Journal of Water Resource and Protection
Vol.6 No.2(2014), Article ID:43192,7 pages DOI:10.4236/jwarp.2014.62020
Chronic Alcohol Consumption and the Development of Skeletal Fluorosis in a Fluoride Endemic Area of the Ethiopian Rift Valley
Grarbet Tehadiso Mahber, Addis Ababa, Ethiopia
Email: redda@ethionet.et
Copyright © 2014 Redda Tekle-Haimanot, Gebeyehu Haile. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Redda Tekle-Haimanot, Gebeyehu Haile. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 25, 2013; revised January 19, 2014; accepted February 13, 2014
Keywords:Fluoride; Skeletal Fluorosis; Ethiopian Rift Valley; Alcohol Consumption
ABSTRACT
This study compared the occurrence of skeletal fluorosis in chronic consumers of locally brewed alcoholic beverages and their matched controls in the Ethiopian Rift Valley. The study revealed that chronic alcohol consumers developed severe forms of crippling skeletal fluorosis quite early in life. The controls were either symptom-free or exhibited mild forms of the fluorosis. The study showed that crippling skeletal fluorosis was directly associated with the large volumes of the locally brewed beer and honey-mead consumption on a daily basis. Chemical analysis of the alcoholic beverages showed that high concentration of fluoride which was much higher than the fluoride in the water was used for the brewing process. From this study one would conclude that in communities residing in high fluoride areas, there should be awareness creation campaigns to point out the relationship of excessive consumption of locally brewed alcoholic drinks and skeletal fluorosis. Regulations should also be put in place to require producers of local alcoholic beverages to use low fluoride water for brewing.
1. Introduction
The Ethiopian Rift valley, a part of the Great African Rift Valley, dissects the country from North to South. The Rift Valley is associated with high fluoride levels in ground waters where deep wells are the major source of drinking water supply. In the most extensive database on fluoride distribution in Ethiopian surface and ground water Tekle-Haimanot et al. [1] reported that 24.5% of the 1438 samples they analyzed had concentration above the 1.5 mg/L optimal concentration recommended by World Health Organization [2]. Regionally, by far the highest fluoride levels were recorded in the Rift Valley, where 33.3% of all samples collected from Rift Valley deep wells exceeded the 1.5% mg/L level [3]. The high fluoride drinking waters have led to the major public health problem of endemic fluorosis in the Rift Valley.
Studies have shown that the prevalence of dental fluorosis in the Ethiopian Rift Valley is above 80% [4-6]. It is often difficult to obtain accurate prevalence figures for skeletal fluorosis because of the big spectrum of the clinical manifestations of the condition which range from mild radiological evidences to that of crippling fluorosis, with or without neurological manifestations. However, available data put the prevalence of skeletal fluorosis in Ethiopia at 40% - 50% among the inhabitants of the high fluoride areas of the Rift Valley [5,7].
The area of the Ethiopian Rift Valley that first attracted the attention of epidemiologists to undertake studies on fluorosis is the Wonji/Shoa Sugar Estate which is situated 120 Km south-east of Addis Ababa. The estate, which was established in 1952, is located on a flat fertile area bordering the Awash River about 1500 m above sea level. The state-owned enterprise is primarily engaged in the production of sugar from sugar cane. The agro-industrial enterprise has two factories (Wonji and Shoa) and 14 plantation villages. At the periphery of the factory villages there are small towns, Gefersa, Kuruftu and Alem Tena. The total population of Wonji Shoa Sugar Estate is presently estimated to be 16,000 while the satellite towns outside the estate have inhabitants of 10,000 (Gefersa), 7000 (Kuruftu) and 7500 (Alem Tena).
Since the establishment of Wonji-Shoa, the inhabitants of all villages and the satellite towns have depended for their drinking water supply on deep wells with fluoride levels ranging from 2 to 18.4 mg/L. At the sugar estates defluoridation plants were installed between 1962 and 1976. However, these plants have not provided adequate low fluoride defluoridated water on a continuous basis due to maintenance problems and interrupted supplies of chemicals and others consumables. Water from the nearby Awash River is used primarily for irrigation although at times, it is used for domestic purposes whenever breakdowns occur in the piped water system. Since five years ago, the sugar estates and the satellite towns have been provided with low fluoride drinking water from the Adama (Nazreth) municipal water supply system which uses treated Awash River with a fluoride level of 1 - 1.5 mg/L. Adama town is situated 13 km from Wonji.
Clinically skeletal fluorosis is the more serious form of fluorosis which occurs when high-fluoride drinking water (>4 mg/l) is ingested over a long period of time. The development of skeletal fluorosis and its severity is directly proportional to the level of fluoride in drinking water and the duration of fluoride intake [8,9]. The changes in skeletal tissue may result in back stiffness, rheumatic pain, limitation of movements and progressive kyphosis due to ossification of spinal ligaments and fusion of the vertebral column. In fluoride endemic regions approximately 10% of those with crippling skeletal fluorosis develop neurological complications due to mechanical compression of nerve roots and the spinal cord by bony growth (osteophytes) [10,11].
Although the major cause of fluorosis is the elevated level of fluoride in drinking water, the ambient temperature of area of residence, the nutritional state of the population, deficiency of calcium and hard manual labor have been found to be important contributory risk factors [8,9,12-15]. The consumption of excess tea whose plant accumulates and stores fluoride has been found to cause dental and skeletal fluorosis. [16,17] In rural China, the use of high fluoride-content coal as an energy resource for heating, cooking and food drying has also been found to result in occurrence of dental and skeletal fluorosis [18]. Another risk factor for fluorosis to be considered in East Africa is the fluoride contaminated salt, trona (Hydrous sodium carbonate), locally known as magadi, which is used as a tenderizer for legumes, vegitables and meat [19,20].
This study has identified yet another risk factor that may be important in the development of skeletal fluorosis in the Ethiopian Rift Valley. One of the authors of this study (GA) who was born in Wonji noticed that more crippling fluorosis occurred among long time heavy alcoholic drinkers compared to non-drinkers who live in similar environment. This led the authors to investigate more closely the relationship between excess alcohol consumption and the development of crippling fluorosis within the community of Wonji-Shoa Sugar Estate in the Rift Valley of central Ethiopia.
2. Ethical Consideration
Permission to undertake the study was obtained from the Health Office of the East Shoa Zone of the Oromia Regional State. Consent for interview and taking pictures was obtained from all participants.
3. Materials and Methods
Twenty two heavy long time alcohol drinkers from Wonji, Shoa and the plantation villages as well as the satellite villages of Gefersa, Kuruftu and Alem Tena that exhibited overt signs of crippling fluorosis were identified. Age and sex matched controls who were not alcoholic consumers were also chosen. The non-drinking controls were school or work mates and neighbors of the drinkers living under the same socio-economic conditions. Both the drinkers and non-drinkers had been residents of Wonji-Shoa Sugar Estate consuming the same high fluoride drinking water for many years. They also consumed similar Ethiopian staple diets.
Face-to-face interviews were undertaken using a structured and tested questionnaire by trained research assistants that were well acquainted with the community. Vital datas of the participants including age, birth place, profession, work place and years of employment were obtained as well as information on the source of their drinking water supply, the alcohol drinking habits and the development of typical symptoms and signs of skeletal fluorosis. Different view photographs of the drinkers with fluorosis and their controls were taken for comparison (Figure 1).
In rural Ethiopia the type of locally produced and consumed alcoholic beverages include a home brewed beer, known as “Tella” sold in tin containers called “Tass” of 750 ml volume. The next popular alcoholic beverage is “Tej”, a honey-based kind of mead drank in funnel necked glass bottle known as “Birelle” with a volume of 300 ml. The third common alcoholic drink, “Katekala” is a locally distilled spirit consumed in 15 ml small glass. The standard modern beer in 250 - 350 ml bottles is also consumed but to a lesser extent because it is more expensive.


Figure 1. Photographs chronic consumers of local alcoholic beverages with crippling skeletal fluorosis compared with matched non-drinkers.
During the survey samples of “Tella”, “Tej”, and the water they were prepared from were collected and taken for fluoride analysis to the Chemistry Department of the Facility of Science of the Addis Ababa University.
The leaves of Rhamnus prinoides, an indigenous shrub known as “Gesho”, is used as a flavoring agent and promoter of fermentation in both “Tella” and “Tej” during brewing from fermented barley, sorghum or finger millet in the case of Tella and honey for Tej. To find if this plant accumulated fluoride, a simple test was carried out. Gesho was soaked in Addis Ababa water (F-1.0 mg/L) for 24 hours. Another sample of the same leaves was boiled in the same type of water for ten minutes. Samples from both tests were taken for fluoride determination. Fluoride concentration was determined using a pH/ISE meter (Orion model, EA 940 Expandable Ion Analyzer, USA) equipped with combination fluoride selective electrode (Orion Model 96-09, USA).
4. Results
As shown in Table 1 the age of drinkers ranged from 34 to 74 years while that of non-drinkers was 36 to 80 years. The male to female ratio of the drinkers was 5:1 and the peak age group for the drinkers was 41 - 50 years. Both the drinkers and non-drinkers were found to have depended on deep wells as their only source of drinking water for over 30 years. The fluoride levels of the water supplies from the deep wells of the Sugar Estate ranged from 2.2 mg/L to 18.5 mg/L. In the last three years (2009 to 2012) the drinking water supply of the Sugar Estate has been changed to treated Awash River piped from the treatment plant of the municipality of nearby town of Adama (Nazareth).
The common alcoholic beverage consumed in large quantities was “Tella” followed by “Tej”. The third commonly drank alcoholic beverage was “Katekala”. Modern beer was also consumed. The survey showed that on a daily basis, 62.5% of the chronic drinkers consumed all of the four types of alcoholic beverages in different combinations. 30% drank three types and (8%) consumed two types. The most popular drink was Tella consumed by all drinkers (100%), followed by Katekala (95.8%), Tej (92%) and beer (75%).
The average consumption of a Tella drinker was 5 Tassas (3500 ml) per day with the highest consumption reaching 15 Tassa (10.5 ml) per day. Similarly, drinkers on the average consumed 5 Birelle (1500 ml) of Tej per-

Table 1. General characteristics of study participants.
day with the highest consumption recorded at 12 Birelle (3600 ml/per day/per person. The consumption of Katekala per person ranged from 1 to 8 glasses per day (average 3 glasses) (Table 2). All drinkers were still drinking up to the day of the interview except for 12.5% that had given up drinking altogether.
In the samples of Tella and Tej from plantation and Wonji and Shoa factory villages as well as the satellite towns the fluoride levels were relatively low but higher than the water samples they were brewed from (Table 3). The low fluoride levels found in the present analysis of alcoholic beverages was because of the recently introduced low fluoride water used in the brewing process. However, the high fluoride levels in the samples of Tella from Sami Bertha village of the Rift Valley (120 Km from Addis Ababa) was more representative of the alcoholic beverages of the Rift Valley where high fluoride water is used for brewing. The findings in Sami Berta were similar to what was discovered by Macdonald & Partners in 1984/85. In 1984 the Ethiopian government state-owned Sugar Corporation commissioned the British firm, Sir M MacDonald & Partners Ltd to undertake a feasibility study to draw up recommendations and outline designs for the provision of safe and reliable low-fluoride portable water supply for Wonji/Shoa. During its extensive consultancy work the company carried out medical surveys on fluorosis cases and analyzed the fluoride content of samples of deep and surface water as well as beverages and food items consumed by the local population. According to the Macdonald & Partners analysis the fluoride content of Tella and Tej were much higher than the water they were prepared from [21].
As shown in Table 4 the commonest complaints of all drinkers and 10% of non-drinkers were stiffness of the

Table 2. Consumption of alcoholic beverages amongchronic alcoholics.
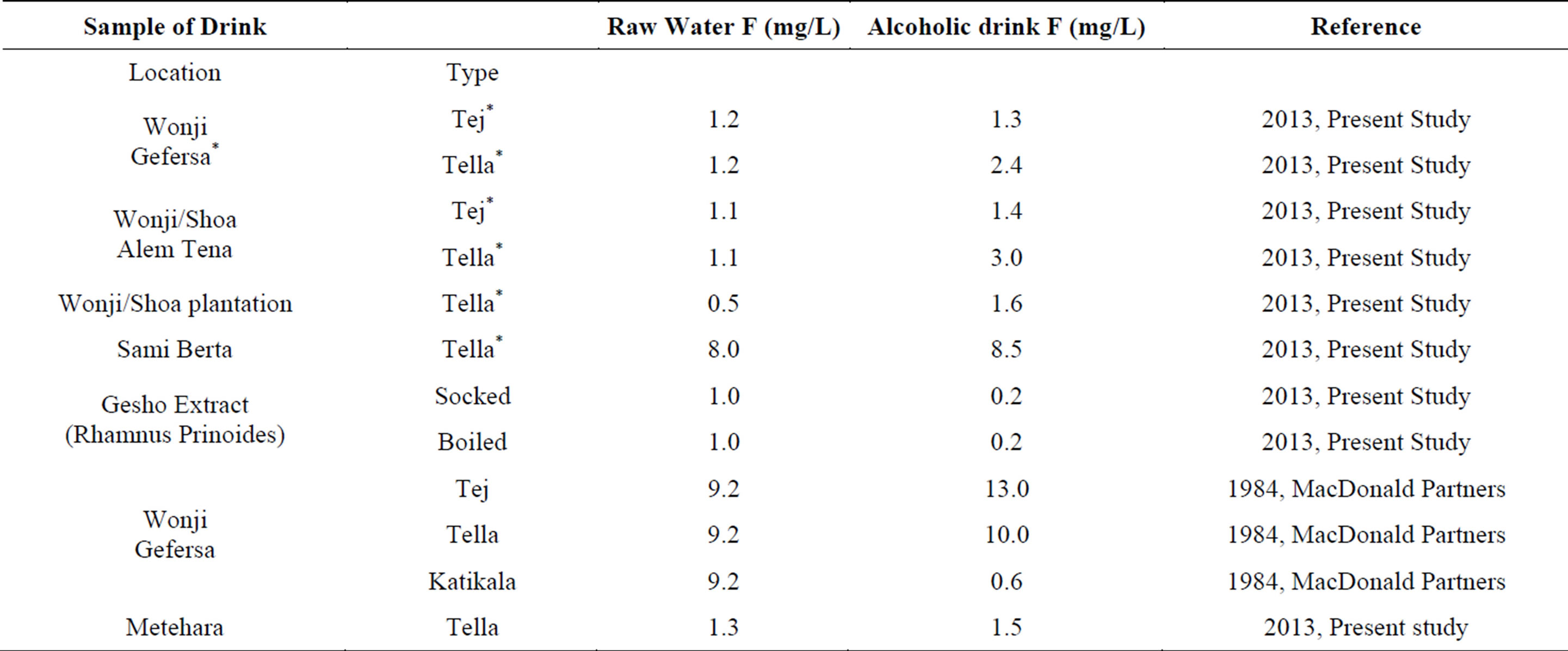
Table 3. Fluoride concentration in alcoholic beverages.
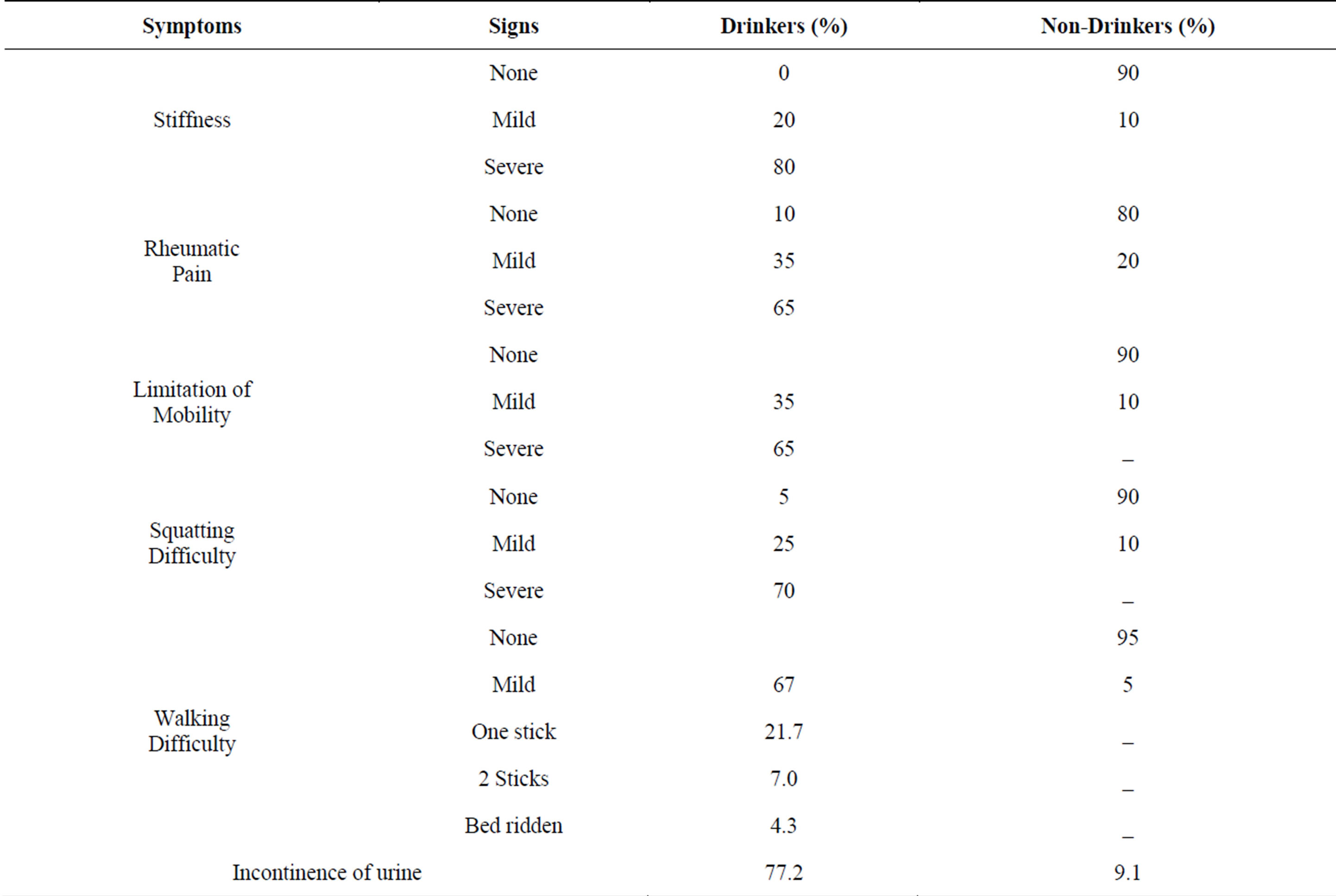
Table 4. Clinical manifestations among drinkers and non-drinkers.
spine and joints with reduced mobility especially at the neck and the joints. Squatting was difficult. Non-drinkers were either symptom free or exhibited mild symptoms. However, all these symptoms and signs were much more pronounced among the drinkers in whom the age of onset was before the age of 30 years in 75% of the cases. In addition, 21% of the drinkers used one stick and 8% two sticks for support during walking and one was bedridden. The most surprising finding of this survey was the reporting of incontinence of urine in 92% of the chronic drinkers with skeletal fluorosis as opposed to only 9.1% among non-drinkers.
5. Discussion
This study shows that excessive long term consumptions of locally alcoholic beverages brewed in high fluoride waters promoted and accelerated the development of skeletal fluorosis in person residing in fluorosis endemic areas. The symptoms and signs of the crippling skeletal fluorosis in the affected individuals were very marked. Alcoholic beverage consumption appears to be the major risk factor for this early onset severe skeletal fluorosis. We believe the possible explanation is related directly to the daily excessive consumption of large volumes of the local alcoholic beverages, particularly Tella and Tej. These drinks are known to have high concentration of fluoride because they are brewed from fermented cereals in water from high fluoride deep wells. In fact, the fluoride levels in the alcoholic beverages were consistently higher that the water they were prepared from. In addition, there are evidences that show cereals grown in high fluoride areas to accumulate excess fluoride [22]. Furthermore, the volumes of the local alcoholic beverages being consumed are very high thus increasing the daily intake of high concentration of fluoride. Following the high level of fluoride, they were discovered in Tella of wonji/Shoa, the British consultancy firm, Sir McDonald & Partner commented that “one or two liters of such Tella per day would be sufficient on their own to create a skeletal fluorosis risk” [21].
In the literature, there is very little reference on the relationship of alcohol consumption and skeletal fluorisis in fluorosis-endemic areas. One such reference is that of Nuguide et al. who found skeletal fluorosis to be more common among drinkers (37.5%) than non-drinkers (19.4%) in the urban slum of Nalgonda, Andra Pradesh, India [23].
As shown in Table 3, Tella and Tej prepared with the water coming from deep wells have dangerous levels of fluoride which predictably will lead to early development of skeletal fluorosis. In addition, most drinkers have poor dietary intake, with low essential vitamins and minerals, notably calcium. These factors are known to contribute to the development of skeletal fluorosis [8,12,13]. The finding that the complications are predominantly in men is most likely due to their common indulgence in alcohol consumption.
Fluorosis will remain a major public health problem in the Ethiopian Rift Valley for a longtime to come. It is estimated that over 10 million people are at risk of developing dental and skeletal fluorosis in Ethiopia, mainly in the Rift Valley. The provision of treated low fluoride surface or defluoridated drinking water to all the inhabitants of fluorosis endemic areas of the Rift Valley will be a Herculean task for the government of Ethiopia. To address the pressing public health issue, the government has set up a Fluorosis Mitigation Office within the Ministry of Water and Energy for guidance on how best to tackle the problem [24]. The country is entertaining to implement in a limited way the expensive and often difficult to maintain defluordation technologies. This again, cannot cover all the fluoride endemic areas. Therefore, the need to depend on deep wells as the major source of drinking water in the Rift Valley will remain for quite some time. Meantime, for lack of a better alternative, the Ethiopian government has proposed to raise the permissible concentration of fluoride in drinking waters coming from deep wells of the Rift Valley to 3 mg/L instead of the internationally accepted WHO standard of 1.5 mg/L [25].
It is therefore important to address risk factors other than high levels fluoride in drinking water. One is the excess fluoride consumption in the food-beverage chain. It is to be emphasized that due attention should be given to the contribution of food as a source of fluoride [26,27]. Excessive tea consumption of tea whose leaves have high levels of fluoride should be avoided. Fortunately in Ethiopia, tea is not a major factor in the causation of fluorosis [28] and the tenderizer, magadi is not used as in other East African countries.
In the same token, excessive consumption of alcoholic drinkers, especially those brewed with high fluoride water and locally produced cereals should be discouraged. Awareness campaigns should be organized within the communities of fluorosis endemic areas to control the over consumption of alcoholic drinks prepared with high fluoride waters. More effectively, local brewers should be required to use low fluoride waters for brewing alcoholic beverages. Municipalities or local administration in the Ethiopia Rift Valley should enforce the regulation through random and regular fluoride analysis of alcoholic beverages, especially Tella and Tej. This is very important because, according to this study, the skeletal fluorosis tends to occur in alcoholic drinkers at a much younger age and with more severity than the general population that is exposed to the same level of fluoride in drinking water.
Acknowledgements
The authors would like to thank all the participants of this study for their cooperation. The Chemistry Department of the Faculty of Science of Addis Ababa University and Dr. Feleke Zewge are gratefully acknowledged for their assistance in the fluoride analysis. We also thank Messrs. Nega Gebre Selassie and Wondimu Mamo who interviewed the participants of the study and Mr. Tatek Kebede who assisted in the preparation of the Tables and Figures. The publication of the paper was facilitated under “the UPGRO Program—Improving Access to Safe Drinking Water: prospection for lowfluoride sources”.
REFERENCES
- R. Tekle-Haimanot, Z. Melaku, H. Kloos, C. Reimann, W. Fantaye, L. Zerihun and K. Bjorvatn, “The Geographical Distribution of Florid Surface and Groundwater in Ethiopia with an Emphasis on the Rift Valley,” Science of the Total Environment, Vol. 367, No. 1, 2005, pp. 182-190. http://dx.doi.org/10.1016/j.scitotenv.2005.11.003
- WHO, “Guidelines for Drinking Water Quality,” 1st Addendum to Vol. 1 Recommendations, 3rd Edition, World Health Organization, Geneva, 2006, p. 595.
- C. Riemann, K. Bjorvatn, B. Frengstad, Z. Melaku, R. Tekle-Haimanot and U. Siewers, “Drinking Water Quality in the Ethiopian Section of the East African Rift Valley,” Science of the Total Environment, Vol. 311, No. 1-3, 2003, pp. 65-80. http://dx.doi.org/10.1016/S0048-9697(03)00137-2
- B. Olsson, “Dental Findings in High Fluoride Areas of Ethiopia,” Community Dental and Oral Epidemiology, Vol. 7, 1979, pp. 51-56. http://dx.doi.org/10.1111/j.1600-0528.1979.tb01185.x
- R. Tekle-Haimanot, F. Asmerom and B. Bedru, “Endemic fluorosis in the Ethiopian Rift Valley,” Tropical and Geographical Medicine, Vol. 39, 1987, pp. 209-217.
- F. Wondwossen, A. N. Astrom, A. Bardsen and K. Bjorvatn, “The Relationship between Dental Caries and Dental Fluorosis in Areas with Moderateand High-Fluoride Drinking Water in Ethiopia,” Community Dent oral Epidemiology, Vol. 32, 2004, pp. 337-344.
- Z. Melaku, G. Assefa, G. Astrom, F. Enquselassie, K. Bjorvatn and R. Tekle Haimanot, “Epidemiological of skeletal fluorosis in wonji Shoa Sugar Estate, Wonji, Ethiopia, A Community Based Survey,” Ethiopian Medical Journal, Vol. 50, 2012, pp. 307-313.
- A. H. Siddiqui, “Fluorosis in Nalgona District, Hyderabad (Deccan),” British Medical Journal, Vol. 1408, 1955, pp. 1408-1414. http://dx.doi.org/10.1136/bmj.2.4953.1408
- S. S. Jolly, B. M. Singh, O. C. Mathur and K. C. Malhotra, “Epidemiological, Clinical, Biochemical Study of Endemic Dental and Skeletal Fluorosis in Punjab,” British Medical Journal, Vol. 4, 1968, pp. 427-429. http://dx.doi.org/10.1136/bmj.4.5628.427
- F. Lester “Fluoride Myelopathy with a Review of the Literature,” Ethiopian Medical Journal, Vol. 12, 1974, pp. 39-49.
- R. Tekle-Haimanot, “Neurological Complications of Endemic Skeletal Fluorosis, with Special Emphasis on Radiculomyelopathy,” Paraplegia, Vol. 28, 1990, pp. 244- 251. http://dx.doi.org/10.1038/sc.1990.31
- J. Littleton, “Paleopathology of Skeletal Fluorosis,” American Journal of Physical Anthropology, Vol. 109, No. 4, 1999, pp. 465-483. http://dx.doi.org/10.1002/(SICI)1096-8644(199908)109:4<465::AID-AJPA4>3.0.CO;2-T
- M. Teotia, S. P. Teotia and K. P. Singh, “Endemic Fluoride Toxicity and Dietary Calcium Deficiency Interaction Syndromes of Metabolic Bone Diseases and Deformities in India,” Indian Journal of Pediatrics, Vol. 65, No. 3, 1998, pp. 371-381. http://dx.doi.org/10.1007/BF02761130
- S. L. Choubisa, L. Choubisa and D. K. Choubisa, “Endemic Fluorosis in Rajasthan,” Indian Journal of Environmental Health, Vol. 43, 2001, pp 177-189
- J. M. Pettifor, C. M. Schnitzler, F. P. Ross and G. P. Moodley, “Endemic Skeletal Fluorosis in Children: Hypocalcemia and the Presence of Renal Resistance in Parathyroid Hormone,” Bon Miner, Vol. 7, No. 3, 1989, pp. 75-88. http://dx.doi.org/10.1016/0169-6009(89)90084-6
- G. N. Opinya, N. Bwibo, J. Valderhaug, J. M. Birkeland and P. Lokken, “Intake of Fluoride through Food and Beverages by Children in a High Fluoride (9 ppm) Area in Kenya,” Discovery and Innovation, Vol. 3, 1991, pp 71-77.
- J. Cao, J. W. Liu, D. Z. Sangbu, S. Yu, Y. Yu and H. Y. Qu, “Dental and Early Stage Skeletal Fluorosis in Children Induced by Fluoride in Brick Tea,” Flouride, Vol. 8, 2005, pp. 44-47.
- M. Ando, M. Tadano, S. Asanuma, K. Tamura, S. Matsushima, T. Watanabe, T. Kondo, S. Sakurai, R. Ji, C. Liang and S. Cao, “Health Effect of Indoor Fluoride Pollution from Coal Burning in China,” Environmental Health Perspectives, Vol. 106, 1998, pp. 239-244. http://dx.doi.org/10.1289/ehp.98106239
- L. Mabelya, W. H. van Palenstein Helderman, M. A. van’t Hof and K. G. Konig, “Dental Fluorosis and the Use of a High Fluoride-Containing Trona Tenderizer,” Community Dentistry & Oral Epidemiology, Vol. 25, No. 2, 1997, pp. 170-117. http://dx.doi.org/10.1111/j.1600-0528.1997.tb00917.x
- M. E. Kaseva, “Contribution of Trona (Magadi) into Excessive Fluorosis—A Case Study in Maji ya Chai Ward, Northern Tanzania,” Science of the Total Environment, Vol. 366, No. 1, 2006, pp. 92-100. http://dx.doi.org/10.1016/j.scitotenv.2005.08.049
- Final Report to the Ethiopian Sugar Corporation, “Supply of Reduced Fluoride Potable Water to Wonji/Shoa and Metehara Sugar Estates,” 1985.
- M. K. Malde, L. Zerihun, K. Julsham and K. Bjorvatn, “Fluoride Content in Selected Food Items from Five Areas of East Africa,” Journal of Food composition and Analysis, Vol. 10, No. 3, 1997, pp. 233-245. http://dx.doi.org/10.1006/jfca.1997.0537
- A. S. Nuguide, G. S. Saiprasad, P. R. Naik and S. Mohanty, “An Epidemiological Study of Fluorosis in an Urban Slum Area of Nalgonda, Andra Pradesh, India,” Indian Journal of Public Health, Vol. 54, 2010, pp. 194- 196. http://dx.doi.org/10.4103/0019-557X.77259
- The National Fluorosis Mitigation Committee (NFMC), Federal Ministry of Water and Energy, Federal Democratic Republic of Ethiopia, 2011
- Ethiopian Guidelines—Specification for Drinking Water quality. The Federal Democratic Republic of Ethiopia Ministry of Health, Addis Ababa, 2002.
- M. K. Malde, L. Zerihun, K. Julshamn and K. Bjorvatn, “Fluoride Intake in Children Living in a High-Fluoride Area in Ethiopia-Intake through Food, International Journal of Paediatric Dentistry, Vol. 13, 2003, pp. 27-34. http://dx.doi.org/10.1046/j.1365-263X.2003.00422.x
- D. Meseret and F. Zewge, “Daily Dietary fluoride intake in rural villages of the Ethiopian Rift Valley, “Toxicology & Environmental Chemistry,” Vol. 95, No. 6, 2013, pp 1056-1068. http://dx.doi.org/10.1080/02772248.2013.827685
- M. K. Malde, L. Zerihun, K. Julshamn and K. Bjorvatn, “Fluoride Intake in Children Living in a High-Fluoride Area in Ethiopia-Intake through Beverages,” International Journal of Paediatric Dentistry, Vol. 13, 2003, pp. 27-34. http://dx.doi.org/10.1046/j.1365-263X.2003.00422.x

