amino acid se- quence similarity. Here, we have used a comprehensive evolutionary analysis to synthesize information from sequence analysis and structural comparisons. This ap- proach leads to a cohesive understanding of evolutionary, structural, and functional relationships of the PITP/rdgB protein families, and may suggest that a re-analysis of PITP protein naming conventions is overdue. 2. MATERIALS AND METHODS 2.1. Identification of Sequences Rat PITP (gi:8393962) was used as a query sequence in a BLASTp (version 2.2.11, database versions as of May 8, 2005) [31] search. The 134 returned sequences were manually culled to remove duplicated or signifi- cantly fragmented sequences, reducing the number to 60 sequences in 26 species (Table 5). The final data set contained both cytosolic PITP and rdgBs of approxi- mately 270-330 residues each, as well as the membrane- associated rdgB/PITPnm proteins, of approximately 1250 residues. Several proteins were determined to be splice variants of existing proteins in the tree; they are annotated in all figures and tables. 2.2. Phylogenetic Analysis Unaligned proteins were processed using the MEME program [32] (implemented in the Wisconsin Package, version 10.4) [33], using the “zero or more” setting, which permits anywhere from zero instances of a block to extreme repetition of blocks, thereby permitting the generation of the most robust matrix of sequence motifs possible. The phylogenetic parsimony tree for the se- quence motifs was made by PAUP* (version 4.0b) [34]. The consensus tree was imported into MacClade (v 4.08) [35] for further character-state analysis. Character-state changes were traced along tree clades and unambiguous changes were examined. A database of sequence motifs and proteins was created to facilitate this study and the 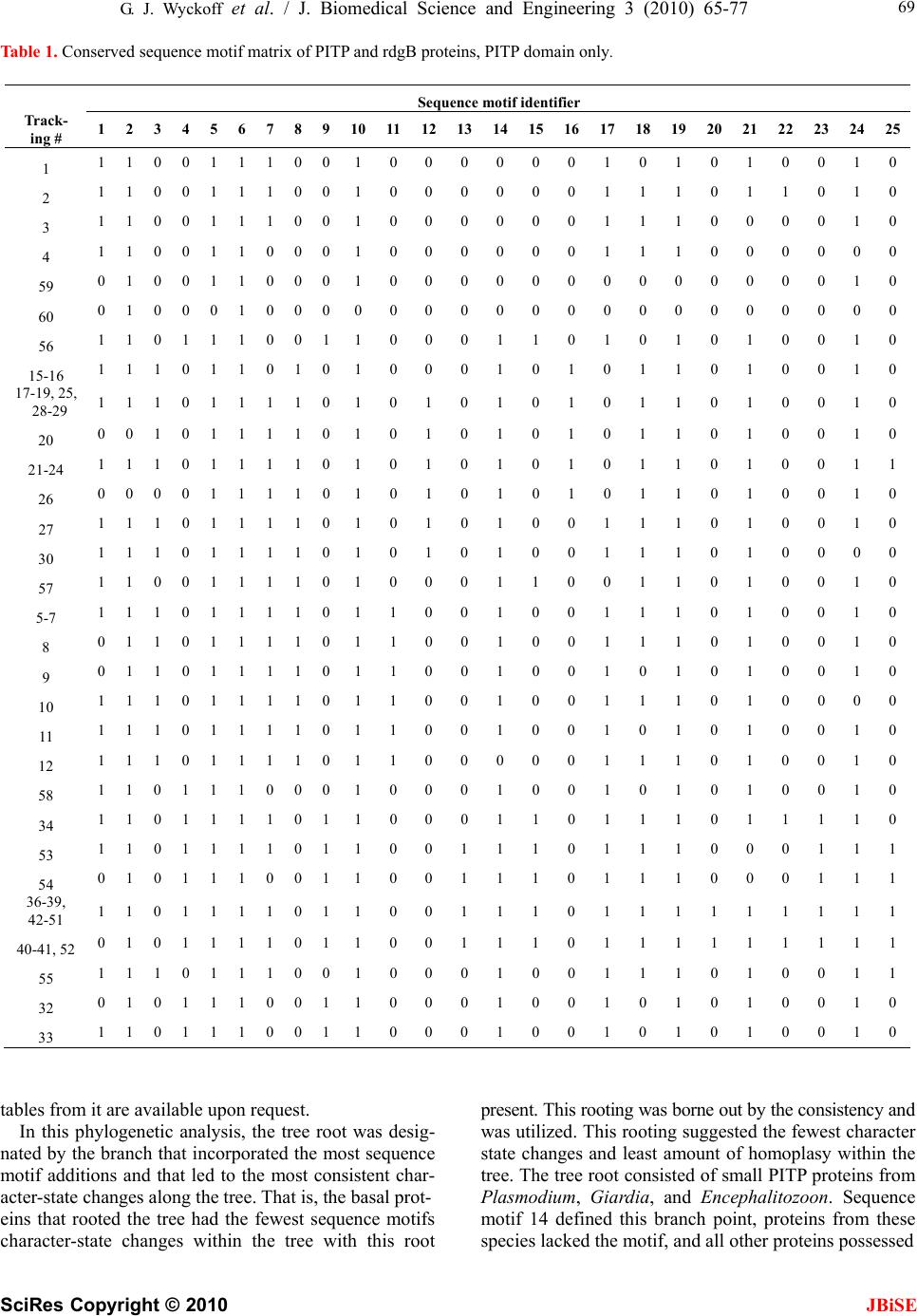 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE 69 Table 1. Conserved sequence motif matrix of PITP and rdgB proteins, PITP domain only. tables from it are available upon request. In this phylogenetic analysis, the tree root was desig- nated by the branch that incorporated the most sequence motif additions and that led to the most consistent char- acter-state changes along the tree. That is, the basal prot- eins that rooted the tree had the fewest sequence motifs character-state changes within the tree with this root present. This rooting was borne out by the consistency and was utilized. This rooting suggested the fewest character state changes and least amount of homoplasy within the tree. The tree root consisted of small PITP proteins from Plasmodium, Giardia, and Encephalitozoon. Sequence motif 14 defined this branch point, proteins from these species lacked the motif, and all other proteins possessed Sequence motif identifier Track- ing # 1 2 3 4 5 6 7 8 9 1011121314151617181920 21 22 232425 1 1 1 0 0 1 1 1 0 0 10000001010 1 0 0 10 2 1 1 0 0 1 1 1 0 0 10000001110 1 1 0 10 3 1 1 0 0 1 1 1 0 0 10000001110 0 0 0 10 4 1 1 0 0 1 1 0 0 0 10000001110 0 0 0 00 59 0 1 0 0 1 1 0 0 0 10000000000 0 0 0 10 60 0 1 0 0 0 1 0 0 0 00000000000 0 0 0 00 56 1 1 0 1 1 1 0 0 1 10001101010 1 0 0 10 15-16 1 1 1 0 1 1 0 1 0 10001010110 1 0 0 10 17-19, 25, 28-29 1 1 1 0 1 1 1 1 0 10101010110 1 0 0 10 20 0 0 1 0 1 1 1 1 0 10101010110 1 0 0 10 21-24 1 1 1 0 1 1 1 1 0 10101010110 1 0 0 11 26 0 0 0 0 1 1 1 1 0 10101010110 1 0 0 10 27 1 1 1 0 1 1 1 1 0 10101001110 1 0 0 10 30 1 1 1 0 1 1 1 1 0 10101001110 1 0 0 00 57 1 1 0 0 1 1 1 1 0 10001100110 1 0 0 10 5-7 1 1 1 0 1 1 1 1 0 11001001110 1 0 0 10 8 0 1 1 0 1 1 1 1 0 11001001110 1 0 0 10 9 0 1 1 0 1 1 1 1 0 11001001010 1 0 0 10 10 1 1 1 0 1 1 1 1 0 11001001110 1 0 0 00 11 1 1 1 0 1 1 1 1 0 11001001010 1 0 0 10 12 1 1 1 0 1 1 1 1 0 11000001110 1 0 0 10 58 1 1 0 1 1 1 0 0 0 10001001010 1 0 0 10 34 1 1 0 1 1 1 1 0 1 10001101110 1 1 1 10 53 1 1 0 1 1 1 1 0 1 10011101110 0 0 1 11 54 0 1 0 1 1 1 0 0 1 10011101110 0 0 1 11 36-39, 42-51 1 1 0 1 1 1 1 0 1 10011101111 1 1 1 11 40-41, 52 0 1 0 1 1 1 1 0 1 10011101111 1 1 1 11 55 1 1 1 0 1 1 1 0 0 10001001110 1 0 0 11 32 0 1 0 1 1 1 0 0 1 10001001010 1 0 0 10 33 1 1 0 1 1 1 0 0 1 10001001010 1 0 0 10  70 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE Table 2. Conserved sequence motif matrix of rdgB proteins, C-terminal of the PITP-domain. Table 3. Gene and protein nomenclature for proteins utilized in this study. the conserved sequence motif. 3. RESULTS 3.1. Characterization of PITP-Like Proteins An initial approach to characterizing the PITP family was relatively standard. Rat PITP was used as the seed for a BLAST search targeting relatively distant protein relatives. BLAST results were imported to Pileup [33] to generate a “first pass” global alignment. Under a variety of different conditions, including weighting end gaps, not weighting end gaps, utilizing low gap opening and extension penalties, high-road and low-road alignment options, alignments were very weak, with notable cases where locally homologous segments identified by BLAST were not aligned in the global matrix. The derived nature of many of the proteins in the PITP family was already apparent; sequence motifs appeared in some classes but not others, rendering standard alignment and analysis methods ineffective. A novel approach was required to identify and analyze the large, divergent PITP family. 3.2. Identification of Conserved Sequence Motifs When a high-confidence global alignment in Pileup proved impossible, unaligned proteins were processed using MEME [32] which identifies conserved sequence motifs in proteins. The program makes identifications based on prior probabilities of amino acid occurrence. Because it performs ungapped determinations, no preced- ing global alignment of sequence motifs was necessary, families with highly derived members. Of the 46 cons- erved sequence motifs detected by MEME, 25 fell Sequence motif identifier Trac- king # 26 27 28 29 30 31 32 333435363738394041 42 43 44 45 46 15 0 0 0 0 1 1 0 0 11 1 1 1 1 10 1 0 0 0 0 16 0 0 0 0 1 1 0 0 11 1 1 1 1 10 1 0 0 0 1 17,21-22, 26, 28 1 1 1 1 1 1 1 1 11 1 1 1 1 11 1 1 1 0 0 18 1 1 1 1 1 1 1 0 11 1 1 1 1 11 1 1 0 0 0 20 0 1 1 0 1 1 0 0 11 1 1 1 1 11 1 1 0 0 0 19 1 0 1 1 1 1 0 0 11 1 1 1 1 11 1 0 0 0 0 23 1 1 1 1 1 1 1 1 11 1 1 1 1 11 1 1 1 1 0 24 1 1 0 1 1 1 1 1 11 1 1 1 1 11 1 1 1 1 0 25 1 1 1 1 0 1 0 0 11 1 1 1 1 11 1 1 0 0 0 27 1 1 1 1 1 1 1 1 11 1 1 1 1 11 1 1 0 0 0 29 1 1 1 0 0 0 0 0 11 1 1 1 1 11 0 0 0 0 0 30 1 0 1 1 1 1 1 0 11 1 1 0 0 00 0 0 0 0 0 PITP PITP rdgBβ rdgB(1) rdgB(2) Gene name Pitpna (mouse) Pitpn (rat) Pitpna (human) Pitpnb (mouse) Pitpnb (rat) Pitpnb (human) pitpnc1 (human) pitpnm1 (mouse) NIR2 rdgB1 (fly) DRES9 pitpnm2 (mouse) NIR3 rdgB2 (fly) Gene sym- bols Pitpna (mouse) Pitpn (rat) Pitpna (human) Pitpnb (mouse) Pitpnb (rat) Pitpnb (human) pitpnc1 (human)pitpnm1 (mouse) pitpnm2 (mouse) Gene ali- ases Pitpn, vib1A Vib1B Rd9; RdgB; DRES9; mpt-1; Pitpnm NIR3; rdgB2; rdgB 2; mKIAA1457 Protein name PITP PITP rdgBβ ; rdgB 1; PITP, cyto- plasmic 1* PITP mem- brane-associated 1 PITP mem- brane-associated 2 Approved gene sym- bol PITPNA PITPNB PITPNC1 PITPNM1 PITPNM2  G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE 71 Table 4. Conserved sequence motifs in the PITP-domain. Motif Identi- fier secondary structure element, PITPa Sequence rangeb: PITP PITPnm1 PITPNC1 Motif sequenceb: PITP PITPnm1 PITPNC1 1 sheet 1 (3-11) 2-9 2-VLLKEYRV-9 1 1-8 1-MLIKEYHI-8 1 1-8 1-MLLKEYRI-8 2 helix A (14-33) 12-24 PVSVDEYQVGQLY 2 11-23 PMSLDEYQVAQLY 2 11-23 PLTVDEYKIGQLY 3 - -------- 3 24-31 MIQKKSRE 3 24-31 MISKHSHE 4 helix A (14-33) 26-36 VAEASKNETGG 4 - ----------- 4 - ----------- 5 sheet 2 (39-49) 37-48 GEGVEVLVNEPY 5 37-48 GSGVEILANRPY 5 36-47 GEGVEVVQNEPF 6 sheet 3 (55-64) 55-64 GQYTHKIYHL 6 56-65 GQYTHKVYHV 6 55-64 GQFTEKRVYL 7 helix B (70-75) 66-73 SKVPTFVR 7 204-211 AKIEQFIH 7 198-205 TRVEQFVH 8 - ----------- 8 69-79 IPGWFRALLPK 8 68-78 LPSWARAVVPK 9 76-86 APEGALNIHEK 9 - ----------- 9 - ----------- 10 sheet 4 (84-91) 87-96 AWNAYPYCRT 10 88-97 SWNAYPYTRT 10 86-95 AWNYYPYTIT 11 - -------- 11 - -------- 11 97-104 YTCSFLPK 12 - -------- 12 98-105 RYTCPFVE 12 - -------- 13 sheet 5 (94-100) 99-106 TNEYMKED 13 - -------- 13 - -------- 14 sheet 6 (108-117) 107-120 FLIKIETWHKPDLG 14 107-120 FSIEIETYYLPDGG 14 105-118 FSIHIETKYEDNKG 15 helix C (132-137) 123-134 ENVHKLEPEAWK 15 - ------------ 15 - ------------ 16 - ----------------------- 16 124-146 NVFNLSGAERRQRILDTIDIVRD 16 - ----------------------- 17 sheet 7 (138-142) 137-144 EAVYIDIA 17 - -------- 17 134-141 EVCFIDIA 18 153-160 DYKAEEDP 18 152-159 EYKAEEDP 18 149-156 YYKESEDP 19 163-173 FKSIKTGRGPL 19 162-172 YHSVKTGRGPL 19 159-169 FKSEKTGRGQL 20 helix E (177-183) 175-183 PNWKQELVN 20 - --------- 20 - --------- 21 sheet 8 (191-201) 190-206 MCAYKLVTVKFKWWGLQ 21 187-203 MCAYKLCKVEFRYWGMQ 21 181-197 MCSYKLVTVKFEVWGLQ  72 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE 22 helix F (206-232) 207-214 NKVENFIH 22 - -------- 22 - -------- 23 helix F (206-232) 217-224 ERRLFTNF 23 - -------- 23 - -------- 24 helix F (206-232) 225-244 HRQLFCWLDKWVDLTMDDIR 24 223-242 HRQAWCWQDEWTELSMADIR 24 216-235 HRQAFAWVDEWYDMTMDEVR 25 helix G (240-261) 245-253 RMEEETKRQL 25 243-252 ALEEETARML 25 - ---------- 26 - -------- 26 294-301 PPGPDASP 26 269-276 RSAPSSAP aSecondary structure nomenclature for rat PITP as defined in [22], parenthesis indicate the complete range of the secondary structure element. bSequences and residue numbering refer to human proteins, PITP is gi:5453908, tracking #38; PITPnm1 is gi:18490106, tracking #21; PITPNC1 is gi:32307140, tracking #7. PITPNC1 is also called rdgB. within PITP-like domains. 3.3. Phylogenetic Analysis With sequence motifs identified, two character-state ma- trices were created. One contained character-state infor- mation on the presence or absence of detected motifs, and one contained aligned amino acid data (Tables 1 and 2). These matrices were utilized for the creation of a character state phylogenetic tree via maximum parsi- mony. The overall shape of the tree was determined by the presence/absence matrix, with the amino acid align- ment data utilized to determine internal clade structures. Gaps, i.e., missing sequence motifs were considered a new state, and thus, the difference between having a specific set of amino acids and not having an amino acid could served as an informative character state. Twelve most parsimonious trees of differing structures were identified by PAUP, but the strict consensus tree structure suggests the likely evolutionary history of this intriguing family of proteins (Figure 3). The tree re- vealed that membrane-associated proteins are derivatives of a group of PITP-like genes, and that many genes pre- viously thought to be related to PITP and PITP via se- quence homology fell outside of clades that contain ex- clusively PITP or PITP genes. 3.4. Identification of Clades Within the Phylogenetic Tree In order to be cladistically sound, nomenclature must be hierarchical; all proteins in the tree were “PITP-like.” The tree lends itself to consideration of the PITP-like family consisting of three large divisions (the classifica- tion nomenclature proposed by Allen-Baume [1] is adapted here): Class I comprised the soluble PITPs, Class IIA were the membrane-associated rdgB proteins (which contain additional domains C-terminal to the PITP-like domain), and Class IIB were the soluble rdgB proteins. Further subdivisions along cladistic lines allowed for the naming of the Alpha clade and the Beta clade within Class I, and an ancestral group of PITP-like proteins, predominantly from protista, that occured in several clades rooting the tree. Table 3 lists the nomenclature commonly utilized for the genes and proteins in this study, as well as the HUGO-approved nomenclature. 3.5. Examination of Conserved Sequence Motifs To understand the functional shifts that have occurred, examination of some of the sequence motifs in each proposed class was necessary. The amino acid sequence of each motif in the human proteins PITP (Class I), PITPNM1 (Class IIA), and PITPNC1, also called rdgB (Class IIB) are given in Table 4. Class I: These proteins are cytosolic and contain se- quence motifs 1-2, 4-7, 9-10, 13-15, and 17-25 (Figure 4). This class was distinguished from the Class II divi- sions by sequence motif 8, which was unique to the Class IIA and IIB clades, and sequence motif 4, which was unique to the non-protista Class I PITPs. A striking observation about sequence motif 8 was that it appears to be an amino acid sequence overlap with sequence motif 9, which defines subnodes within Class I. In the structure of rat PITP, the residues defined by sequence motif 9 incorporate helix B, which is a putative mem- brane insertion helix [25]. There was no unique con- served sequence motif that distinguishes the functionally divergent alpha and the beta clades. Class IIA: These proteins are membrane-associated and typically are 160 kDa. In this analysis they were distinguished by the unique presence of sequence motifs 34-40, which consisted of amino acids residues 831-844, 856-866, 899-920, 937-951, 993-1015, 1022-1038, and 1040-1086 in the human PITPnm1 protein. The division had two major subnodes, one consisting of non-mamma-  G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE 73 Table 5. Proteins used in phylogenetic analysis. aindicated as a predicted protein, bindicated as a hypothetical or a putative protein lian proteins and containing the canonical rdgB protein from D. melanogaster, and the other subnode containing the mammalian sequences PITPNM1 and PITPNM2. There were 21 conserved sequence motifs outside the gi # Tracking # Species common Protein designations 55243798 19 Anopheles gambiae mosquito 48135931 20 Apis mellifera honeybee 39590557 16 Caenorhabditis briggsaaenematode 39590636 33 Caenorhabditis briggsaaenematode CB09751 b (NCBI-COG: PITP) 17556182 32 Caenorhabditis elegans 17554244 15 Caenorhabditis elegans nematode PITP DDHD (NCBI-COG: PITP) 48059855 58 Caenorhabditis elegans nematode Y71G12B.17 b (NCBI-COG: PITP) 57091327 40 Canus familiaris dog similar to PITP a 41055500 49 Danio rerio zebrafish PITP 41055576 51 Danio rerio zebrafish similar to PITP 28422482 52 Danio rerio zebrafish 8307957 56 Dictyostelium discoideumslime moldPITP 2 8307955 57 Dictyostelium discoideumslime moldPITP 1 24641869 17 Drosophila melanogasterfruit fly CG11111-PB, isoform B, rdgB 62484257 12 Drosophila melanogasterfruit fly CG17818-PA, rdgBβ 7300495 35 Drosophila melanogasterfruit fly CG5269-PA PITP, vib 20151901 34 Drosophila melanogasterfruit fly SD01527p, vib 54642914 18 D.pseudoobscura fly GA10766-PA, Dpse\GA10766 19170839 60 Encephalitozoon cuniculi PITP 50758480 41 Gallus gallus chicken similar to PITP a 50756343 31 Gallus gallus chicken similar to PITP, membrane-associated 2 a 50755397 13 Gallus gallus chicken similar to rdgBβ a 53134209 47 Gallus gallus chicken 50757849 8 Gallus gallus chicken similar splicing variant rdgBβ a 29250063 59 Giardia lamblia similiar to D. discoideum PITP1 18490106 21 Homo sapiens human PITPNM1 24308237 27 Homo sapiens human PITPNM2 5453908 38 Homo sapiens human PITP 6912594 43 Homo sapiens human PITPβ 32307140 7 Homo sapiens human PITP, rdgBβ 1 6679337 36 Mus musculus mouse PITP 9790159 45 Mus musculus mouse PITPβ 22003862 5 Mus musculus mouse rdgB 6679339 23 Mus musculus mouse PITPNM1 47124324 25 Mus musculus mouse PITPNM2 2137007 39 Oryctolagus cuniculus rabbit PITP 55660937 42 Pan troglodytes chimp PITP a 55644759 54 Pan troglodytes chimp similar to PITP a 55639157 30 Pan troglodytes chimp similar to PITP membrane-associated 2 a 55636463 24 Pan troglodytes chimp similar to PITP membrane-associated 1 a 56493706 2 Plasmodium berghei PITP b 56509884 4 Plasmodium chabaudi protein b 23619433 3 Plasmodium falciparum PITP b 23485539 1 Plasmodium yoelii yoelii PITP 2 55731914 46 Pongo pygmaeus orangutan protein b 56605814 22 Rattus norvegicus rat PITPnm, PI membrane-associated a 62658898 26 Rattus norvegicus rat similar KIAA1457, PITPnm2a 8393962 37 Rattus norvegicus rat PITP, PITPn 16758568 44 Rattus norvegicus rat PITP 62657241 6 Rattus norvegicus rat rdgB 56756428 55 Schistosoma japonicum Unknown, similiar to vib (fly) 47228496 53 Tetraodon nigroviridis pufferfish 47228470 28 Tetraodon nigroviridis pufferfish 47223953 9 Tetraodon nigroviridis pufferfish 47206861 14 Tetraodon nigroviridis pufferfish 47226436 29 Tetraodon nigroviridis pufferfish 47937798 48 Xenopus laevis frog MGC84500 proteinb 49256286 10 Xenopus laevis frog MGC84224 protein 62860160 11 Xenopus tropicalis frog protein LOC549393 b 38512077 50 Xenopus tropicalis frog PITPβ  74 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE PITP domain, with sequence motifs 34-37 observed in all analyzed proteins in this class. In human pitpnm1, the FFAT domain was found at the end of sequence motif 27, with a derived motif sequence of EFFDCLD. The DDHD region contained sequence motifs 30-35, which comprises 35% of the defined DDHD range, and the LSN2 region contained sequence motifs 39 and the N-terminal portion of sequence motif 40, which is 23% of this region. Lev, et al. defined six, short hydrophobic regions based on hydrophathy plots that were originally postulated to be transmembrane domains [9] then later to mediate membrane association [7]. None of the six re- gions fell in a conserved sequence motif identified in this study On the other hand, sequence motif 26 ap- peared in multiple repeats throughout the non-PITP do- main in sequence motifs 29, 32-33, and 44-45. This is a Ser rich motif of eight amino acids, the significance of this observation is currently unclear. Class IIB: The proteins in this class are historically referred to as rdgBs and are monomeric, cytosolic pro- teins of ~330 amino acids. They were distinguished by the unique presence of sequence motif 11, which con- sists of residues 97-104 in the human PITPNC1 se- quence. Sequence motif 11 is interesting; although it was only present in proteins in this division, it overlaped in sequence with motif 12 and motif 13. Sequence motif 12 was only observed in class IIA, whereas sequence motif 13 was unique to Class I, alpha and beta clades. Another notable feature of this division was PITP1 from D. dis- coideum. It grouped outside the other rdgBproteins and rooted the class IIA clade. 4. DISCUSSION 4.1. Phylogenetics Ocaka et al [36] provide an extensive analysis of the chromosomal location of the PITPN genes in humans with a phylogenetic tree rooted with C. elegans PITP as the chosen outgroup. The analysis presented here differs in two respects. First, the original gene-duplication event leading to the evolution of soluble and mem- brane-associated PITP proteins likely occurred not early in animal evolution, but instead was initiated in some protists. D. discoideum has been shown to have two genes, pitA and pitB, and expression has been demon- strated for both proteins, PITP1 and PITP2 [21]. The phylogeny shown here indicates that PITP1 is a class IIB protein, perhaps representative of an ancestral precursor to class IIA proteins. Second, data presented here indi- cate that the Class IIB proteins are derived from Class I proteins, and the membrane-associated Class IIA pro- teins are derived from the class IIB proteins. Previous cladograms and dendrograms have indicated different derivization patterns. Ocaka et al [36] indicate that mammalian PITPNC1 (Class IIB in the present analysis) shares a most recent common ancestor (MRCA) with PITPNA and PITPNB; more so than with any of the PITPNMs. Fullwood et al. present a dendrogram in which the membrane-associated PITPs and the soluble PITPs split from a shared derivation from Class IIB pro- teins (rdgB). An increased sample size, an unbiased tree root, and the use of conserved sequence motifs for global alignment in the present study lead to a different interpretation of the evolution of this protein family. The phylogenetic tree produced here is indicative of the evolutionary history of these sequence motifs, and not of the proteins as a whole. This is important, because in some proteins, large sequences with no known se- quence motif patterns are observed (such as in the Plas- modium yoelli “PITP1” protein) that may be the result of gene conversion, unequal crossing-over, or repeat ex- pansion during the evolution of specific proteins. Such large insertions and deletions in protein sequence are difficult to utilize in any phylogenetic analysis; they are purely derived characters that are phylogenetically un- informative. However, the existence of these derived sequences suggests that either the function or regulation of proteins in this family has changed dramatically, in- dicative of the type of specific adaptation often seen within protein families. 4.2. Functional Shifts in PITP Proteins Alignment and subsequent phylogenetic analysis of di- vergent members of protein families is a problem com- pounded by shifts in primary, secondary, and tertiary structure that occur during protein evolution and func- tional diversification. Global alignments of complete protein coding regions often fail when functional units (such as regulatory regions, transmembrane domains, DNA-binding domains, protein-protein interaction do- mains, etc.) are gained or lost over evolutionary time. This issue complicates the otherwise straightforward phylogenetic analysis of related proteins. Additional complications arise in organismal surveys in which en- tire genomes are sequenced and annotated by automated algorithms based on homology. BLAST and FASTA searches find protein regions with high local identity, which can suggest functional similarity. However, out- side of these identified regions, major changes can occur in protein function that obscure identity and make global protein alignment difficult or impossible. Without cor- responding functional data, these genes are often named inconsistently. For example, a gene found in one species with locally high homology to a protein region in an- other species is often named “Similar to…” or “Species X homologue of Species Y protein”. Because BLAST is a local homology search tool, it is inadequate for identi- fication and annotation of species orthologs. For this reason, many of the genes identified in species genome projects are insufficiently annotated; phylogenetic analysis of all proteins labeled as “Phosphatidylinositol transfer proteins, Beta” for example will yield a spurious  G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE 75 alignment and therefore a misleading tree. As demon- strated in this study, analysis of the PITP/rdgB family was complicated by both issues. It should be noted that full-length sequences produce similar phylogenetic trees for alignments of many related sets of proteins (all Class I or Class II proteins, for example). The resultant phylogenetic tree reveals that the gen- eral naming convention for the PITP family needs to be critically examined. Many of the proteins in this family cannot be named exclusively by local sequence identity, as their functions appear to be significantly altered from previously studied proteins. Many annotated genes are named in a way that might not be truly indicative of their function. In other words, sequence homology in local segments has obscured the greater functional diversifica- tion within this family. For example, although the mem- brane-associated proteins (Class IIB) are shown to be derived and monocladistic, the tree root appears to be within the PITP family, suggesting that many other func- tions are derived characteristics from this single-domain protein. At best, this tree demonstrates the need for bet- ter functional and structural classification of PITP-like proteins before final assignment of their names. An additional feature of the strict consensus tree is the hypothesis it suggests about the evolution of PITP pro- tein family members. First, no PITP proteins are found within prokaryotes or within yeast. Yeast have an analo- gous enzyme, Sec14p, but it appears to have an inde- pendent evolutionary origin. The slime-mold and Plas- modium sequences represent the most primitive species in this PITP family tree. In contrast, the multiplication of forms within mammals is impressive. Humans, mice, and rats have well-characterized genomes and each have a variety of PITP family-member proteins. 4.3. Conserved Sequence Motifs The use of conserved sequence motifs in a cladistic analysis of the PITP family highlights several regions for consideration. Two regions are of particular interest: one containing sequence motifs 7, 8, and/or 9, and the other containing sequence motifs 11, 12, or 13. These two regions form loops at the surface of the protein near the ends of the acyl chains of the bound phospholipid (Fig- ure 2(b) and 2(c)). Sequence motif 7 is the only motif present that is not found in the same primary sequence space in the three main protein classes. In human PITP, the amino acid sequence is at residues 66-73, while it occurs at residues 204-211 and 198-205 in human PITPNM1 and PITPNC1, respectively. Sequence motif 8 is not present in class I PITPs, and partially overlaps with sequence motif 9, which is exclusive to the al- pha/beta clades of class I. Sequence motif 7 in the class I PITP's partially overlaps with sequence motif 8 of class IIA and IIB. Sequence motif 7 contains the putative helix insertion loop proposed to anchor PITP to the mem- brane during lipid exchange [25]. It is tempting to specu- late that this helix has adapted a different conformation or perhaps has migrated across the lipid-binding cavity to near residues 200-208 relative to the PITP struc- tures. The second region of interest contains sequence mo- tifs 11, 12, or 13. These three motifs are present in ap- proximately the same space in primary sequence, but each is unique to the three family classes. Sequence mo- tif 11 is unique to class IIB, whereas Sequence motif 12 is unique to class IIA, and sequence motif 13 is unique to class I. The role of this loop in the structure or the function of any class of PITP is currently unexplored. A concern with the novel phylogenetic analysis tech- nique used in this study is the proportion of the sequence data actually used in the evolutionary analysis and whether amino acids that have been structurally and functionally shown to affect phospholipid transfer and signal transduction capabilities are included in the alignment matrices. In human PITP, 214 of the 270 amino acids (79.3%) are included in the conserved se- quence motifs. Most site-directed mutagenic analysis of the role of specific residues in the phospholipid transfer, specificity, and PLC reconstitution capabilities have been done on human or rat PITP and PITP. Residues in rat PITP that have been studied and shown to play a functional role in PITPs include Thr59, Lys61, Glu86, Asn90, Tyr103, and the double Trp pair at 203-204 [37,38]. These residues are all in conserved sequence motifs. Thr59 and Lys 61 map to sequence motif 6, which is the only sequence motif present in all se- quences in this study. Interestingly, sequence motif 9, containing Glu 86, is present only in Class I proteins. Tyr103 has been shown to diminish PLC reconstitution without affecting phospholipid transfer. This residue is a Tyr only in the Class I proteins, and is a conservative substitution to a Phe in the class IIA and class IIB pro- teins. Examination of these amino acids and sequence motifs from an evolutionary perspective enables a more thorough understanding of their importance in shaping PITP function and structure. 4.4. Functional Specialization across Evolution Functional shifts within the PITP family are difficult to assess in light of the relative paucity of experimental analysis of different family members. In rat, PITPs are broadly expressed and have been detected in at lease 20 tissues [39]. Unigene expression profiles (http://www. ncbi.nlm.nih.gov/entrez/query.fcgi?db=unigene) for ma- mmalian species indicate a slightly more general and ubiquitous expression for the alpha isoform than the beta isoform. Mouse PITP -/- embryonic stem cells are em- bryonically lethal, indicating essential functions in cell viability [29]. In contrast, mice deficient in PITP de- velop normally to term, but fail to thrive neonatally [30].  76 G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 JBiSE These observations and the EST expression profiles support the argument that PITP isoforms are not redun- dant, and have different functions [29]. One implication of the consensus tree is that, given that the ancestral proteins have putative roles in cellular function, e.g., PITP2 in Dictyostelium, they may be more widely ex- pressed than the more derived family members, which appear to have roles only in specific tissues or at specific developmental times. PITPnm1 in mice, for example, is highly expressed primarily in the retina, with weaker expression in the brain and some suggestion (from rat data) of central nervous system expression. Other studies in humans showed PITPnm2 and PITPnm3 expression in brain and retina. The phylogenetic analysis described here has demon- strated the utility of the use of conserved sequence mo- tifs in detecting evolutionary relationships among pro- teins in large and/or diverse functions. This method uses short conserved sequence motifs instead of single char- acter amino acids. The presence and absence of motifs becomes an important data point. The use of sequence motifs in phylogenetic analysis as described here should be applicable to the analysis of a wide range of protein families, particularly large, diverse families where re- shuffling of domains occurred over evolutionary time. 5. ACKNOWLEDGEMENTS The authors wish to acknowledge Lee Likins, Christine Malcom, Justin Paschall, and Ming Yang for comments and assistance. Funding for this study was provided in part by a University of Missouri Research Board grant and NIH R15HD055668-01A1 (to GJW). REFERENCES [1] Allen-Baume, V., Segui, B. and Cockcroft, S. (2002) Current thoughts on the phosphatidylinositol transfer protein family. FEBS Letters, 531, 74-80. [2] Cockcroft, S. and Carvou, N. (2007) Biochemical and biological functions of class I phosphatidylinositol trans- fer proteins. Biochim. Biophys. Acta, 1771, 677-691. [3] Hsuan, J. and Cockcroft, S. (2001) The PITP family of phosphatidylinositol transfer proteins. Genome Biol., 2, 3011.1-3011.8. [4] Routt, S.M. and Bankaitis, V.A. (2004) Biological func- tions of phosphatidylinositol transfer proteins. Biochem. Cell Biology, 82, 254-262. [5] Thomas, G.M.H. and Pinxteren, J.A. (2000) Phosphati- dylinositol transfer proteins: One big happy family or strangers with the same name? Mol. Cell Biol. Res. Comm., 4, 1-9. [6] Wirtz, K.W.A. (2006) Phospholipid transfer proteins in perspective. FEBS Lett., 580, 5436-5441. [7] Trivedi, D. and Padinjat, R. (2007) RdgB proteins: func- tions in lipid homeostasis and signal transduction. Bio- chim. Biophys. Acta, 1771, 692-699. [8] Loewen, C.J., Roy, A. and Levine, T.P. (2003) A con- served ER targeting motif in three families of lipid bind- ing proteins and in Opi1p binds VAP. EMBO J. 22, 2025-2035. [9] Lev, S., Hernandez, J., Martinez, R., Chen, A., Plowman, G. and Schlessinger, J. (1999) Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mole. Cell. Biol., 19, 2278-2288. [10] Milligan, S.C., Alb, J.G. Jr., Elagina, R. B., Bankaitis, V. A. and Hyde, D.R. (1997) The phosphatidylinositol transfer protein domain of Drosophila retinal degenera- tion B protein is essential for photoreceptor cell survival and recovery from light stimulation. J. Cell Biol., 139, 351-363. [11] Vihtelic, T.S., Goebl, M., Milligan, S., O'Tousa, J.E. and Hyde, D.R. (1993) Localization of Drosophila retinal degeneration B, a membrane associated phosphatidy- linositol transfer protein. J. Cell Biol., 122, 1013-1022. [12] Lu, C., Vihtelic, T.S., Hyde, D.R. and Li, T. (1999) A neuronal-specific mammalian homolog of the Drosophila retinal degeneration B gene with expression restricted to the retina and dentate gyrus. J. Neurosci., 19, 7317-7325. [13] Fullwood, Y., dos Santos, M. and Hsuan, J.J. (1999) Cloning and characterization of a novel human phos- phatidylinositol transfer protein, rdgBb. J. Biol. Chem., 274, 31553-31558. [14] Takano, N., Owada, Y., Suzuki, R., Sakagami, H., Shi- mosegawa, T. and Kondo, H. (2003) Cloning and char- acterization of a novel variant (mM-rdgB b1) of mouse M-rdgBs, mammalian homologs of Drosophila retinal degeneration B gene proteins, and its mRNA localization in mouse brain in comparison with other M-rdgBs. J Neurochem, 84, 829-839. [15] Skinner, H.B., Alb ,J.G.Jr., Whitters, E.A., Helmkamp, G. M.Jr. and Bankaiitis V. A. (1993) Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO, 12, 4775-4784. [16] Tanaka, S. and Hosaka, K. (1994) Cloning of a cDNA encoding a second phosphatidylinositol transfer protein of rat brain by complementation of the yeast sec14 muta- tion. J. Biochem (Tokyo), 115, 981-984. [17] Cunningham, E., Tan, S.K., Swigart, P., Hsuan, J., Bankaitis, V. and Cockcroft, S. (1996) The yeast and mammalian isoforms of phosphatidylinositol transfer protein can all restore phospholipase C-mediated inositol lipid signaling in cytosol-depleted RBL-2H3 and HL-60 cells. Proc. Natl. Acad. Sci. USA, 93, 6589-6593. [18] Hay, J.C. and Martin, T.F.J. (1993) Phosphatidylinositol transfer protein is required for ATP-dependent priming of Ca2+-activated secretion. Nature, 366, 572-575 [19] Jones, S.M., Alb, J.G.J., Phillips, S. E., Bankaitis and V.A., Howell, K.E. (1998) A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J. Biol. Chem. 273, 10349-10354. [20] Ohashi, M., de Vries, K.J., Frank, R., Snoek, G., Bankaitis, V., Wirtz, K. and Huttner, W.B. (1995) A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature, 377, 544-547. [21] Swigart, P., Insall, R., Wilkins, A. and Cockcroft, S. (2000) Purification and cloning of phosphatidylinositol transfer proteins from Dictyostelium discoideum: homo-  G. J. Wyckoff et al. / J. Biomedical Science and Engineering 3 (2010) 65-77 SciRes Copyright © 2010 77 JBiSE logues of both mammalian PITPs and Saccharomyces cerevisiae Sec14p are found in the same cell. Biochem. J., 347, 837-843. [22] Yoder, M.D., Thomas, L.M., Tremblay, J.M., Oliver, R. L., Yarbrough, L.R. and Helmkamp, G.M.Jr. (2001) Structure of a multifunctional protein: mammalian phos- phatidylinositol transfer protein complexed with phos- phatidylcholine. J. Biol. Chem., 276, 9246-9252. [23] Vordtriede, P.B, Doan, C.N., Tremblay, J.M., Helmkamp, G.M.J. and Yoder, M.D. (2005) Structure of PITPb in complex with phosphatidylcholine: Comparison of struc- ture and lipid transfer to other PITP isoforms. Biochem- istry, 44, 14760-14771. [24] van Tiel, C.M., Schouten, A., Snoek, G.T., Gros, P., Wirtz, K.W.A. (2002) The structure of phosphatidylinositol transfer protein a reveals sites for phospholipid binding and membrane association with major implications for its function. FEBS, 531, 69-73. [25] Schouten, A., Agianian, B., Westerman, J., Kroon, J., Wirtz, K.W.A. and Gros, P. (2002) Structure of apo- phosphatidylinositol transfer protein a provides insight into membrane association. EMBO J., 21, 2117-2121. [26] Sha, B., Phillips, S.E., Bankaitis, V.A. and Luo, M. (1998) Crystal structure of the Saccharomyces cerevisiae phos- phatidylinositol transfer protein. Nature, 391, 506-510. [27] Romanowski, M.J., Soccio, R.E., Breslow, J.L. and Bur- ley, S.K. (2002) Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) contain- ing a StAR-related lipid transfer domain. Proc. Natl. Acad. Sci. USA, 99, 6949-6954. [28] Hamilton, B.A., Smith, D.J., Mueller, K.L., Kerrebrock, A.W., Bronson, R.T., van Berkel, V., Daly, M.J., Kruglyak, L, Reeve, M.P., Nemhauser, J.L., Hawkins, T. L. Rubin, E.M. and Lander, E.S. (1997) The vibrator mutation causes neurodegeneration via reduced expres- sion of PITPa: positional complementation cloning and extragenic suppression. Neuron, 18, 711-722. [29] Alb, J.G.Jr., Phillips, S.E., Rostand, K., Cui, X., Pinx- teren, J., Cotlin, L., Manning, T, Guo, S, York, J.D., Sontheimer, H., Collawn, J.F., Bankaitis, V.A. (2002) Genetic ablation of phosphatidylinositol transfer protein function in murine embryonic stem cells. Mol. Biol. Cell, 13, 739-754. [30] Alb, J.G.J., Cortese, J.D, Phillips, S.E., Albin, R.L., Nagy, T.R., Hamilton, B.A. and Bankaitis, V.A. (2003) Mice lacking phosphatidylinositol transfer protein-a exhibit spinocerebellar degeneration, intestinal and hepatic stea- tosis, and hypoglycemia. J. Biol. Chem. 278, 33501- 33518. [31] Altschul, S.F., Gish, W., Miller, W., Myers, E.W., Lipman, D.J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403-410. [32] Bailey, T.L. and Elkan, C.P. (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. AAAI Press, Menlo Park, CA, 28-36. [33] Accelrys, I. (2005) Wisconsin Package v.10.3. Accelrys, Inc, San Diego, CA. [34] Swofford, D.L. (2003) PAUP*. Phylogenetic analysis using parsimony and other methods. Version 4. Sinauer Associates, Sunderland, Massachusetts. [35] Maddison, D.R. and Maddison, W.P. (2005) MacClade v.4.08. Sinauer Associates, Sunderland, Massachusetts. [36] Ocaka, L., Spalluto, C., Wilson, D.I, Hunt, D.M., Halford, S. (2005) Chromosomal localization, genomic organiza- tion and evolution of the genes encoding human phos- phatidylinositol transfer protein membrane- associated (PITPNM) 1, 2 and 3. Cytogenet Genome Res., 108, 293-202. [37] Alb,J.G. Jr., Gedvilaite, A., Cartee, R.T., Skinner, H.B., Bankaitis, V.A. (1995) Mutant rat phosphatidylinosi- tol/phosphatidylcholine transfer proteins specifically de- fective in phosphatidylinositol transfer: Implications for the regulation of phospholipid transfer activity. Proc. Natl. Acad. Sci. USA, 92, 8826-8830. [38] Tilley, S.J., Skippen, A., Murray-Rust, J., Swigart, P.M., Stewart, A., Morgan, C.P., Cockcroft, S. and McDonald, N.Q. (2004) Structure-function analysis of phosphatidy- linositol transfer protein alpha bound to human phos- phatidylinositol. Structure, 12, 317-326. [39] Venuti, S.E. and Helmkamp, G.M.J. (1988) Tissue distri- bution, purification and characterization of rat phos- phatidylinositol transfer protein. Biochim Biophys Acta, 946, 119-128. [40] Lev, S. (2004) The role of the Nir/rdgB protein family in membrane trafficking and cytoskeleton remodeling. Exp. Cell Res., 297, 1-10. |

