Paper Menu >>
Journal Menu >>
 Possible roles of electrical synapse in temporal information processing: a computational study Possible roles of electrical synapse in temporal information processing: a computational study Xu-Long Wang, Xiao-Dong Jiang & Pei-Ji Liang Department of Biomedical Engineering, Shanghai Jiao Tong University. * Correspondence should be addressed to Pei-Ji Liang (pjliang@sjtu.edu.cn). and motor tasks. Neuroscientists roughly categorize ABSTRACT temporal information processing in the neural system into four different time scales: microseconds, millisec- Temporal information processing in the onds, seconds and circadian rhythm, which serve for range of tens to hundreds of milliseconds is different physiological functions and rely on differ- critical in many forms of sensory and motor ent neural mechanisms. The process within the scale tasks. However, little has been known about of millisecond is perhaps the most sophisticated and the neural mechanisms of temporal informa-the least well understood one among these categories. tion processing. Experimental observations Behavioral tasks with temporal information process- indicate that sensory neurons of the nervous ing falling within this scale include speech discrimi- system do not show selective response to nation in the auditory system, motion information temporal properties of external stimuli. On processing in the visual systems, and movement the other hand, temporal selective neurons in coordination in the motor system [1-3]. the cortex have been reported in many Information processing in neural systems normally species. Thus, processes which realize the consists of a number of successive stages. Neural temporal-to-spatial transformation of activities in a certain stage are mostly determined by neuronal activities might be required for neural activities of the preceding stages and our temporal information processing. In the perception of the world in the brain is based on the present study, we propose a computational spatio-temporal patterns of neuronal activities model to explore possible roles of electrical produced at sensory stages [4-5]. Physiological synapses in processing the duration of observations indicate that neurons in the sensory external stimuli. Firstly, we construct a levels do not respond selectively to the temporal small-scale network with neurons intercon-properties of external stimuli. Temporal information nected by electrical synapses in addition to is thus suggested to be contained in the temporal chemical synapses. Basic properties of this patterns of neuronal activities in the sensory layer. small-scale neural network in processing On the other hand, neurons which show selective duration information are analyzed. Secondly, response to specific temporal properties, especially a large-scale neural network which is more the duration content, have been reported in the cortex biologically realistic is further explored. Our of many species [6-10]. Temporal information is results suggest that neural networks with therefore suggested to be transformed into the electrical synapses functioning together spatially distributed neuronal activities in the cortex with chemical synapses can effectively work and neural mechanisms which contribute to the for the temporal-to-spatial transformation of temporalto-spatial transformation of neuronal neuronal activities, and the spatially distrib-activities are required. uted sequential neural activities can poten-Electrical synapse is another type of widely tially represent temporal information.distributed neuronal connection in the neural systems in addition to chemical synapse [11-12]. Functional role of electrical synapse has been identified in fine motor coordination which requires temporal infor- mation processing in milliseconds scale [13]. In the 1. INTRODUCTIONpresent work, we try to explore possible neural Biological neural systems are endowed with the mechanisms of electrical synapse in processing the ability to process temporal information given the duration content of external stimuli via computa- inherent temporal nature of sensory environments tional approach. Briefly, we construct neural net- Keywords: Model; Neural network; Electrical synapse; Temporal information processing J. Biomedical Science and Engineering, 2008, 1, 27-36Scientific Research Publishing JBiSE Published Online May 2008 in SciRes. http://www.srpublishing.org/journal/jbise SciRes Copyright © 2008 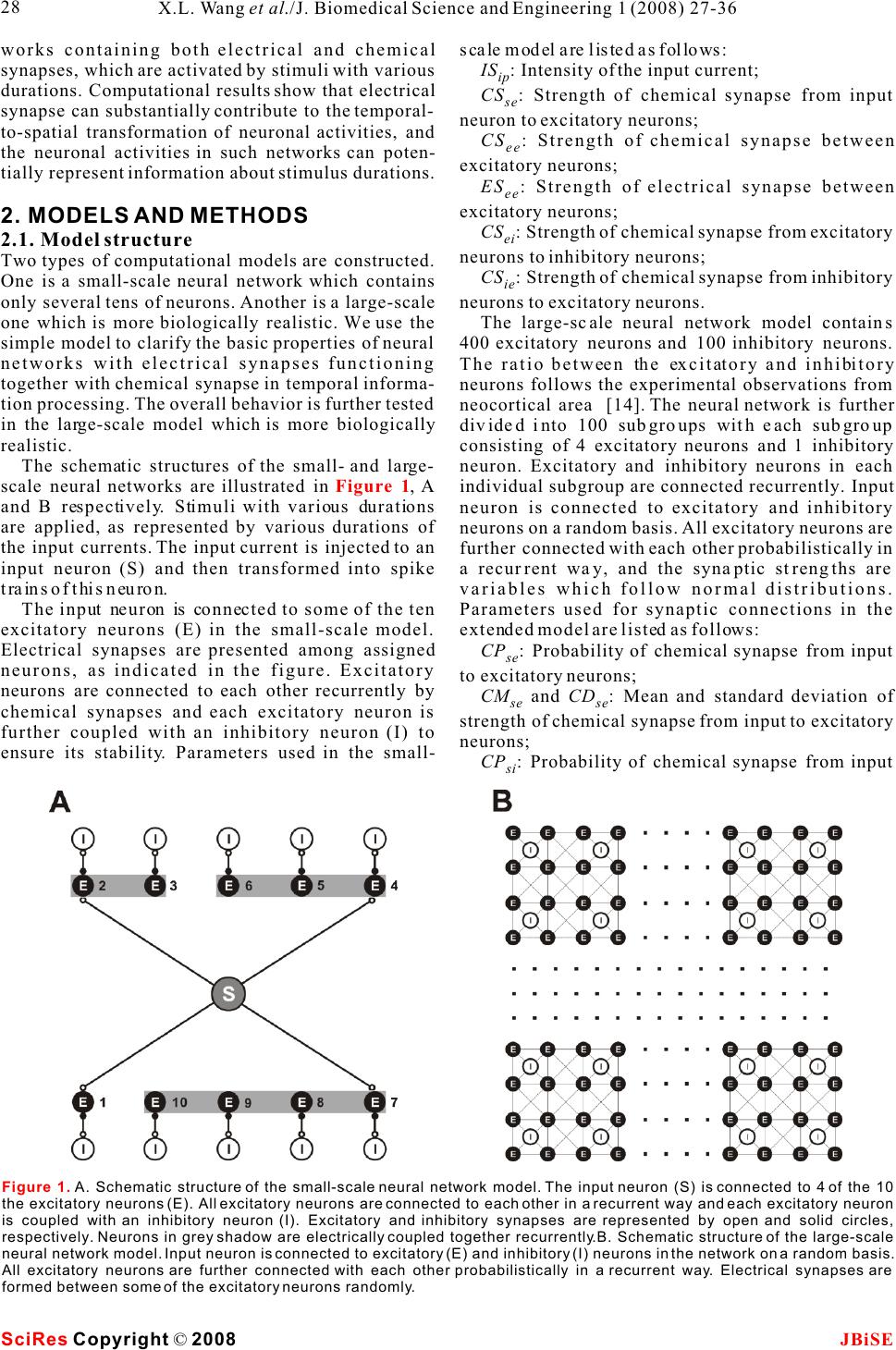 works containing both electrical and chemical scale model are listed as follows: synapses, which are activated by stimuli with various IS : Intensity of the input current; ip durations. Computational results show that electrical CS : Strength of chemical synapse from input se synapse can substantially contribute to the temporal-neuron to excitatory neurons; to-spatial transformation of neuronal activities, and CS : Strength of chemical synapse between ee the neuronal activities in such networks can poten-excitatory neurons; tially represent information about stimulus durations.ES: Strength of electrical synapse between ee excitatory neurons; 2. MODELS AND METHODSCS : Strength of chemical synapse from excitatory ei 2.1. Model structureneurons to inhibitory neurons; Two types of computational models are constructed. CS : Strength of chemical synapse from inhibitory One is a small-scale neural network which contains ie only several tens of neurons. Another is a large-scale neurons to excitatory neurons. one which is more biologically realistic. We use the The large-scale neural network model contains simple model to clarify the basic properties of neural 400 excitatory neurons and 100 inhibitory neurons. networks with electrical synapses functioning The ratio between the excitatory and inhibitory together with chemical synapse in temporal informa-neurons follows the experimental observations from tion processing. The overall behavior is further tested neocortical area [14]. The neural network is further in the large-scale model which is more biologically divided into 100 subgroups with each subgroup realistic. consisting of 4 excitatory neurons and 1 inhibitory The schematic structures of the small- and large-neuron. Excitatory and inhibitory neurons in each scale neural networks are illustrated in , A individual subgroup are connected recurrently. Input and B respectively. Stimuli with various durations neuron is connected to excitatory and inhibitory are applied, as represented by various durations of neurons on a random basis. All excitatory neurons are the input currents. The input current is injected to an further connected with each other probabilistically in input neuron (S) and then transformed into spike a recurrent way, and the synaptic strengths are trains of this neuron.variables which follow normal distributions. The input neuron is connected to some of the ten Parameters used for synaptic connections in the excitatory neurons (E) in the small-scale model. extended model are listed as follows: Electrical synapses are presented among assigned CP: Probability of chemical synapse from input se neurons, as indicated in the figure. Excitatory to excitatory neurons; neurons are connected to each other recurrently by CM and CD: Mean and standard deviation of se se chemical synapses and each excitatory neuron is strength of chemical synapse from input to excitatory further coupled with an inhibitory neuron (I) to neurons; ensure its stability. Parameters used in the small-CP : Probability of chemical synapse from input si Figure 1 Figure 1. A. Schematic structure of the small-scale neural network model. The input neuron (S) is connected to 4 of the 10 the excitatory neurons (E). All excitatory neurons are connected to each other in a recurrent way and each excitatory neuron is coupled with an inhibitory neuron (I). Excitatory and inhibitory synapses are represented by open and solid circles, respectively. Neurons in grey shadow are electrically coupled together recurrently.B. Schematic structure of the large-scale neural network model. Input neuron is connected to excitatory (E) and inhibitory (I) neurons in the network on a random basis. All excitatory neurons are further connected with each other probabilistically in a recurrent way. Electrical synapses are formed between some of the excitatory neurons randomly. SciRes JBiSE Copyright © 2008 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 28  to inhibitory neurons;potentials of excitatory and inhibitory synapses, CM and CD : Mean and standard deviation of respectively; si si I represents the current passing through strength of chemical synapse from input to inhibitory esyn neurons;electrical synapses. CP : Probability of chemical synapse between In addition, when the membrane potential reaches ee a threshold (V), the neuron fires an action potential, excitatory neurons;th CM and CD: Mean and standard deviation of and the membrane potential is immediately reset to ee eethe equilibrium potential (V) after a firing lasting strength of chemical synapse between excitatory eq neurons;time (T). fire CM and CD : Mean and standard deviation of Parameter values chosen for the I-F neuron model ei ei strength of chemical synapse from excitatory to are listed in . These values are mostly adopted inhibitory neurons;from Troyer and Miller (1997) [15], except that the CM and CD : Mean and standard deviation of firing lasting time of inhibitory neurons is chosen as ie ie4 to ensure the neurons' inhibitory effect on the strength of chemical synapse from inhibitory to activities of excitatory neurons. excitatory neurons; EP : Probability of electrical connection ee12.2.2 Description of synaptic current between excitatory neurons within one subgroup;The chemical synapses are modeled as follows [16- EP : Probability of electrical connection ee217]: between excitatory neurons in different subgroups; EM and ED: Mean and standard deviation of ee ee strength of electrical synapse between excitatory neurons. where g(t) and g(t) in eqns (2) and (3) are ex in . presented by g(t)g(t) here, with g representing 2.2. Mathematical description of neurons and csyn csyn synaptic strength which is modified by a factor of g(t): synapses 2.2.1 Description of integrate-and-fire neuron Neurons are described in an integrate-and-fire manner (I-F neuron) [5]. Membrane potential of the input neuron (V), excitatory neuron (V), and where sEx inhibitory neuron (V) can be determined as follows: In in which =15 ms , E= - 40 mV, and(u) syn thr follows a step function: The electrical synapses are described as follows: Where g represents the synaptic strength. We csyn whereadopt this abstract function which simply depicts that C represents the membrane capacitance; the current passing through the electrical synapses is V denotes the equilibrium membrane potential; eq generally dependent on the membrane potential g is the leak conductance; leak difference between the pre-synaptic and post- g and g represent the conductance of excitatory synaptic neurons [18]. ex in and inhibitory synapses, respectively; E and E represent the reversal membrane 3. RESULTS ex in Table 1 (2) (3) (1) (4) (5) (6) (7) SciRes JBiSE Copyright © 2008 29 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 Table 1. Parameter values for the I-F neuron model. The firing lasting time (T) for sensory and excitatory neurons is set as fire 1.75 ms whereas that for inhibitory neuron is set as 4 ms. C (pF) 0.5 Veq (mV) -74 Vth (mV) -54 gleak (S) 0.025 Eex (mV) 0 Ein mV) -74 Tfire (ms) 1.75/4 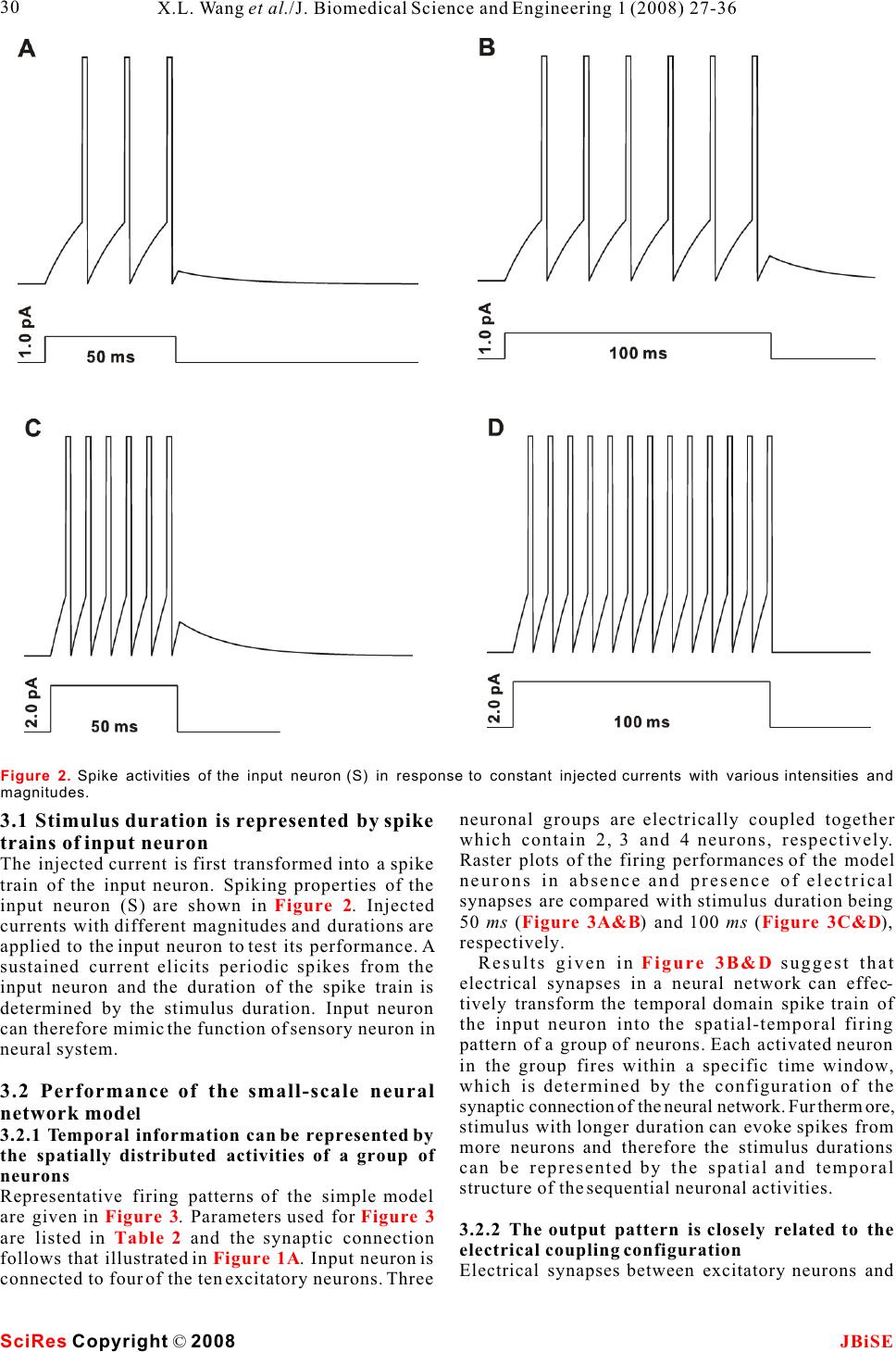 neuronal groups are electrically coupled together 3.1 Stimulus duration is represented by spike which contain 2, 3 and 4 neurons, respectively. trains of input neuronRaster plots of the firing performances of the model The injected current is first transformed into a spike neurons in absence and presence of electrical train of the input neuron. Spiking properties of the synapses are compared with stimulus duration being input neuron (S) are shown in . Injected 50 ms () and 100 ms (), currents with different magnitudes and durations are respectively. applied to the input neuron to test its performance. A Results given in suggest that sustained current elicits periodic spikes from the electrical synapses in a neural network can effec- input neuron and the duration of the spike train is tively transform the temporal domain spike train of determined by the stimulus duration. Input neuron the input neuron into the spatial-temporal firing can therefore mimic the function of sensory neuron in pattern of a group of neurons. Each activated neuron neural system.in the group fires within a specific time window, which is determined by the configuration of the 3.2 Performance of the small-scale neural synaptic connection of the neural network. Furthermore, network modelstimulus with longer duration can evoke spikes from 3.2.1 Temporal information can be represented by more neurons and therefore the stimulus durations the spatially distributed activities of a group of can be represented by the spatial and temporal neurons structure of the sequential neuronal activities. Representative firing patterns of the simple model are given in . Parameters used for 3.2.2 The output pattern is closely related to the are listed in and the synaptic connection electrical coupling configuration follows that illustrated in . Input neuron is Electrical synapses between excitatory neurons and connected to four of the ten excitatory neurons. Three Figure 2Figure 3A&BFigure 3C&D Figure 3B&D Figure 3Figure 3 Table 2 Figure 1A SciRes JBiSE Copyright © 2008 30 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 Figure 2. Spike activities of the input neuron (S) in response to constant injected currents with various intensities and magnitudes. 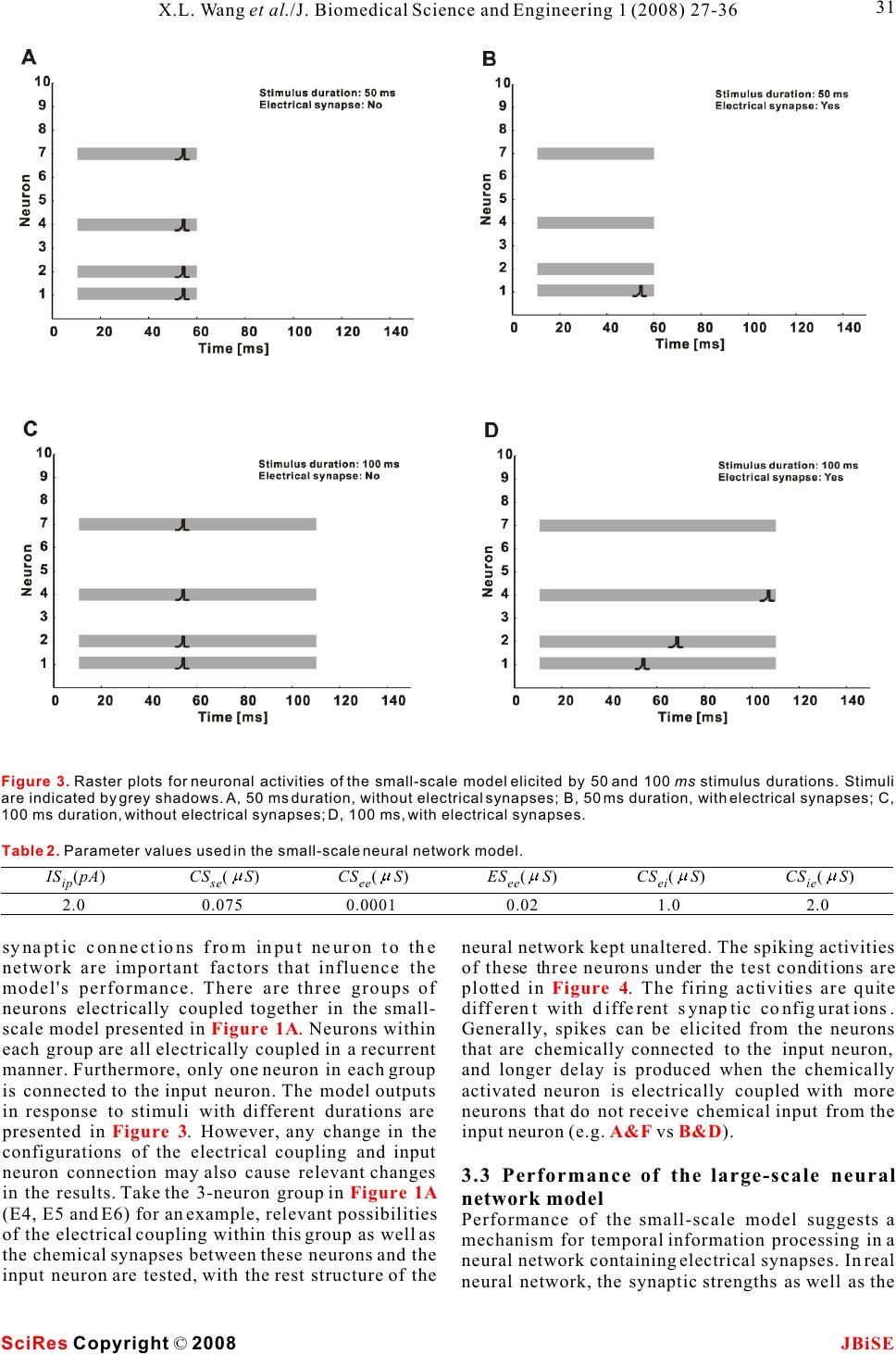 SciRes JBiSE Copyright © 2008 31 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 Figure 3. Raster plots for neuronal activities of the small-scale model elicited by 50 and 100 ms stimulus durations. Stimuli are indicated by grey shadows. A, 50 ms duration, without electrical synapses; B, 50 ms duration, with electrical synapses; C, 100 ms duration, without electrical synapses; D, 100 ms, with electrical synapses. Table 2. Parameter values used in the small-scale neural network model. IS(pA) ip 2.0 CS (S) se 0.075 CS (S) ee 0.0001 ES (S) ee 0.02 CS (S) ei 1.0 CS (S) ie 2.0 synaptic connections from input neuron to the neural network kept unaltered. The spiking activities network are important factors that influence the of these three neurons under the test conditions are model's performance. There are three groups of plotted in . The firing activities are quite neurons electrically coupled together in the small-different with different synaptic configurations. scale model presented in . Neurons within Generally, spikes can be elicited from the neurons each group are all electrically coupled in a recurrent that are chemically connected to the input neuron, manner. Furthermore, only one neuron in each group and longer delay is produced when the chemically is connected to the input neuron. The model outputs activated neuron is electrically coupled with more in response to stimuli with different durations are neurons that do not receive chemical input from the presented in . However, any change in the input neuron (e.g. vs ). configurations of the electrical coupling and input neuron connection may also cause relevant changes 3.3 Performance of the large-scale neural in the results. Take the 3-neuron group in network model (E4, E5 and E6) for an example, relevant possibilities Performance of the small-scale model suggests a of the electrical coupling within this group as well as mechanism for temporal information processing in a the chemical synapses between these neurons and the neural network containing electrical synapses. In real input neuron are tested, with the rest structure of the neural network, the synaptic strengths as well as the Figure 4 Figure 1A Figure 3A&FB&D Figure 1A 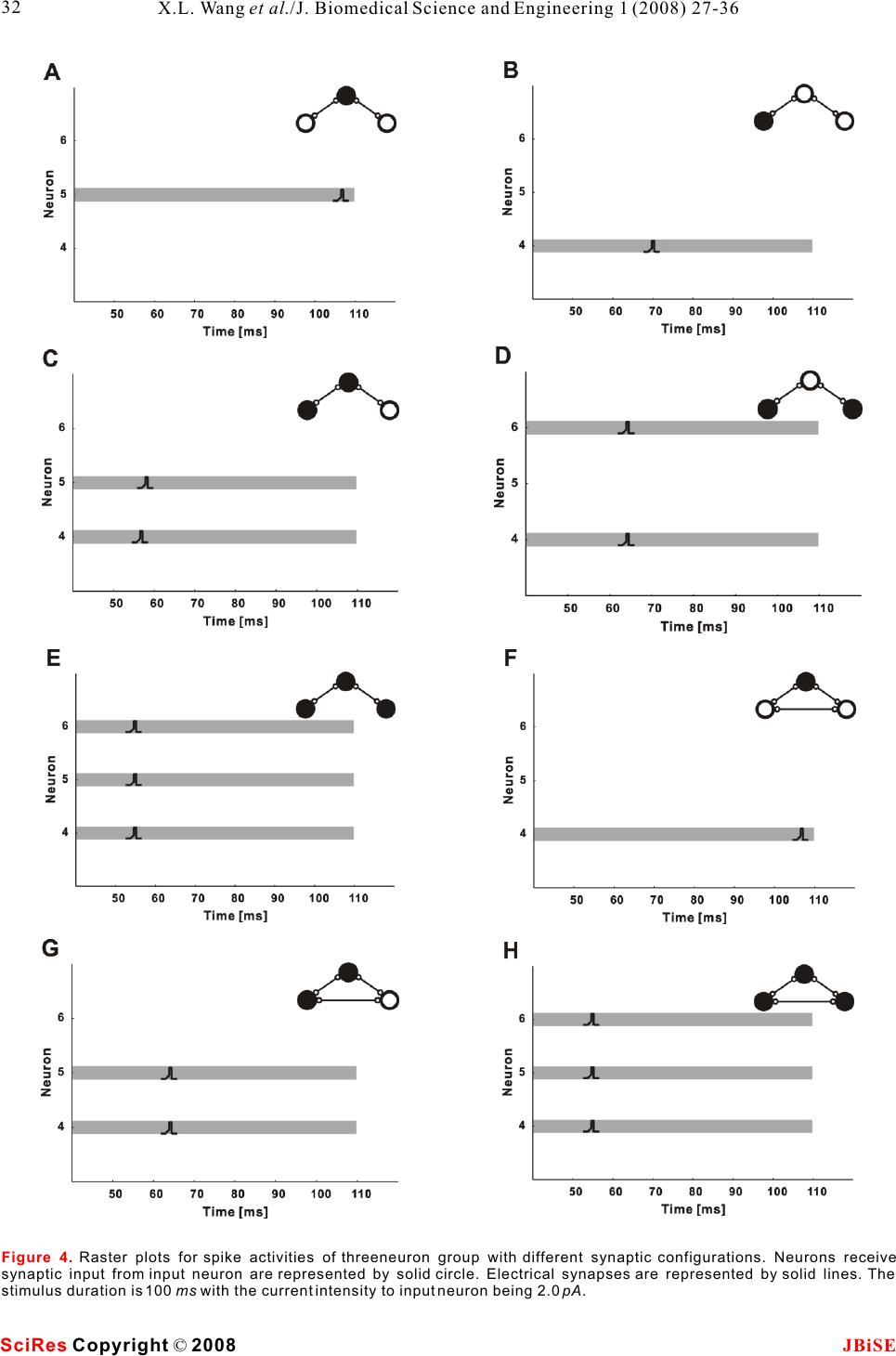 SciRes JBiSE Copyright © 2008 32 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 Figure 4. Raster plots for spike activities of threeneuron group with different synaptic configurations. Neurons receive synaptic input from input neuron are represented by solid circle. Electrical synapses are represented by solid lines. The stimulus duration is 100 ms with the current intensity to input neuron being 2.0 pA. 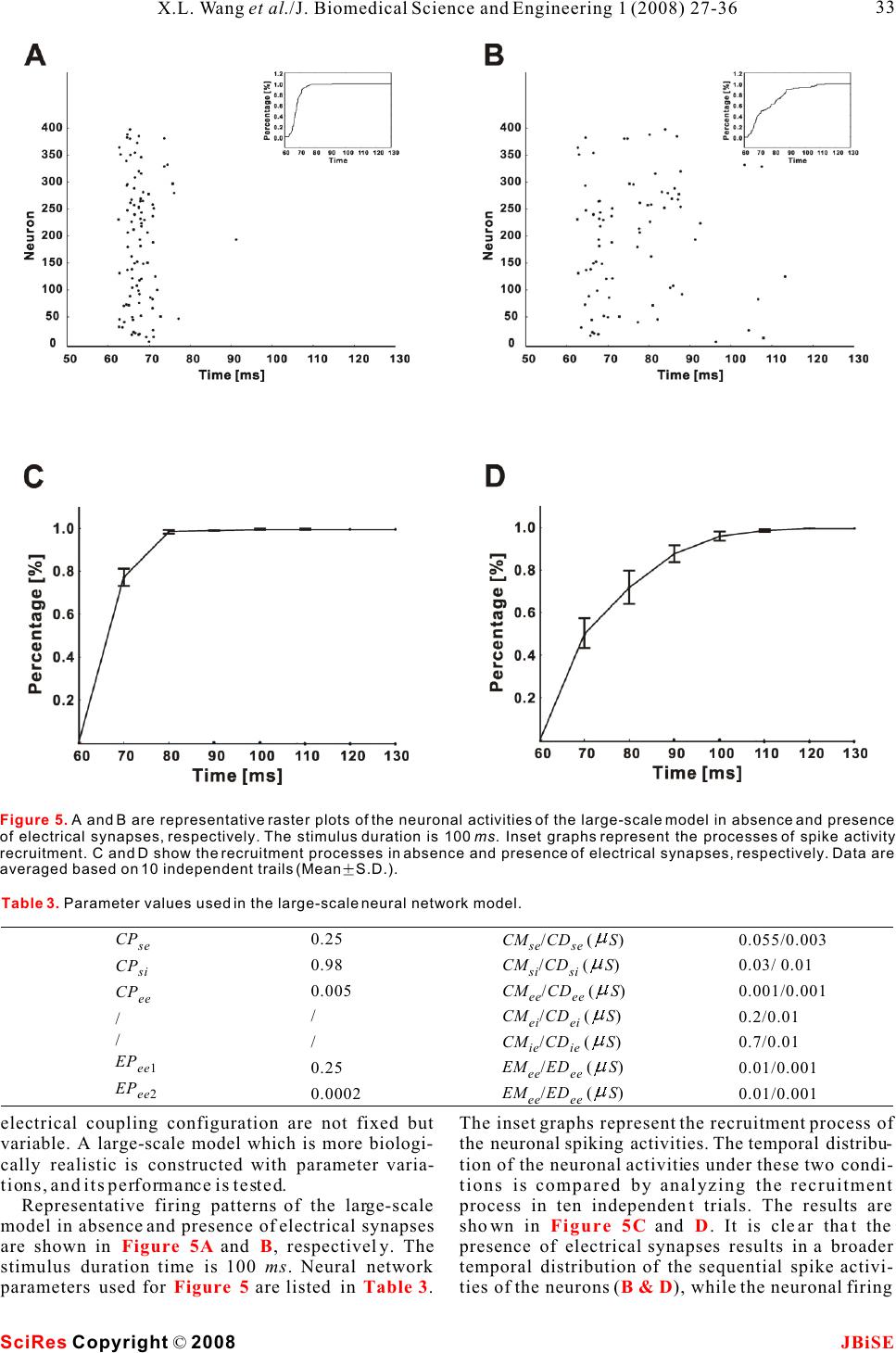 electrical coupling configuration are not fixed but The inset graphs represent the recruitment process of variable. A large-scale model which is more biologi-the neuronal spiking activities. The temporal distribu- cally realistic is constructed with parameter varia-tion of the neuronal activities under these two condi- tions, and its performance is tested.tions is compared by analyzing the recruitment Representative firing patterns of the large-scale process in ten independent trials. The results are model in absence and presence of electrical synapses shown in and . It is clear that the are shown in and , respectively. The presence of electrical synapses results in a broader stimulus duration time is 100 ms. Neural network temporal distribution of the sequential spike activi- parameters used for are listed in . ties of the neurons (), while the neuronal firing Figure 5CD Figure 5AB Figure 5Table 3B & D Figure 5. A and B are representative raster plots of the neuronal activities of the large-scale model in absence and presence of electrical synapses, respectively. The stimulus duration is 100 ms. Inset graphs represent the processes of spike activity recruitment. C and D show the recruitment processes in absence and presence of electrical synapses, respectively. Data are averaged based on 10 independent trails (MeanS.D.). SciRes JBiSE Copyright © 2008 33 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 Table 3. Parameter values used in the large-scale neural network model. CPse CPsi CPee / / EPee1 EPee2 0.25 0.98 0.005 / / 0.25 0.0002 CM /CD (S) se se CM /CD (S) si si CM /CD (S) ee ee CM /CD (S) ei ei CM /CD (S) ie ie EM /ED (S) ee ee EM /ED (S) ee ee 0.055/0.003 0.03/ 0.01 0.001/0.001 0.2/0.01 0.7/0.01 0.01/0.001 0.01/0.001 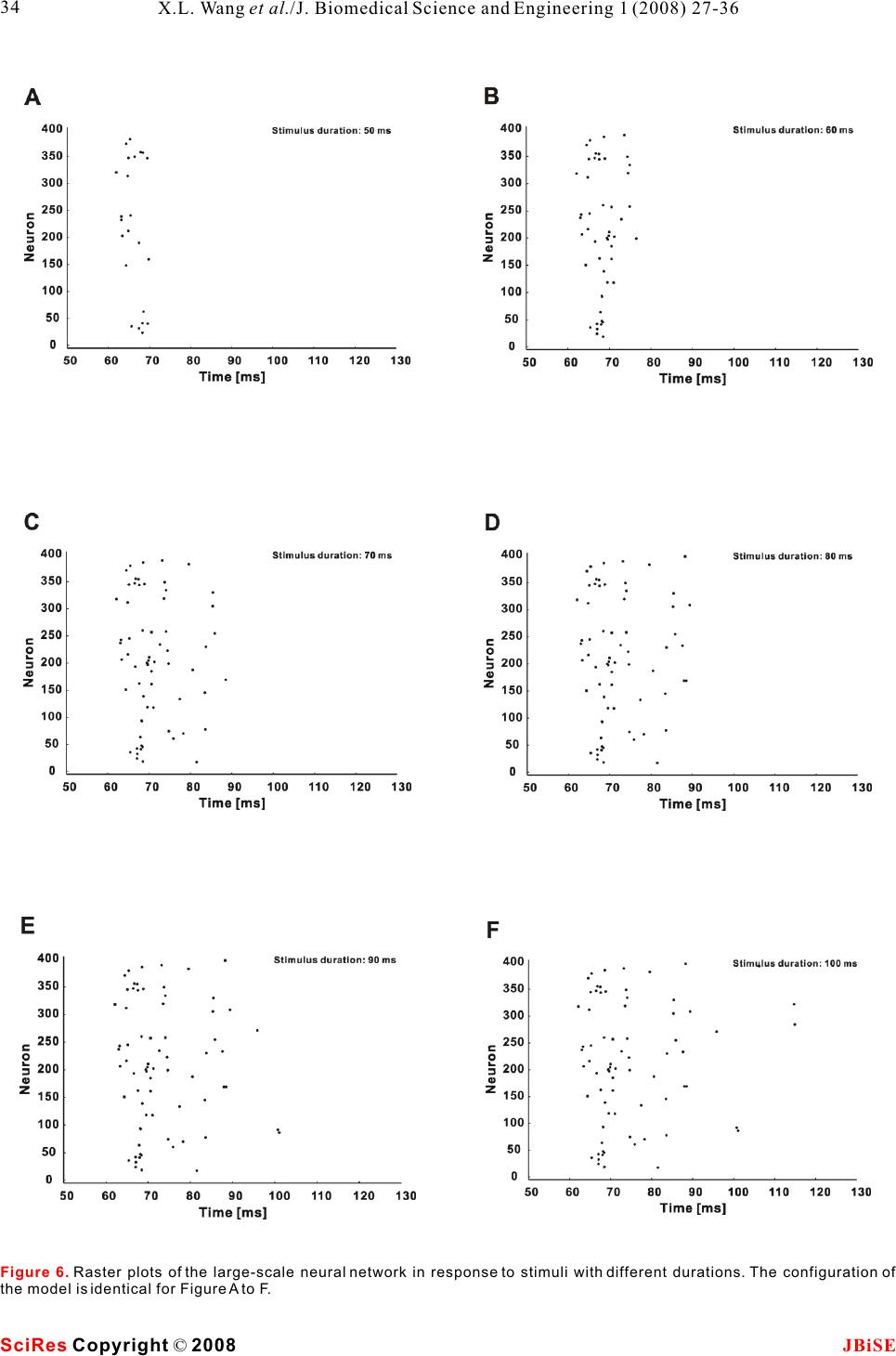 Figure 6. Raster plots of the large-scale neural network in response to stimuli with different durations. The configuration of the model is identical for Figure A to F. SciRes JBiSE Copyright © 2008 34 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 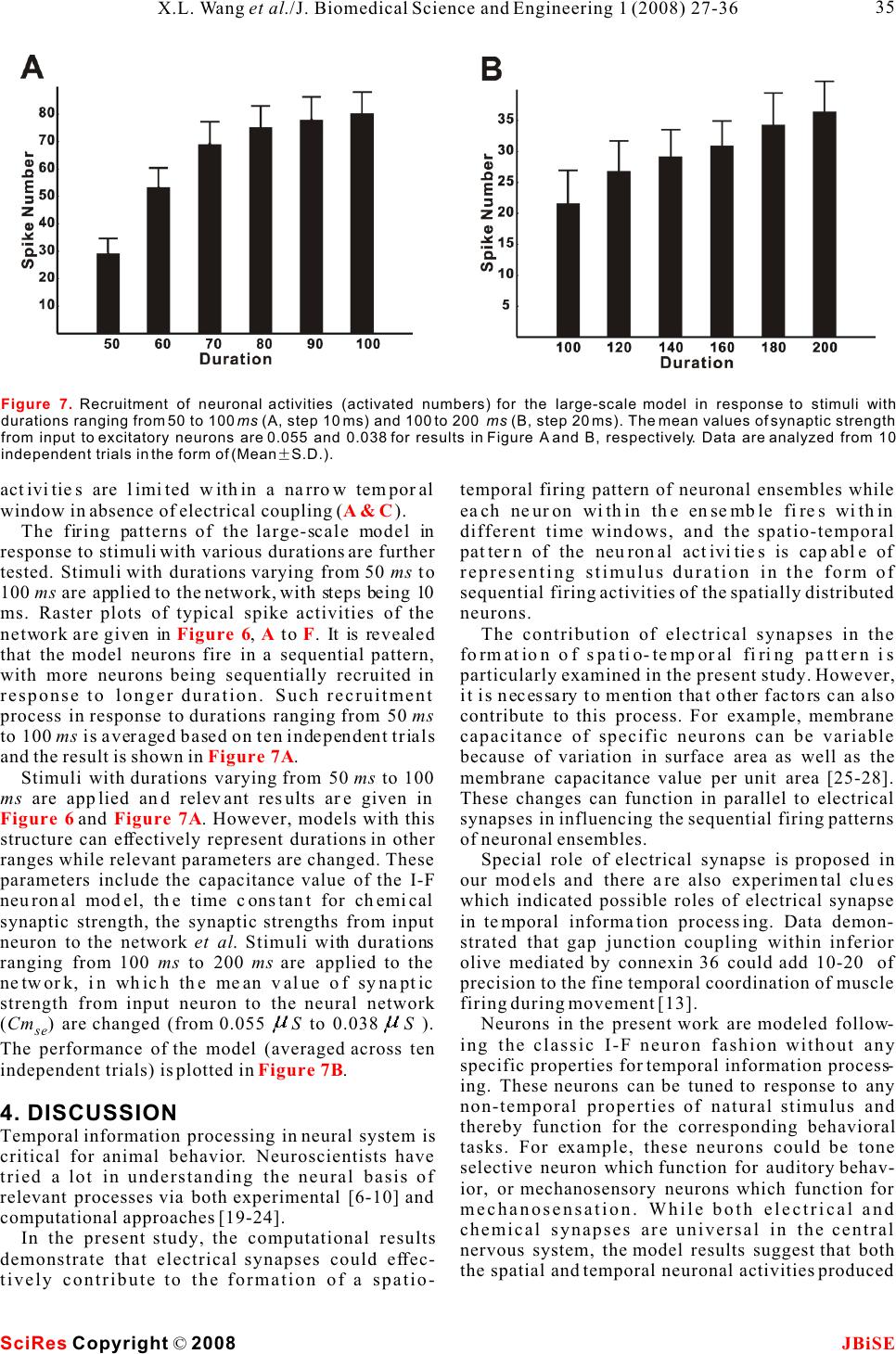 activities are limited within a narrow temporal temporal firing pattern of neuronal ensembles while window in absence of electrical coupling ().each neuron within the ensemble fires within The firing patterns of the large-scale model in different time windows, and the spatio-temporal response to stimuli with various durations are further pattern of the neuronal activities is capable of tested. Stimuli with durations varying from 50 ms to representing stimulus duration in the form of 100 ms are applied to the network, with steps being 10 sequential firing activities of the spatially distributed ms. Raster plots of typical spike activities of the neurons. network are given in , to . It is revealed The contribution of electrical synapses in the that the model neurons fire in a sequential pattern, formation of spatio-temporal firing pattern is with more neurons being sequentially recruited in particularly examined in the present study. However, response to longer duration. Such recruitment it is necessary to mention that other factors can also process in response to durations ranging from 50 ms contribute to this process. For example, membrane to 100 ms is averaged based on ten independent trials capacitance of specific neurons can be variable and the result is shown in.because of variation in surface area as well as the Stimuli with durations varying from 50 ms to 100 membrane capacitance value per unit area [25-28]. ms are applied and relevant results are given in These changes can function in parallel to electrical and . However, models with this synapses in influencing the sequential firing patterns structure can effectively represent durations in other of neuronal ensembles. ranges while relevant parameters are changed. These Special role of electrical synapse is proposed in parameters include the capacitance value of the I-F our models and there are also experimental clues neuronal model, the time constant for chemical which indicated possible roles of electrical synapse synaptic strength, the synaptic strengths from input in temporal information processing. Data demon- neuron to the network et al. Stimuli with durations strated that gap junction coupling within inferior ranging from 100 ms to 200 ms are applied to the olive mediated by connexin 36 could add 10-20 of network, in which the mean value of synaptic precision to the fine temporal coordination of muscle strength from input neuron to the neural network firing during movement [13]. (Cm) are changed (from 0.055 S to 0.038 S ). Neurons in the present work are modeled follow- se ing the classic I-F neuron fashion without any The performance of the model (averaged across ten specific properties for temporal information process- independent trials) is plotted in .ing. These neurons can be tuned to response to any non-temporal properties of natural stimulus and 4. DISCUSSIONthereby function for the corresponding behavioral Temporal information processing in neural system is tasks. For example, these neurons could be tone critical for animal behavior. Neuroscientists have selective neuron which function for auditory behav- tried a lot in understanding the neural basis of ior, or mechanosensory neurons which function for relevant processes via both experimental [6-10] and mechanosensation. While both electrical and computational approaches [19-24].chemical synapses are universal in the central In the present study, the computational results nervous system, the model results suggest that both demonstrate that electrical synapses could effec-the spatial and temporal neuronal activities produced tively contribute to the formation of a spatio- A & C Figure 6AF Figure 7A Figure 6Figure 7A Figure 7B Figure 7. Recruitment of neuronal activities (activated numbers) for the large-scale model in response to stimuli with durations ranging from 50 to 100 ms (A, step 10 ms) and 100 to 200 ms (B, step 20 ms). The mean values of synaptic strength from input to excitatory neurons are 0.055 and 0.038 for results in Figure A and B, respectively. Data are analyzed from 10 independent trials in the form of (MeanS.D.). SciRes JBiSE Copyright © 2008 35 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36  dependent plasticity. Phys Rev E Stat Nonlin Soft Matter Phys at the sensory layer of neural system could be 2003, 68:011908. processed together by sharing the same neural circuit. [22]Buonomano DV & Merzenich MM. Temporal information Temporal content of external stimulus could be read transformed into a spatial code by a neural network with out from spike patterns of neuronal ensembles in the realistic properties. Science 1995, 267:1028-1030. [23]Mauk MD & Donegan NH. A model of Pavlovian eyelid brain. conditioning based on the synaptic organization of the cerebellum. Learn Mem 1997, 4:130-158. [24]Medina JF, Garcia KS, Nores WL, Taylor NM & Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a ACKNOWLEDGEMENTS large-scale computer simulation. J Neurosci 2000, 20:5516- The authors would like to thank Dr. Shi-Yong Huang for helpful 5525. discussion. This work was supported by grants from the Hi-Tech [25]Chitwood RA, Hubbard A. & Jaffe DB. Passive electrotonic Research and Development Program of China (No. 2006AA01Z125).properties of rat hippocampal CA3 interneurones. J Physiol 1999, 515 ( Pt 3):743-756. [26]Gentet LJ, Stuart GJ & Clements JD. Direct measurement of REFERENCE specific membrane capacitance in neurons. Biophys J 2000, [1]Buonomano DV& Karmarkar UR. How do we tell time? 79:314-320. Neuroscientist 2002, 8:42-51.[27]Major G., Larkman AU, Jonas P., Sakmann B. & Jack JJ. [2]Mauk MD & Buonomano DV. The neural basis of temporal Detailed passive cable models of whole-cell recorded CA3 processing. Annu Rev Neurosci 2004, 27:307-340.pyramidal neurons in rat hippocampal slices. J Neurosci 1994, [3]Ivry RB & Spencer RM. The neural representation of time. Curr 14:4613-4638. Opin Neurobiol 2004, 14:225-232.[28]Thurbon D., Luscher HR, Hofstetter T. & Redman SJ. Passive [4]deCharms RC & Zador A.Neural representation and the cortical electrical properties of ventral horn neurons in rat spinal cord code. Annu Rev Neurosci 2000, 23:613-647.slices. J Neurophysiol 1998, 80:2485-2502. [5]Dayan, P. & Abbott, LF () Theoretical Neuroscience, MIT Press 2001. [6]Casseday JH, Ehrlich D & Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science 1994, 264:847-850. [7]Galazyuk AV & Feng AS. Encoding of sound duration by neurons in the auditory cortex of the little brown bat, Myotis lucifugus. J Comp Physiol 1997, [A] 180:301-311. [8]He J., Hashikawa T., Ojima H. & Kinouchi Y. () Temporal integration and duration tuning in the dorsal zone of cat auditory cortex. J Neurosci 1997, 17:2615-2625. [9]Ehrlich D, Casseday JH & Covey E. Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol 1997, 77:2360-2372. [10]Fremouw T, Faure PA, Casseday JH & Covey E. Duration selectivity of neurons in the inferior colliculus of the big brown bat: tolerance to changes in sound level. J Neurophysiol 2005, 94:1869-1878. [11]Connors BW & Long MA. Electrical synapses in the mamma- lian brain. Annu Rev Neurosci 2004, 27:393-418. [12]Sohl G., Maxeiner S. & Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci 2005, 6:191-200. [13]Placantonakis DG, Bukovsky AA, Zeng XH, Kiem HP & Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc Natl Acad Sci U S A 2004, 101:7164-7169. [14]Beaulieu C., Kisvarday Z., Somogyi P., Cynader M. & Cowey A. Quantitative distribution of GABA-immunopositive and - immunonegative neurons and synapses in the monkey striate cortex (area 17). Cereb Cortex 1992, 2:295-309. [15]Troyer TW & Miller KD. Physiological gain leads to high ISI variability in a simple model of a cortical regular spiking cell. Neural Comput 1997, 9:971-983. [16]Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol 1967, 30:1138-1168. [17]Nowotny T., Rabinovich MI, Huerta R. & Abarbanel HD. Decoding temporal information through slow lateral excitation in the olfactory system of insects. J Comput Neurosci 2003, 15:271-281. [18]Kopell N. & Ermentrout B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc Natl Acad Sci U S A 2004, 01:15482-15487. [19]Hooper SL, Buchman E. & Hobbs KH. A computational role for slow conductances: single-neuron models that measure duration. Nat Neurosci 2002, 5:552-556. [20]Buonomano DV. Decoding temporal information: A model based on short-term synaptic plasticity. J Neurosci 2000, 20:1129-1141. [21]Nowotny T, Rabinovich MI & Abarbanel HD. Spatial representation of temporal information through spike-timing- SciRes JBiSE Copyright © 2008 36 X.L. Wang et al./J. Biomedical Science and Engineering 1 (2008) 27-36 |

