Natural Science
Vol.5 No.5(2013), Article ID:32107,7 pages DOI:10.4236/ns.2013.55077
Cellulosic ethanol and its co-products from different substrates, pretreatments, microorganisms and bioprocesses: A review
![]()
1Laboratory of Bioengineering, Faculty of Engineering, Federal University of Grande Dourados, Dourados, Brazil; *Corresponding Author: ggf@ufgd.edu.br
2Laboratory of Food Technology, Faculty of Engineering, Federal University of Grande Dourados, Dourados, Brazil
Copyright © 2013 Fabiano Avelino Gonçalves et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 21 March 2013; revised 22 April 2013; accepted 5 May 2013
Keywords: Cellulosic Ethanol; Pretreatments; Fermentation
ABSTRACT
Cellulosic ethanol involves the following production steps: physical and/or chemical pretreatment, biological treatment, fermentation and distillation. First three steps are also the bottlenecks for the production of cellulosic ethanol and its co-products. Their production still pose some difficulties in terms of pretreatment, the high cost of enzymes for substrate hydrolysis, the formation of inhibitory compounds in the hydrolyzate, the lack of efficient and viable microorganisms for industrial fermentation of hexose and pentose among others. The solution or minimization of these difficulties may lead to numerous socio-environmental, political, and economic advantages for cellulosic ethanol production. This paper highlights the potential of different substrates, pretreatments, microorganisms and bioprocesses for cellulosic ethanol production.
1. INTRODUCTION
The difference between the production of cellulosic ethanol and sugarcane-based ethanol lies basically in the conversion of polymeric compounds that are presented in cellulosic biomass into fermentable sugars and in the fermentation of hexoses and pentoses. Cellulosic ethanol involves the following production steps: physical and/or chemical pretreatment, biological treatment, fermentation and distillation [1-3].
One of the processes to obtain ethanol from cellulose and hemicellulose is the enzymatic hydrolysis or the chemical hydrolysis of polysaccharides into disaccharides and monosaccharides for further fermentation. However, the recalcitrance of this lignocellulosic material requires pretreatment to facilitate enzymatic action [4,5]. Several methods of plant biomass pretreatment have been studied, e.g., milling [6], acid [7-9], irradiation [10], hydrothermal [11,12], hydrothermal alkaline [13], pyrolysis [14], steam explosion [15,16], catalyzed steam explosion [17, 18], carbon dioxide [19], chlorine dioxide, nitrogen and sulfuric acid [20], organosolvation [21,22], microwave and alkaline treatments [23] and biological treatments [24-27].
The production of ethanol from lignocellulosic compounds, which originated basically in Germany and Russia more than 80 years ago, involves saccharification by acid hydrolysis [28]. Today, the production of ethanol from low added value carbohydrates is a reality in some countries. However, higher medium-term expectations for the viability of cellulosic ethanol focus on the possible use of microbial metabolism in the degradation and saccharification of the plant cell wall to minimize the presence of inhibitors during fermentation [3] and maximize the fermentation of hexoses and pentoses [29]. Table 1 lists different substrates, microorganisms, pretreatments and fermentation processes for cellulosic ethanol production discussed by various researchers.
2. PERSPECTIVES AND CELLULOSIC ETHANOL CO-PRODUCTS
The industrial-scale production of cellulosic ethanol from plant biomass is expected benefit society and the environment in numerous ways. These benefits of eco-
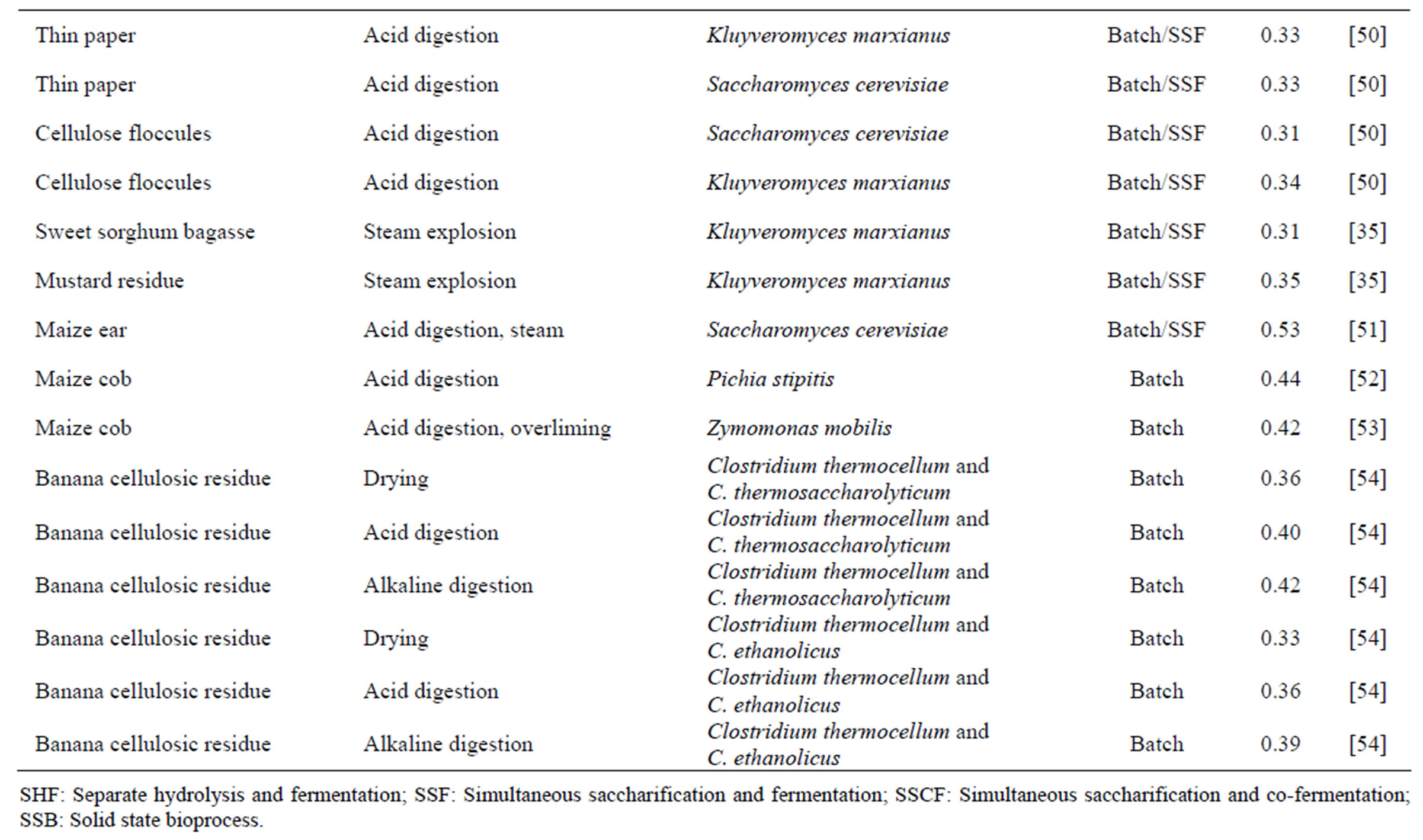
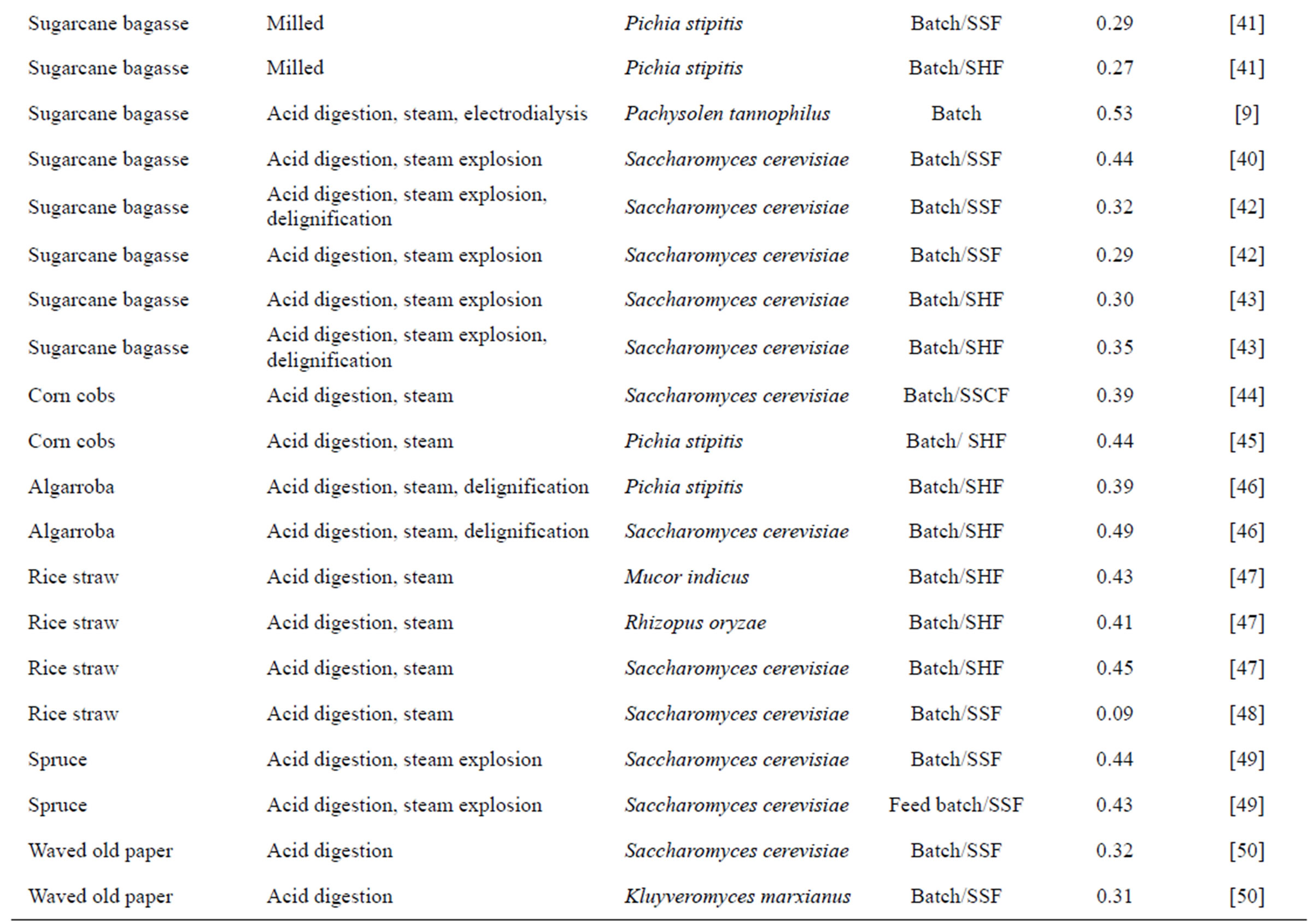
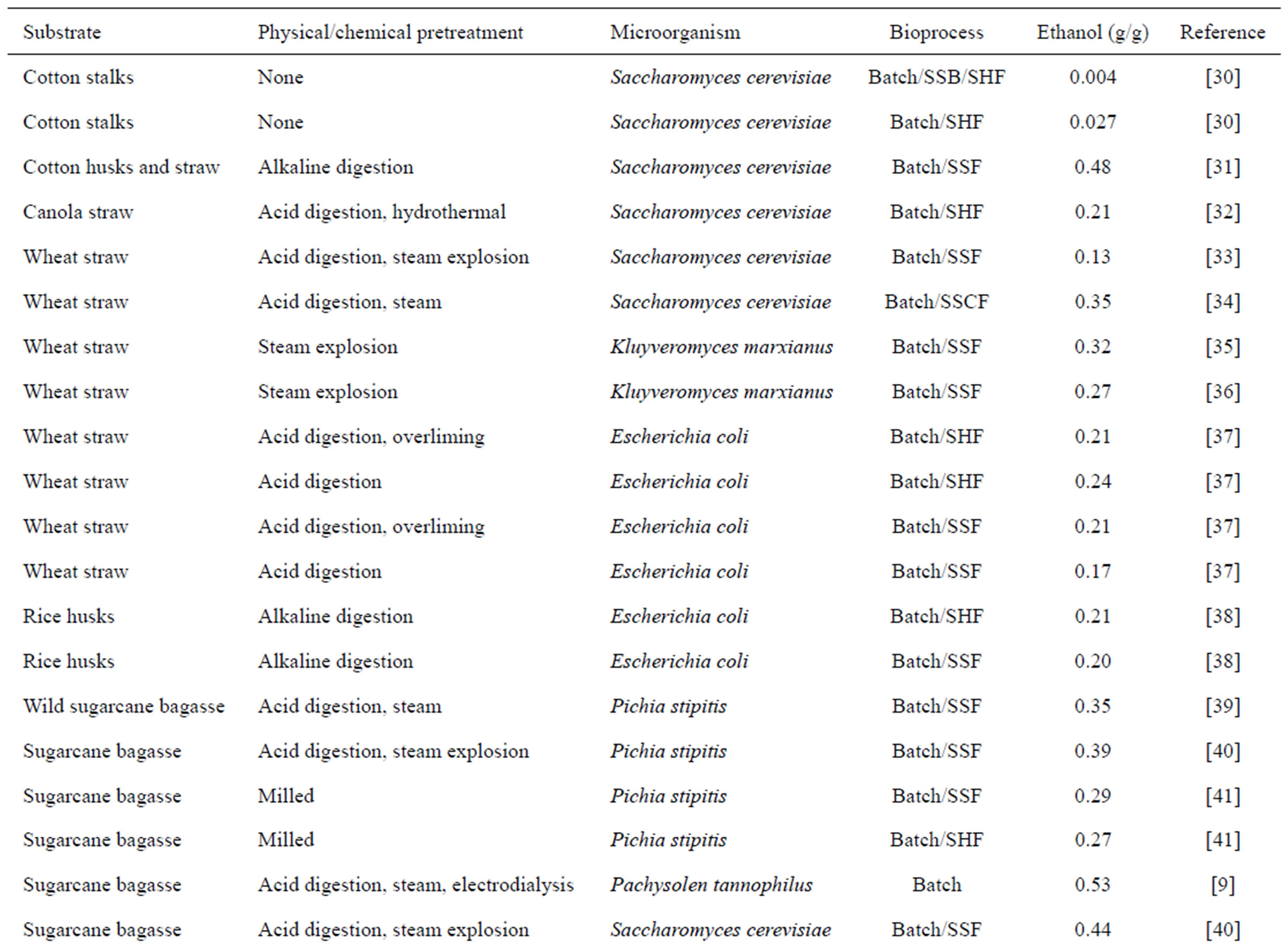
Table 1. Production of cellulosic ethanol from different pretreatments, microorganisms and bioprocesses.
efficiency include the use of organic waste of plant origin, which will allow for augmented biofuel production, optimization of production units (ethanol production from sugarcane residues in the harvesting off-season), extension of landfill service life and reduction of organic pollution. However, so far there is no consolidated biotechnological process for cellulosic ethanol production, especially in terms of pretreatment, and chemical and biochemical hydrolysis, or in pentose fermentation [2,3,55,56].
Moreover, one of the difficulties of the process is the action of lignin, which is able to adsorb cellulolytic and hemicellulolytic enzymes, impairing enzymatic hydrolysis [5]. In the pretreatment process, delignification of the substrate by an alkaline substance is desirable to reduce the amount of lignin [57]. One way to produce biodegradable plants is by modifying them genetically to reduce their lignin content, which affects pretreatment and enzymatic hydrolysis [58].
The physicochemical pretreatment of plant biomass releases inhibitory compounds that affect the fermentation process, e.g., furfural, hydroxymethylfurfural, phenolic compounds and acetic acid [59,60]. Moreover, some of these processes produce vinasse and excessive amounts of wastewater.
Higher expectations concerning the viability of cellulosic ethanol focus on the possible use of microbial metabolism to degrade plant biomass into disaccharides and monosaccharides, thereby enabling fermentation [5]. The efficient use of xylose together with hexose allows for significantly lower cellulosic ethanol production costs [61]. The use of microorganisms that produce cellulases, hemicellulases and ligninases, allied to the use of low cost substrates, may help reduce the price of commercial enzymes [5].
Industrially viable microorganisms should be obtained by genetic engineering [62,63] or prospection [64,65]. Such microorganisms should increase the efficiency of fermentation of hydrolyzed substances containing hexoses and pentoses, and augment the resistance to high sugar concentrations, as well as the presence of inhibitory compounds generated during hydrolysis and fermentation, as in ethanol production.
After plant biomass has been pretreated, the ligninrich fraction that is separated from the hemicellulose can be burned to supply energy to biorefineries or it can be converted into synthesis gas (syngas) [66]. Moreover, lignin can be degraded into smaller fractions and used in the production of polyurethane foam, phenolic resin and epoxy, as a source of phenol and ethylene [67], or be converted into carbon fibers [68]. Lignin can also be employed to produce phenol, acetic acid, vanillin and phenol-formaldehyde resins [69]. The kinetics of lignin pyrolysis may differ according to the procedures utilized in pretreatment and delignification [14], affecting its use. Furfural can be hydrolyzed into maleic acid or form resins with the addition of phenol or urea [70]. The hydroxymethylfurfural produced can be cleaved into formic and levulinic acids, and t and the latter can be used as feedstock for the production of polyesters [71]. Acetic acid can be used as a chemical reagent or in the form of vinegar. Vinasse, in turn, can be used as a soil fertilizer.
The use of plant biomass in ethanol production augments the generation of many co-products, e.g., ethanol steam for hydrogen production to obtain fuel cells [72, 73], the production of ethylene, ethylene glycol, acetaldehyde, acetate, ethyl acetate, glycols, acrylates, ethyl chloride, butane, propylene and butadiene [71], and the production of ethane resulting from ethanol dehydration, which is precursor of a wide range of products such as polyethylene, polypropylene and vinyl polychloride. In Brazil, these ethanol co-products are expanding due the increasing production of ethanol [74]. Moreover, the increasing use of ethanol to replace methanol in biodiesel production [75], as well the conversion of ethanol into ethylene for the production of bioplastics, contributes to boost the demand for ethanol. Lastly, the carbon dioxide generated during ethanol production can be used as carbon source for microbial cultivations and converted into microbial protein biomass [66,76].
3. FINAL REMARKS
The production of cellulosic ethanol still poses some difficulties in terms of pretreatment, the high cost of enzymes for substrate hydrolysis, the formation of inhibitory compounds in the hydrolyzate, the lack of efficient and viable microorganisms for industrial fermentation of hexose and pentose, the gasification of carbon dioxide generated during fermentation, the removal of lignin, furfural and hydroxymethylfurfural from enzymatic and fermentation processes and its proper utilization, as well as adequate reuse of water during the process. The solution or minimization of these difficulties may lead to numerous socioenvironmental, political, and economic advantages for cellulosic ethanol production.
4. ACKNOWLEDGEMENTS
The authors are indebted to the Brazilian research funding agencies CNPq and FUNDECT for their financial support.
REFERENCES
- Martin, C., Galbe, M., Wahlbom, C.F., Hahn-Hägerdal, B. and Jönsson, L.J. (2002) Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme and Microbial Technology, 31, 274-282. doi:10.1016/S0141-0229(02)00112-6
- Sun, Y. and Cheng, J. (2002) Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresource Technology, 83, 1-11. doi:10.1016/S0960-8524(01)00212-7
- Gonçalves, F.A., Sanjinez-Argandoña, E.J. and Fonseca G.G. (2011) Utilization of agro-Industrial residues and municipal waste of plant origin for cellulosic ethanol production. Journal of Environmental Protection, 2, 1303- 1309. doi:10.4236/jep.2011.210150
- Adsul, M.G., Ghule, J.E., Shaikh, H., Singh, R., Bastawde, K.B., Gokhale, D.V. and Varma, A.J. (2005) Enzymatic hydrolysis of delignified bagasse polysaccharides. Carbohydrates Polymers, 62, 6-10. doi:10.1016/j.carbpol.2005.07.010
- Bon, E.P.S., Gírio, F. and Pereira, N. (2008) Enzymes in ethanol production. In: Bon, E.P.S., Ferrara, M.A. and Corvo, M.L., Eds., Enzymes in Biotechnology: Production, Applications and Markets, Interciência, Rio de Janeiro, 241-271.
- Mais, U., Esteghlalian, A. and Saddler, J. (2002) Influence of mixing regime on enzymatic saccharification of steam-exploded softwood chips. Applied Biochemistry and Biotechnology, 98, 463-472. doi:10.1385/ABAB:98-100:1-9:463
- Taherzadeh, M.J. and Karimi, K. (2007) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources, 2, 472-499.
- Zhao, X.B., Wang, L. and Liu, D.H. (2007) Effect of several factors on peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis. Journal of Chemical Technology and Biotechnology, 82, 1115-1121. doi:10.1002/jctb.1775
- Cheng, K., Cai, B., Zhang, J., Ling, H., Zhou, Y., Ge, J. and Xu, J. (2008) Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochemical Engineering Journal, 38, 105-109. doi:10.1016/j.bej.2007.07.012
- Kumakura, M., Kojima, T. and Kaetsu, I. (1982) Pretreatment of lignocellulosic wastes by combination of irradiation and mechanical crushing. Biomass, 2, 299-308. doi:10.1016/0144-4565(82)90015-4
- Yu, G., Yano, S., Inoue, H., Inoue, S., Endo, T. and Sawayama, S. (2009) Pretreatment of rice straw by a hot-compressed water process for enzymatic hydrolysis. Applied Biochemistry and Biotechnology, 160, 539-551. doi:10.1007/s12010-008-8420-z
- Boussarsar, H., Rogé, B. and Mathlouthi, M. (2009) Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresource Technology, 100, 6537-6542. doi:10.1016/j.biortech.2009.07.019
- Yu, Q., Zhuang, X., Yuan, Z., Wang, Q., Qi, W., Wang, W., Zhang, Y., Xu, J. and Xu, H. (2010) Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresource Technology, 101, 4895-4899. doi:10.1016/j.biortech.2009.11.051
- Jiang, G., Nowakowski, D.J. and Bridgwater, A.V. (2010) A systematic study of the kinetics of lignin pyrolysis, Thermochimica Acta, 498, 61-66. doi:10.1016/j.tca.2009.10.003
- Ballesteros, I., Negro, M.J., Oliva, J.M., Cabañas, A., Manzanares, P. and Ballesteros, M. (2006) Ethanol production from steam-explosion pretreated wheat straw. Applied Biochemistry and Biotechnology, 129/132, 496-508. doi:10.1385/ABAB:130:1:496
- Chen, H. and Liu, L. (2007) Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Bioresource Technology, 98, 666-676. doi:10.1016/j.biortech.2006.02.029
- Alizadeh, H., Teymouri, F., Gilbert, T.I. and Dale, B.E. (2005) Pretreatment of switchgrass by ammonia fiber explosion (AFEX). Applied Biochemistry and Biotechnology, 121/124, 1133-1141. doi:10.1385/ABAB:124:1-3:1133
- Sassner, P., Galbe, M. and Zacchi, G. (2006) Bioethanol production based on simultaneous saccharification and fermentation of steam-pretreated Salix at high dry-matter content. Enzyme and Microbial Technology, 39, 756-762. doi:10.1016/j.enzmictec.2005.12.010
- Kim, K.H. and Hong, J. (2001) Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresource Technology, 77, 139-144. doi:10.1016/S0960-8524(00)00147-4
- Fan, L.T., Lee, Y.H. and Gharpuray, M.M. (1982) The nature of lignocellulosics and their pretreatment for enzymatic hydrolysis. Advances in Biochemical Engineering, 22, 158-183. doi:10.1007/3540116982_4
- Zhao, X., Cheng, K. and Liu, D. (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Applied Microbiology and Biotechnology, 82, 815- 827. doi:10.1007/s00253-009-1883-1
- Zhao, X., Peng, F., Cheng, K. and Liu, D. (2009) Enhancement of the enzymatic digestibility of sugarcane bagasse by alkali-peracetic acid pretreatment. Enzyme and Microbial Technology, 44, 17-23. doi:10.1016/j.enzmictec.2008.07.011
- Ma, H., Liu, W.W., Chen, X., Wu, Y.J. and Yu, Z.L. (2009) Enhanced enzymatic saccharification of rice straw by microwave pretreatment. Bioresource Technology, 100, 1279- 1284. doi:10.1016/j.biortech.2008.08.045
- Kurakake, M., Ide, N. and Komaki, T. (2007) Biological pretreatment with two bacterial strains for enzymatic hydrolysis of office paper. Current Microbiology, 54, 424- 428. doi:10.1007/s00284-006-0568-6
- Taherzadeh, M.J. and Karimi, K. (2007) Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources, 2, 707-738.
- Li, L., Li, X.Z., Tang, W.Z. Zhao, J. and Qu, Y.B. (2008) Screening of a fungus capable of powerful and selective delignification on wheat straw. Letters in Applied Microbiology, 47, 415-420. doi:10.1111/j.1472-765X.2008.02447.x
- Roslan, M.A., Yee, P.L., Shah, U.K.M., Aziz, S.A. and Hassan, M.A. (2011) Production of bioethanol from rice straw using cellulases by local Aspergillus sp. International Journal of Agricultural Research, 6, 188-193. doi:10.3923/ijar.2011.188.193
- Bastos, V.D. (2007) Ethanol, alcoholchemistry and biorefineries. BNDES Setorial, Rio de Janeiro, 25, 5-38.
- Stambuk, B.U., Eleutherio, E.C.A., Florez-Pardo, L.M., Souto-Maior, A.M. and Bon, E.P.S. (2008) Brazilian potential for biomass ethanol: challenge of using hexose and pentose co-fermenting yeast strains, Journal of Science and Industrial Research, 67, 918-926. doi:35400018591835.0070
- Agbogbo, F.A. and Wenger, K.S. (2007) Production of ethanol from corn stover hemicellulose hydrolyzate using Pichia stipitis. Journal of Industrial Microbiology and Biotechnology, 34, 723-727. doi:10.1007/s10295-007-0247-z
- Mohaghegi, A., Dowe, N., Schell, D., Chou, Y.E., Eddy, E. and Zhang, M. (2004) Performance of a newly developed integrant of Zymomonas mobilis for ethanol production on corn stover hydrolysates. Biotechnology Letters, 26, 321-325. doi:10.1023/B:BILE.0000015451.96737.96
- Reddy, H.K.Y., Srijana, M., Reddy, M.D. and Reddy, G. (2010) Coculture fermentation of banana agro-waste to ethanol by cellulolytic thermophilic Clostridium thermocellum CT2. African Journal of Biotechnology, 9, 1926- 1934.
- Palonen, H., Tjerneld, F., Zacchi, G. and Tenkanen, M. (2004) Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. Journal of Biotechnology, 107, 65-72. doi:10.1016/j.jbiotec.2003.09.011
- Lin, Y. and Tanaka, S. (2006) Ethanol fermentation from biomass resources: Current state and prospects. Applied Microbiology and Biotechnology, 69, 627-642. doi:10.1007/s00253-005-0229-X
- Gouveia, E.R., Nascimento, R.T., Souto-Maior, A. and Rocha, G.J.M. (2009) Validation of methodology for the chemical characterization of sugar cane bagasse. Química Nova, 32, 1500-1503. doi:10.1590/S0100-40422009000600026
- Buckeridge, M.S., Silva, G.B. and Cavalari, A.A. (2008) Cell. In: Kerbauy, G.B., Ed., Plant Physiology, 2nd Edition, Guanabara Koogan, Rio de Janeiro, 165-181.
- Almeida, J.R.M., Modig, T., Petersson, A., HahnHägerdal, B., Lidén, G. and Gorwa-Grauslund, M.F. (2007) Increased tolerance and conversion of inhibitors in lingocellulosic hydrolysates by Saccharomyces cerevisiae. Journal of Chemical Technology and Biotechnology, 82, 340-349. doi:10.1002/jctb.1676
- Wikandari, R., Millati, R., Syamsiyah, S., Muriana, R. and Ayuningsih, Y. (2010) Effect of furfural, hydroxylmethylfurfural and acetic acid on indigenous microbial isolate for bioethanol production. Agricultural Journal, 5, 105-109. doi:10.3923/aj.2010.105.109
- Olsson, L. and Hahn-Hägerdal, B. (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme and Microbial Technology, 18, 312-331. doi:10.1016/0141-0229(95)00157-3
- Lau, M.W., Gunawan, C., Balan, V. and Dale, B.E. (2010) Comparing the fermentation performance of Escherichia coli KO11, Saccharomyces cerevisiae 424A (LNH-ST) and Zymomonas mobilis AX101 for cellulosic ethanol production. Biotechnology for Biofuels, 3, 11. doi:10.1186/1754-6834-3-11
- Sanchez, R.G., Karhuma, K., Fonseca, C., Nogué, V.S., Almeida, J.R.M., Larsson, C. U., Bengtsson, O., Bettiga, M., Hahn-Hägerdal, B. and Gorwa-Grauslund, M.F. (2010) Improved xylose and arabinose utilization by an industrial recombinant Saccharomyces cerevisiae strain using evolutionary engineering. Biotechnology for Biofuels, 3, 13. doi:10.1186/1754-6834-3-13
- Almeida, J.R., Karhuma, K., Bengtsson, O., GorwaGrauslund, M.F. (2009) Screening of Saccharomyces cerevisiae strains with respect to anaerobic growth in non-detoxified lignocellulose hydrolysates. Bioresource Technology, 100, 3674-3677. doi:10.1016/j.biortech.2009.02.057
- Lotfi, A., Ghanbary, M.A.T., Ranjbar, G.A. and Asgharzadeh, A. (2010) Screening of some Zygomycetes for cellulase activity. African Journal of Biotechnology, 9, 4211- 4216.
- Fang, X., Yano, S., Inoue, H. and Sawayama, S. (2009) Strain improvement of Acremonium cellulolyticus for cellulase production by mutation. Journal of Bioscience and Bioengineering, 107, 256-261. doi:10.1016/j.jbiosc.2008.11.022
- Lora, J.H. and Glasser, W.G. (2002) Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. Journal of Polymers and the Environment, 10 39-48. doi:10.1023/A:1021070006895
- Kadla, J.F., Kubo, S., Venditti, R.A., Gilbert, R.D., Compere, A.L. and Griffith, W. (2002) Lignin-based carbon fibers for composite fiber applications. Carbon, 40, 2913-2920. doi:10.1016/S0008-6223(02)00248-8
- Benar, P. (1996) Lignins acetosolv and formacell from eucalyptus and sugar cane bagasse: Isolation, fractionation, characterization and use as a component of phenolic resins resol type. Ph.D. Thesis, University of Campinas, Campinas.
- Mckillip, W.J., Collin, G. and Höke, H. (1989) Furan and derivatives. In: Elvers, B., Hawkins, S., Ravenscroft, M., Rounsaville, J.F. and Schulz, G. Eds., Ullmann’s Encyclopedia of Industrial Chemistry, 5th Edition, VCH Publishers, 119-121.
- Schuchardt, U.L.F., Ribeiro, M.L. and Gonçalves, A.R. (2001) The petrochemical industry in the next century: How to replace petroleum as raw material. Química Nova, 24, 247-251. doi:10.1590/S0100-40422001000200016
- Benito, M., Sanz, J.L., Isabel, R., Padilha, R., Arjona, R. and Daza, L. (2005) Bio-ethanol steam reforming: Insights on the mechanism for hydrogen production. Journal of Power Sources, 151, 11-17. doi:10.1016/j.jpowsour.2005.02.046
- Maia, T.A., Bellido, J.D.A., Assaf, E.M. and Assaf, J.M. (2007) Hydrogen production by ethanol steam reforming using Cu/Ni/-Al2O3. Química Nova, 30, 339-345. doi:10.1590/S0100-40422007000200019
- BNDES, Brazilian Development Bank (2008) Bioethanol from cane sugar: Energy for sustainable development. Rio de Janeiro, BNDES.
- García, M.A., Gonzalo, A., Sánchez, L.J.A., Arauzo, J.A. and Simões, C. (2009) Methanolysis and ethanolysis of animal fats: A comparative study of the influence of alcohols. Chemical Industry and Chemical Engineering Quarternaly, 15, 1-18. doi:10.2298/CICEQ100224058G
- Silva, J.P.A., Mussatto, S.I. and Roberto, I.C. (2010) The influence of initial xylose concentration, agitation, and aeration on ethanol production by Pichia stipitis from rice straw hemicellulosic hydrolysates. Applied Biochemistry and Biotechnology, 162, 1306-1315. doi:10.1007/s12010-009-8867-6
- Shi, D.J., Wang, C.L. and Wang, K.M. (2009) Genome shuffling to improve thermotolerance, ethanol tolerance and ethanol productivity of Saccharomyces cerevisiae. Journal of Industrial Microbiology and Biotechnology, 36, 139-147. doi:10.1007/s10295-008-0481-z
- Jeihanipour, A. and Taherzadeh, M.J. (2009) Ethanol production from cotton-based waste textiles. Bioresource Technology, 100, 1007-1010. doi:10.1016/j.biortech.2008.07.020
- Lu, X.B., Zhang, Y.M. and Angelidaki, I. (2009) Optimization of H2SO4 catalyzed hydrothermal pretreatment of rapeseed straw for bioconversion to ethanol: Focusing on pretreatment at high solids content. Bioresource Technology, 100, 3048-3053. doi:10.1016/j.biortech.2009.01.008
- Linde, M., Jakobsson, E.L., Galbe, M. and Zacchi, G. (2008) Steam pretreatment of dilute H2SO4 impregnated wheat straw and SSF with low yeast and enzyme loading for bioethanol production. Biomass Bioenergy, 32, 326- 332. doi:10.1016/j.biombioe.2007.09.013
- Olofsson, K., Wiman, M. and Liden, G. (2010) Controlled feeding of cellulases improves conversion of xylose in simultaneous saccharification and co-fermentation for bioethanol production. Journal of Biotechnology, 145, 168-175. doi:10.1016/j.jbiotec.2009.11.001
- Ballesteros, M., Oliva, J.M., Negro, M.J., Manzanares, P. and Ballestros, I. (2004) Ethanol from lignocellulosic materials by a simultaneous sacharification and fermentation process (SSF) with Kluyveromyces marxianus CECT 10875. Process Biochemistry, 39, 1843-1848 doi:10.1016/j.procbio.2003.09.011
- Tomás-Pejó, E., García-Aparicio, M., Negro, M.J., Oliva, J.M. and Ballesteros, M. (2009) Effect of different cellulose dosages on cell viability and ethanol production by Kluyveromyces marxianus in SSF processes. Bioresource Technology, 100, 890-895. doi:10.1016/j.biortech.2008.07.012
- Saha, B.C., Iten, L.B., Cotta, M.A. and Wu, Y.V. (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochemistry, 40, 3693-3700. doi:10.1016/j.procbio.2005.04.006
- Saha, B.C. and Cotta, M.A. (2007) Enzymatic hydrolysis and fermentation of lime pretreated wheat straw to ethanol. Journal of Chemical Technology and Biotechnology, 82, 913-919. doi:10.1002/jctb
- Scordia, D., Cosentino, S.L. and Jeffries, T.W. (2010) Second generation bioethanol production from Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hack. Bioresource Technology, 101, 5358-5365. doi:10.1016/j.biortech.2010.02.03
- Carrasco, C., Baudel, H.M., Sendelius, J., Modig, T., Roslander, C., Galbe, M., Hahn-Hägerdal, B., Zacchi, G. and Liden, G. (2010) SO2 catalyzed steam pretreatment and fermentation of enzymatically hydrolyzed sugarcane bagasse. Enzyme and Microbial Technology, 46, 64-73. doi:10.1016/j.enzmictec.2009.10.016
- Buaban, B., Inoue, H., Yano, S., Tanapongpipat, S., Ruanglek, V., Champreda, V., Pichyangkura, R., Rengpipat, S. and Eurwilaichitr, L. (2010) Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipites. Journal of Bioscience and Bioengineering, 110, 18-25. doi:10.1016/j.jbiosc.2009.12.003
- Santos, J.R.A. and Gouveia, E.R. (2009) Production of bioethanol from sugarcane bagasse. Revista Brasileira de Produtos Agroindustriais, 11, 27-33.
- Santos, J.R.A., Souto-Maior, A.M., Gouveia, E.R. and Martín, C. (2010) Comparison of SHF and SSF processes from sugar cane bagasse for ethanol production by Saccharomyces cerevisiae. Química Nova, 33, 904-908. doi:10.1590/S0100-40422010000400027
- Öhgren, K., Bengtsson, O., Gorwa-Grauslund, M.F., Galbe, M., Hahn-Hägerdal B., and Zacchi, G. (2006) Simultaneous saccharification and cofermentation of glucose and xylose in steam-pretreated corn stover at high fiber content with Saccharomyces cerevisiae TMB3400. Journal of Biotechnology, 126, 488-498. doi:10.1016/j.jbiotec.2006.05.001
- Lee, J.W., Rodrigues, C.L.B.R., Kim, H.J., Choi, I.G. and Jeffries, T.W. (2010) The roles of xylan and lignin in oxalic acid pretreated corncob during separate enzymatic hydrolysis and ethanol fermentation. Bioresource Technology, 101, 4379-4381. doi:10.1016/j.biortech.2009.12.112
- Gupta, R., Sharma, K.K. and Kuhad, R.C. (2009) Separate hydrolysis and fermentation (SHF) of Prosopis juliflora a wood substrate, for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis NCIM 3498. Bioresource Technology, 100, 1214-1220. doi:10.1016/j.biortech.2008.08.033
- Abedinifar, S., Karimi, K., Khanahmadi, M. and Taherzadeh, M.J. (2009) Ethanol production by Mucor indicus and Rhizopus oryzae from rice straw by separate hydrolysis and fermentation. Biomass Energy, 33, 828-833. doi:10.1016/j.biombioe.2009.01.003
- Sukumaran, R.K., Singhania, R.R., Mathew, G.M. and Pandey, A. (2009) Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renewable Energy, 34, 421-424. doi:10.1016/j.renene.2008.05.008
- Bertilsson, M., Olofsson, K. and Lidén, G. (2009) Prefermentation improves xylose utilization in simultaneous saccharification and co-fermentation of pretreated spruce. Biotechnology for Biofuels, 2, 8. doi:10.1186/1754-6834-2-8
- Kadár, Z., Szengyel, Z. and Reczey, K. (2004) Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Industrial Crops and Products, 20, 103-110. doi:10.1016/j.indcrop.2003.12.015
- Shen, Y., Zhang, Y., Ma, T., Bao, X., Du, F., Zhuang, G. and Qu, Y. (2008) Simultaneous saccharification and fermentation of acid-pretreated corncobs with a recombinant Saccharomyces cerevisiae expressing β-glucosidase. Bioresource Technology, 99, 5099-5103. doi:10.1016/j.biortech.2007.09.046

