Health
Vol.08 No.04(2016), Article ID:63971,10 pages
10.4236/health.2016.84039
Application of Precision-Cut Rat Liver Slice to Study the Influence of Monocrotaline, Tussilago farfara Alkaloids on the Expression of Cytochrome P450 Enzymes
Hailin Wang1*, Lianqiang Hui1*, Chun Li1, Ting Liu1#, Chang’an Yu2, Chunyu Cao1, Ran Hao1, Yi Zhang1
1Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
2China-Japanese Friendship Hospital, Beijing, China

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 12 January 2016; accepted 26 February 2016; published 29 February 2016
ABSTRACT
Precision-cut liver slice has been successfully used to study the mechanism of drug-induced hepatotoxicity, the prediction of liver toxicity, the discovery of early hepatic toxicity biomarker and the metabolism of drug in liver. We detected the expression of CYP3A4, CYP2B1 + CYP2B2 and CYP2E1 in precision-cut liver slice after co-cultured with monocrotaline or Tussilago farfara alkaloids to investigate the hepatotoxicity mechanism of those drugs. After co-culturing with monocrotaline or Tussilago farfara alkaloids for 6 hours, the expression of CYP3A4 in the microsome of precision- cut liver slices was detected by Western blot, and the expressions of CYP2B1 + CYP2B2 and CYP2E1 were detected by immunofluorescence. The results showed that monocrotaline induced the expression of CYP3A4 and CYP2B1 + CYP2B2, and Tussilago farfara alkaloids obviously up-re- gulated the expression of CYP2E1 and CYP3A4. Thus, we conclude that the up-regulation of CYP3A4, CYP2B1 + CYP2B2 and CYP2E1 may be one of the toxic mechanisms of liver injury of those drugs.
Keywords:
Precision-Cut Liver Slices, Monocrotaline, Tussilago farfara Alkaloids, Hepatotoxity, Cytochrome P450 Enzymes

1. Introduction
Precision-cut liver slice is an advanced technology between organ and cell levels, integrating in vivo and in vitro detecting techniques, which is simple, rapid, economic and can be used to predict drug toxicity [1] - [5] . Its advantage is the preservation of liver tissue structure. A piece of liver slice contains the cells of all types in liver tissue, and perfectly preserves the contact between matrix and cell. At the same time, it avoids the damage of protease to cell in the process of cell separation. The metabolic ability of precision-cut liver slices is close to intact organs, so it is very suitable for pharmacokinetic studies, and is an ideal technology platform for the study of drug metabolic enzymes.
Since precision-cut liver slice was reported in the early 1980s [6] , it has became more and more mature and perfect along with the improvement of culture method [1] [7] [8] . Precision-cut liver slice has been successfully used in the study of drug toxicity mechanism and prediction, the discovery of early toxicity markers and drug metabolism and transformation. In addition, it is increasingly and widely used in drug absorption mediated by transport protein, the absorption and efficacy of targeted agents, the regulation and transportation of drug metabolic enzymes and so on, so it attracts a lot of attention [9] .
The liver is the main organ of drug metabolism in vivo; a series of drug metabolic processes take place in liver, such as polymerization, oxidation, reduction, hydroxylation, demethylation and so on. Thus, the liver is most vulnerable to drug damage. According to the data published by FDA in 2008, liver toxicity studies were in the first place in all adverse drug reactions [10] .
Pyrrolizidine alkaloids (PAs) are a group of alkaloids widely distributed in nature, about 3% of the flowering plants containing PAs. PAs currently known as the most important plant liver toxic ingredients, are mainly distributed in many plants of Compositae (Senecioneae and Eupatorieae), Boraginaceae (all genera) and legume (Crotalaria), also called hepatotoxic pyrrolizidine alkaloids (HPAs), which can cause hepatic hemorrhage, liver cells necrosis, venous occlusion disorder and so on; some of them may also cause other organs damage, such as heart, lung, kidney and brain; others may have obvious carcinogenic, mutagenic and teratogenic effects [11] . The structure of PAs is composed of two basic parts, namely necine and necic acid, and PAs with allyl alcohol ester structure formed by 1, 2 bits of unsaturated double bond have strong liver toxicity to human.
In China, there are at least 40 kinds of common used traditional Chinese medicine (TCM) contained PAs, including Rattlebush and Tussilago farfara. TCM contained PAs can cause direct and dose-dependent liver injury. Studies show that those TCM can transform to unstable metabolites (such as pyrrole derivatives) under the action of cytochrome P450s (CYP450s), which can injure the sinusoidal endothelial cell to cause hepatic blood flow disorder [12] . 12 rats were firstly fed with 32% Tussilago farfara-containing feed for 4 days, then fed with 16% Tussilago farfara-containing feed for more than 380 days. The results showed 8 rats with liver angiosarcoma, 1 rat with liver cell adenoma, 1 rat with hepatocellular carcinoma, and 1 rat with bladder papilloma. Liver angiosarcoma occurred in only 1 of 10 rats fed with 8% Tussilago farfara-containing feed for 600 days, while there was no tumor occurring in rats fed with 4% Tussilago farfara-containing feed for 600 days and the control group [13] . In order to ensure clinical medication safety, WHO specially designs safety guide on the HPAs; for example the Germany ministry of health requires that the oral daily dose of preparation containing the HPAs is not more than 1 μg, external use does not exceed 100 μg per day, and the highest dose permission of coltsfoot leaf as a herbal tea is 10 μg per day [14] . Research has shown that monocrotaline has obvious mutagenic effect [15] . Muller et al. evaluated the cytotoxity of 5 PAs including monocrotaline by cultivating primary rat hepatocytes and V79 cells, and found that cytotoxicity of those PAs would be obvious after incubation for 18 hours [16] . Study has found that MCTP of monocrotaline metabolites can induce DNA cross-linking in pulmonary endothelial cells, which is the cytotoxic mechanism of monocrotaline, and that the DNA cross-linking is closely related to the dose of MCTP [17] .
The current study suggests that the active metabolite of CYP 450, mainly electronic material and some active oxygen free radicals, can damage cellular macromolecules such as DNA, proteins, and interfere with intracellular homeostasis, leading to apoptosis or necrosis [18] [19] . CYP members participated in drug-induced liver injury are mainly CYP2E1, CYP3A4, CYP2B, etc. [20] -[22] . CYP3A4 is the most important component of human liver microsomal CYP450s, approximately accounting for 30% - 40% of total CYP450s, and playing an important role in many metabolisms of endogenous and exogenous compounds. CYP2B can catalyze substrate metabolism to form non-active and easy excretive metabolites to play a detoxification role. CYP2B can also activate certain drugs (such as anti-cancer drug: cyclophosphamide) by metabolism. CYP2E1 accounts for 6.6% of total hepatic CYP, which is an important metabolic enzyme for many low molecular weight organic compounds and drugs.
Our past research has found that co-culture of monocrotaline and precision-cut liver slices for 24 hours can damage the liver cell membrane to a certain degree, cause LDH leakage increased and protein content decreased, and those changes become aggravated with the increase of monocrotaline dose [23] . Co-cultured Tussilago farfara alkaloids with liver slices for 6 hours show obvious liver toxicity: can increase the leakage of LDH, ALT, GGT and decrease the protein content [24] . Based on the previous results, this research further tests the expression of CYP3A4, CYP2B and CYP2E1 after monocrotaline or Tussilago farfara alkaloids co-cultured with precision-cut liver slices for 6 hours to observe regulatory effect of those TCM contained PAs on CYP450s.
2. Materials
Animals, supplies, and equipments. Young adult male (180 - 200 g) Sprague-Dawley rats were purchased from Beijing Vital River Laboratory Animal Technology Co. (Beijing, CHN). Penicillin and streptomycin were purchased from HyClone (Logan, UT). Waymouth’s medium was purchased from Gibco (Grand island, NY). Four methyl thiazolyl tetrazolium (MTT) and bovine serum albumin (BSA) were imported and packed by Kehai Junzhou (Beijing, CHN). Hepes was purchased from Amresco (Solon, OH). Dexamethasone was purchased from Solarbio (Beijing, CHN). Agarose was purchased from OXOID (Basingstoke, UK). Ethanolamine was purchased from Acros organics (Belgium, USA). Transferrin was purchased from Sigma (St. Louis, MO). One-step rapid WB reagent (rabbit), western blot internal positive control (tubulin) and tissue protein extraction reagents were all purchased from Beijing ComWin Biotech Co. Ltd. (Beijing, CHN). BCA protein assay kit was purchased from Pierce (Rockford, IL). CYP2B1 + CYP2B1, CYP3A4, CYP2E1 antibodies were purchased from Abcam (Hong Kong, CHN). CYP2E1 antibodies were purchased from Protein tech (Chicago, IL). Goat anti-rabbit-TRITC and goat anti-mouse-FITC were purchased from ZSGB-Bio (Beijing, CHN). Antifade solution and DAPI were purchased from Boster Bio-Engineering (Wuhan, CHN). Monocrotaline was imported and packed by Shanghai Green Bird Technologies (Shanghai, CHN), purity > 99%. 1000 Plus Vibracome was purchased from the Vibratome Company (St. Louis, MO). Electrophoresis apparatus was purchased from Biorad (Hercules, CA). Optima L-90K ultra low speed centrifuge was purchased from Beckman coulter (Indianapolis, IN).
3. Methods
The extraction of total alkaloids from Tussilago farfara. 1 kg Tussilago farfara was extracted by reflux extraction with 90% ethanol for three times, 2 hours each time. Then, filtered the extract, combined the filtrate, removed ethanol completely by vacuum recovery. The pH value of water solution was adjusted to 2 - 3 with 2.5% HCl. The solution was extracted by CHCl3 three times. Then the chloroform solution was discarded. The pH value of remaining aqueous layer was adjusted to 10 - 11 with 5% NaOH. The solution was extracted by CHCl3 three times. Recovered chloroform to get the total alkaloids 1 g. The total alkaloids were used directly for LC-MS analysis, and two main chromatographic peaks (tR 25.573 min and 28.133 min) in the mass spectrum were deduced to be pyrrolizidine alkaloids of senkirkine type on the basis of mass fragmentation and literature data [25] .
The test was performed on a Waters Acquity H-class UPLC/Xevo G2-S Q-TOF system, with MassLynx V4.1. software. Chromatographic separation was carried on a Waters ACQUITYUPLC BEH C18 column (2.1 × 100 mm, 1.7 μm), using methanol (A)-0.1% formic acid aqueous solution (B) (5% - 38%, 40 min) in gradient elution mode. The flow rate was set at 0.2 mL∙min−1, the column temperature was set to 25˚C and the sample injection was 0.2 μL. The eluent was detected with UV at 254 nm and mass spectrometry sequentially. The mass spectrometry parameters were as below: electrospray ionization (ESI), detection in positive mode; capillary: 2 kv; Auto MS2, m/z 50 - 1200. The UPLC/UV (254 nm) chromatogram and total ion chromatogram of Tussilago farfara total alkaloids were shown in Figure 1.
The preparation of liver slices. The rats were decapitated. Liver was removed in a sterile environment and placed in oxygenated ice-cold Kerbs-Henseleit buffer (KHB) continuously gassed with 95% O2 and 5% CO2. Then, we took a piece of liver tissue about 20 × 15 × 4 mm3 in right medial lobe. The liver tissue was placed in 6-well plates and embedded with 2% agarose. The liver blocks were fixed to the specimen mounting block of vibration slicer with specimen adhesive, then the specimen mounting block was taken to the specimen bath contained KHB. Adjusted the blade angle to about 18˚, the knife forward speed to minimum, the knife amplitude to maximum, the slice thickness to 200 μM to make liver slices, and kept the buffer temperature maintained at 2˚C - 4˚C by circulating pump. Complete liver slices were selected and cored using a 6-mm biopsy punch. Then, the rat liver slices were maintained in BPM medium [1] (Waymouth MB 752/1 basal medium supplemented with 27 mM NaHCO3, 25 mM Hepes, 25 mM glucose, 0.6 g/mL insulin, 0.4 mM sodium pyruvate, 65.5 μM ethanolamine, 100 μg/mL transferrin, 0.1 μM dexamethasone, 10 nM glucagon, 0.08% BSA, penicillin, streptomycin, and gentamycin) at 37˚C in an atmosphere of 95% O2/5% CO2 for 6 hours.
The grouping information. Set up six groups: negative control group, solvent control group, 0.05% monocrotaline group, 0.2% monocrotaline group, 0.05% Tussilago farfara total alkaloids group and 0.2% Tussilago farfara total alkaloids group.
The preparation of drugs. Monocrotaline and Tussilago farfara total alkaloids were first dissolved in DMSO, then added into the culture medium, the final concentration of DMSO was 0.5%, the final concentration of both monocrotaline and Tussilago farfara total alkaloids were 0.05% and 0.2%. The negative control was the culture medium without DMSO, the solvent control was the culture medium containing 0.5% DMSO.
The morphological observation of the liver slices. Liver slices cultured in medium for 6 hours and 0 hour were fixed in 10% formalin solution for 15 hours, then dehydrated in an ethylalcohol series, embedded in paraffin, sectioned at 4 μm and stained with Hematoxylin and Eosin to make pathological sections. Then the pathological sections were observed using Olympus microscopyto find the survival state of the liver slices cultured for 6 hours. When liver cells showed cytoplasm irreversible eosinophilic changes, or nuclear pyknosis,karyorrhexis and karyolysis, it was belived that the liver cells lost their activity.
Preparation of liver slices microsomes. 0.15 M KCl buffer was added to the liver slice by 1:4 W/V to make liver homogenates. The prepared liver homogenate was centrifuged in ultracentrifuge at 9000 r∙min−1 for 15 min. The supernatant was also centrifuged at 150,000 r∙min−1 for 60 min. Then the supernatant was discarded, the pink precipitate was liver microsomes. The precipitate was suspended in 1 mL 0.15 M KCl buffer. Microsomal protein concentrations were determined by BCA assay.
The expression of CYP3A4 protein in liver slices microsomes. The microsomes were centrifuged at 12,000 rpm for 5 min at 4˚C, then the supernatant was discarded. Added 200 ul protein lysis buffer, mixed with high speed suspension, placed in a 4˚C water bath for 40 min, then centrifuged in a refrigerated centrifuge at 12,100 rpm for 20 min at 4˚C. The supernatant was sucked in a new EP tube, then added the loading buffer. The protein was denatured at 100˚C for 3 min for protein detection by western blot. Protein lysate samples were separated on 10% SDS-PAGE gels, then transferred to NC membranes. After blocking, the membranes were reacted with the first antibody and antibody reaction solution in fast WB kit. Then the membranes were washed and added ECL to produces a light emission. This light emission was imaged onto photographic film and the film was developed and fixed. Using an image acquisition system to obtain image and IPP (Image Pro Plus) software to analyse the integral optical density (IOD) and area of the images, calculate the mean optical density (ROD) of tubulin and the target protein of each group, obtain the ratio as the protein expression of each group.
The expressions of CYP2B1 + CYP2B2, CYP2E1 in liver slices by immunofluorescence. The cultured liver slices were fixed in 10% formalin solution. After routine gradient alcohol dehydration, paraffin embedding and cutting into sections (4 μm), the liver paraffin sections were made. After sections were deparaffinized in xylene and rehydrated in graded alcohol dilutions, antigen retrieval was performed by microwave. Non-specific binding was blocked using goat serum at room temperature for 30 minutes. Then the sections were incubated with primary antibodies (CYP2B1 + CYP2B2 and CYP2E1) at a dilution of 1:25 for 12 hours at 4˚C, and were then thoroughly rinsed in PBS (0.01 M, pH 7.2 - 7.4). Light was avoided in next procedure. Two secondary antibodies (goat anti-rabbit-TRITC and goat anti-mouse-FITC) both at a dilution of 1:100 were added and incubated for 2 hours at room temperature. After rinsing with PBS, DAPI was incubated with the sections for 2 minutes at room temperature. The sections were rinsed in PBS, then mounted with antifade solution. The images were observed at 400× magnification by fluorescence microscope and the average optical density of images was determined by IPP image analysis software. The expression of each group immunofluorescence was analysed with one-way analysis of variance (ANOVA). If the variance was homogeneous, the data was analysed by LSD test; if not, the Tamhane’s T2 test was applied. Probability values of P < 0.05 were considered significant.
4. Results
4.1. Histological Observation of Liver Slices after Cultured for 6 Hours by Optical Microscope
After cultured for 6 hours, the cell survival state of liver slices was good by light microscope observation.
Figure 1. The UPLC and total ion chromatogram of Tussilago farfara alkaloids, as well as the primary and secondary mass spectra of peak 1 (tR 28.133 min) and peak 2 (tR 25.573 min) (Note 1: The UPLC/UV (254 nm) (up) and total ion chromatogram (down) of Tussilago farfara alkaloids; 2 and 3: the primary and secondary mass spectra of peak 1 and peak 2; peak 1 and 2 are deduced to be pyrrolizidine alkaloids of senkirkine type on the basis of mass fragmentation and literature data [25] ).
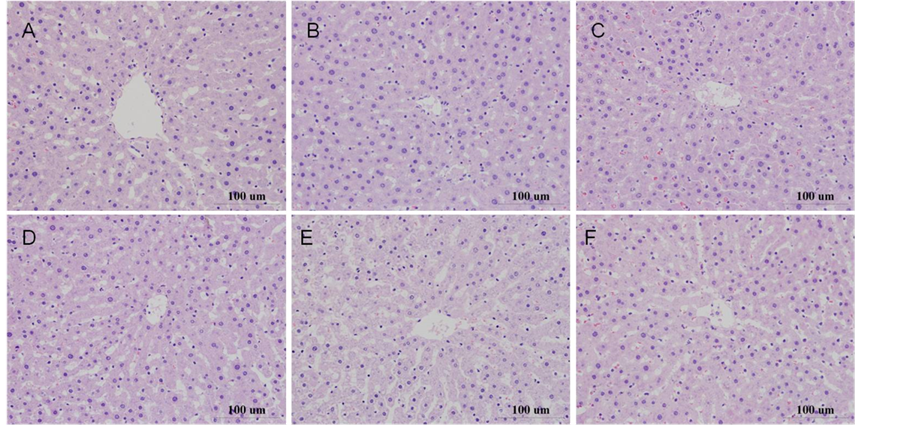
compared with fresh liver tissue sections (cultured for 0 hour) prepared by the same vibration cutting machine, the liver structure of all groups is clear, the liver cell is in good condition, no degeneration, necrosis and inflammation, there was no significant morphological differences in liver slices cultured for 6 hours, the results are showed in Figure 2.
4.2. The Effect of Monocrotaline, Tussilago farfara Alkaloids on the Expression of CYP3A4
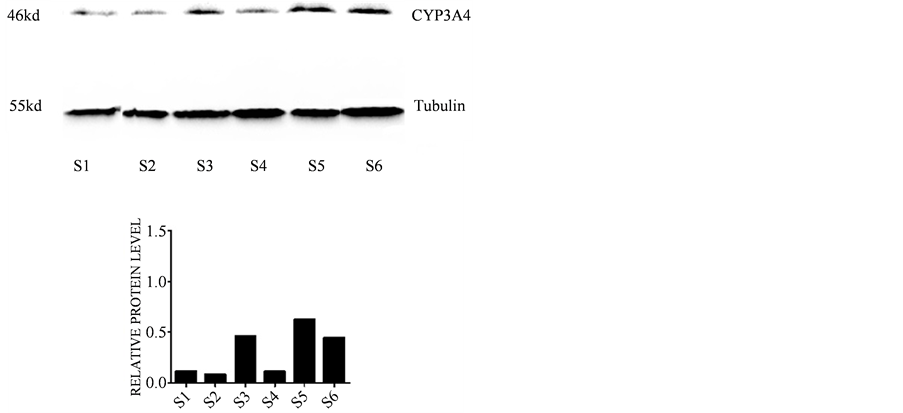
There was no significant difference in mean optical density value between the control group and 0.5% DMSO group. Compared with 0.5% DMSO group, the expression of CYP3A4 was significantly up-regulated after incubated with monocrotaline or Tussilago farfara alkaloids for 6 hours., the increases rate of CYP3A4 protein expression in four drug groups S3-S6 was 44.8%, 36%, 64.4%, 43.0% respectively. The results are showed in Figure 3.
Figure 2. Histopathology pictures after incubation for 6 hours, HE × 400 (Note: (A) medium control; (B) DMSO control; (C) 0.05% Monocrotaline; (D) 0.2% Monocrotaline; (E) 0.05% Tussilago farfara alkaloids; (F) 0.2% Tussilago farfara alkaloids).
Figure 3. The effects of different drugs on the expression of CYP3A4 in microsomes of rat liver (Note: S1: medium control, S2: 0.5% DMSO control, S3: 0.2% Monocrotaline, S4: 0.05% Monocrotaline, S5: 0.2% Tussilago farfara alkaloids, S6: 0.05% Tussilago farfara alkaloids).
4.3. The Effect of Monocrotaline, Tussilago farfara Alkaloids on the Expression of CYP2B1 + CYP2B2, CYP2E1 by Immunofluorescence
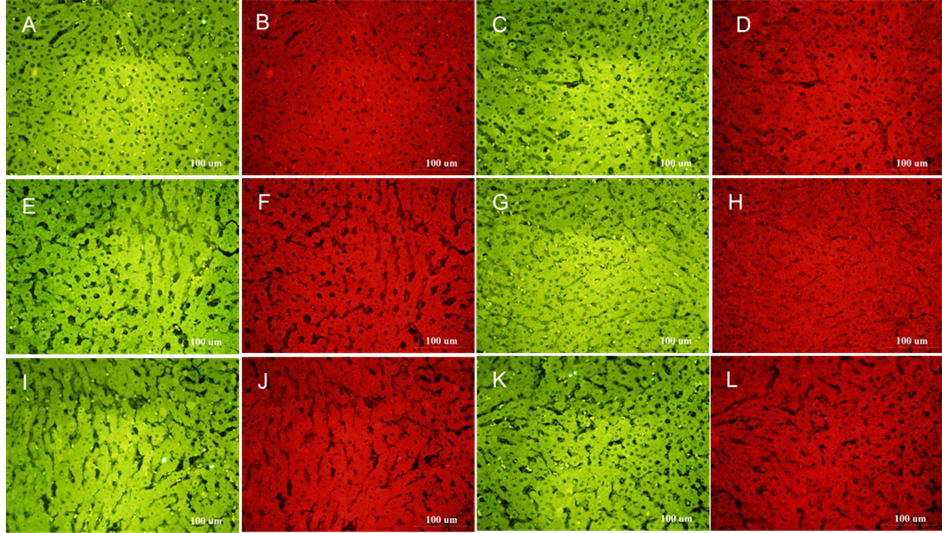
There was no significant difference in the mean optical density value between the control group and 0.5% DMSO group (P > 0.05). After incubation for 6 hours, compared with the DMSO group, the expression of CYP2B1 + CYP2B2 was significantly increased after incubating with 0.2% monocrotaline (P < 0.05), the expression of CYP2E1 in 0.05% and 0.2% Tussilago farfara alkaloids groups was significantly up-regulated (P < 0.05, P < 0.01 respectively). The results are showed in Table 1 and Figure 4.
5. Discussion
There is a close relationship between the occurrence of drug-induced liver injury and the level of CYP450s
Table 1. The effect of monocrotaline, Tussilago farfara alkaloids on the expression of CYP2B1 + CYP2B2 and CYP2E1 by immunofluorescence (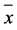 ± S, n = 6).
± S, n = 6).
Note: Compared with DMSO control, *P < 0.05, **P < 0.01.
Figure 4. The effects of different drugs on the expression of CYP2B1 + CYP2B2 and CYP2E1 by immunofluorescence. (Note: 1. The expression of CYP2B1 + CYP2B2 is green fluorescence, the expression of CYP2E1 is red fluorescence. 2. (A) (B) Medium control; (C) (D) DMSO control; (E) (F) 0.05% Monocrotaline; (G) (H) 0.2% Monocrotaline; (I) (J) 0.05% Tussilago farfara alkaloids; (K) (L) 0.2% Tussilago farfara alkaloids).
activity in liver. CYP450s is a superfamily of monooxygenase, which mainly existed in the microsomes of liver and intestine. They are main phase I drug metabolizing enzymes in body, catalyzing a lot of metabolism of endogenous and exogenous substance. Hepatic CYP450s system plays an important role in drug metabolism. It can change the drug structure from hydrophobic to hydrophilic, which makes it more easily excreted [26] .
Human genome sequence has revealed about 107 human P450 genes: 59 active and about 48 pseudogenes, and mainly 10 genes encode protease involved in drug metabolism, in which the most important enzymes for drug metabolism are CYP2C9, CYP2C19, CYP2D6 and CYP3A4, and about 34% of drug metabolism are catalyzed by CYP3A4. The most important isoforms responsible for the biotransformation of chemicals and especially for the metabolic activation of pre-carcinogens are CYP1A, CYP1A2, CYP1B1, CYP2A6, CYP2E1 and CYP3A4, and more than 70 different chemicals with diverse structures are metabolised by CYP2E1 [27] [28] . CYP2B is mainly existed in the form of CYP2B1 in SD rats [29] , the induction of CYP2B by Phenobarbital has been studied in rat liver slices [1] .
Culture system should be able to keep the metabolic activity of liver slices within the time co-cultured with drugs to mostly reflect the metabolic characteristics of pharmaceutical itself. Differential decrease of CYP activity may have a great impact on the accuracy of quantitative prediction of drug metabolism [30] . Some researchers have shown that metabolism of testosterone, lidocaine and antipyrine of rat liver slices remained close to initial levels during cultured in WME media for 24 hours [31] . Total CYP content in liver slices incubated in dynamic organ culture with Waymouth’s medium is maintained over 8 hours [4] . However, other researchers reported CYP activity in human liver slices is rapidly decreased to levels < 50% of initial levels after cultured for 4 hours [32] . In our study, we chose BPM medium in which the activity of liver slices can maintain 96 hours and the levels of CYP2B and 2E1 protein are at 1.8 and 1.9-fold higher than the slices maintained in the serum-con- taining medium respectively [1] . The new BPM medium provides suitable conditions for maintaining CYP2B and 2E1 in liver slices and supports the investigation of drug-induced modulation of these enzymes. Our study confirms that there is no significant difference in morphology of liver slices cultivated for 6 hours compared with the fresh-cut liver slices, indicating liver cells of liver slices can maintain good status at least 6 hours in the culture system.
Liver injury induced by containing PAs drugs is closely related of the CYP450 activity, it has been reported that some traditional Chinese medicine which contain pyrrolizidine alkaloids such as Crotalaria sessiliflora L., Senecio scandens Buch-Ham. ex D. Don, Heliotropium europaeum L. and Ephedra sinica Stapf, could cause dose-dependent hepatic injury, research suggests that mechanism of liver injury may be related with unstable metabolites such as pyrrole derivatives which is transformed in the role of cytochrome P450. These unstable metabolites could damage liver sinusoidal endothelial cell, cause liver blood disorder [33] .
In our previous studies had reported liver toxicity of monocrotaline and Tussilago farfara alkaloids by precision-cut liver slice technique, the results showed that LDH leakage was significantly increased and protein content was obviously decreased after monocrotaline co-culture for 24 h with final concentration 0.02, 0.1 and 0.5 g∙L−1, and LDH and ALT leakage were significantly increased with final concentration 0.5 g∙L−1. GGT leakage was significantly increased and protein content was obviously decreased with final concentration 2.0 g∙L−1 after co-culture for 6 hours with the total Alkaloid of Tussilago farfara [23] [24] . Through this experiment, the results show that Monocrotaline mainly induce CYP3A4 and CYP2B1 + CYP2B2, Tussilago farfara alkaloids (containing PAs by LC-MS analysis) can significantly upregulate the expression of CYP2E1 and CYP3A4. The study suggests that the mechanism of liver injury is closely related to the active metabolite of CYP450. The upregulation of CYP450s expression will cause a corresponding increase in enzyme activity, thus enhancing the drug metabolism, which increase the toxic metabolites to cause liver damage. However, what kind of metabolites and what kind of metabolic pathway have not been clarified in this study, these research works are in progress.
6. Conclusion
In summary, we conclude that the up-regulation of CYP3A4, CYP2B1 + CYP2B2 and CYP2E1 may be one of the toxic mechanisms of liver injury of monocrotaline and Tussilago farfara total alkaloids.
Acknowledgements
The authors thank department of animal management of our center for helping us feed animals (SPF). This work was supported by ICMM Grant ZZ2006106 and NSFC Grant 90709043.
Declaration of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Cite this paper
HailinWang,LianqiangHui,ChunLi,TingLiu,Chang’anYu,ChunyuCao,RanHao,YiZhang, (2016) Application of Precision-Cut Rat Liver Slice to Study the Influence of Monocrotaline, Tussilago farfara Alkaloids on the Expression of Cytochrome P450 Enzymes. Health,08,370-379. doi: 10.4236/health.2016.84039
References
- 1. Catania, J.R., McGarrigle, B.P., Rittenhouse-Olson, K. and Olson, J.R. (2007) Induction of CYP2B and CYP2E1 in Precision-Cut Rat Liver Slices Cultured in Defined Medium. Toxicology in Vitro, 21, 109-115.
http://dx.doi.org/10.1016/j.tiv.2006.08.001 - 2. Neyrinck, A.M., Gomez, C. and Delzenne, N.M. (2004) Precision-Cut Liver Slices in Culture as a Tool to Assess the Physiological Involvement of Kupffer Cells in Hepatic Metabolism. Comparative Hepatology, 3, S45.
http://dx.doi.org/10.1186/1476-5926-2-S1-S45 - 3. Lake, B.G., Charzat, C., Tredger, J.M, . Renwick, A.B., Beamand, J.A. and Price, R.J. (1996) Induction of Cytochrome P450 Isoenzymes in Cultured Precision-Cut Rat and Human Liver Slices. Xenobiotica, 26, 297-306.
http://dx.doi.org/10.3109/00498259609046709 - 4. Lerche-Langrand, C. and Toutain, H.J. (2000) Precision-Cut Liver Slices: Characteristics and Use for in Vitro Pharmaco-Toxicology. Toxicology, 153, 221-253.
http://dx.doi.org/10.3109/00498259609046709 - 5. Lupp, A., Danz, M. and Müller, D. (2001) Morphology and Cytochrome P450 Isoforms Expression in Precision-Cut Rat Liver Slices. Toxicology, 161, 53-66.
http://dx.doi.org/10.1016/S0300-483X(01)00333-X - 6. Krumdieck, C.L., dos Santos, J.E. and Ho, K.J. (1980) A New Instrument for the Rapid Preparation of Tissue Slices. Analytical Biochemistry, 104, 118-123.
http://dx.doi.org/10.1016/0003-2697(80)90284-5 - 7. Behrsing, H.P., Vickers, A.E. and Tyson, C.A. (2003) Extended Rat Liver Slice Survival and Stability Monitored Using Clinical Biomarkers. Biochemical and Biophysical Research Communications, 312, 209-213.
http://dx.doi.org/10.1016/0003-2697(80)90284-5 - 8. van Midwoud, P.M., Groothuis, G.M., Merema, M.T. and Verpoorte, E. (2010) Microfluidic Biochip for the Perifusion of Precision-Cut Rat Liver Slices for Metabolism and Toxicology Studies. Biotechnology and Bioengineering, 105, 184-194.
http://dx.doi.org/10.1002/bit.22516 - 9. de Graaf, I.A., Olinga, P., de Jager, M.H., Merema, M.T., de Kanter, R., van de Kerkhof, E.G. and Groothuis, G.M. (2010) Preparation and Incubation of Precision-Cut Liver and Intestinal Slices for Application in Drug Metabolism and Toxicity Studies. Nature Protocols, 5, 1540-1551.
http://dx.doi.org/10.1002/bit.22516 - 10. Geng, X.C., Shen, L.Z., Li, B. and Wang, J.Z. (2011) Progress of Biomarkers for Hepatic Toxicity. Chinese Pharmaceutical Journal, 46, 721-725.
- 11. Wang, J., Wang, C.H. and Wang, Z.T. (2007) Advancement of Investigation on Cytotoxicity and Mechanism of Pyrrolizidine Alkaloids. International Journal of Pharmaceutical Research, 34, 246-249, 258.
- 12. Liu, P. (2004) Liver Injuries Caused by Chinese Herbs. Chinese Journal of Hepatology, 12, 243.
- 13. Zeng, M.Y., Li, M.M. and Zhao, X.W. (1996) Traditional Chinese Medicine Containing Pyrrolizidine Alkaloids and Its Toxicity(2)-Tussilago farfara L. and Petasites japonicas miq.etc. Traditional Chinese Drug Research and Clinical Pharmacology, 7, 51-52.
- 14. Keller, K., Hansel, R., De Smet, P.A.G.M. and Chandler, R.F. (1992) Adverse Effects of Herbal Drugs. Vol. 1, Springer-Verlag, Berlin Heidelberg, 191-206, 211-214.
- 15. Williams, G.M., Mori, H., Hirono, I. and Nagao, M. (1980) Genotoxicity of Pyrrolizidine Alkaloids in the Hepatocyte Primary Culture/DNA-Repair Test. Mutation Research/Genetic Toxicology, 79, 1-5.
http://dx.doi.org/10.1016/0165-1218(80)90141-X - 16. Müller, L., Kasper, P. and Kaufmann, G. (1992) The Clastogenic Potential in Vitro of Pyrrolizidine Alkaloids Employing Hepatocyte Metabolism. Mutation Research/Genetic Toxicology, 282, 169-176.
http://dx.doi.org/10.1016/0165-7992(92)90091-U - 17. Wagner, J.G., Petry, T.W. and Roth, R.A. (1993) Characterization of Monocrotalinepyrrole-Induced DNA Cross-Linking in Pulmonary Artery Endothelium. American Journal of Physiology, 264, L517-L522.
- 18. Zhang, G.S. (2003) Cytochrome P450 and Drug Induced Liver Injury. Journal of Jinzhou Medical College, 5, 45-47.
- 19. Kaplowitz, N. (2002) Biochemical and Cellular Mechanisms of Toxic Liver Injury. Seminars in Liver Disease, 22, 137-144.
http://dx.doi.org/10.1055/s-2002-30100 - 20. Vuilleumier, N., Rossier, M.F. and Chiappe, A. (2006) CYP2E1 Genotype Andi-Soniazid-Induced Hepatotoxicity in Patients Treated for Latent Tuber-Culosis. European Journal of Clinical Pharmacology, 62, 423-429.
http://dx.doi.org/10.1007/s00228-006-0111-5 - 21. Wolf, K.K., Wood, S.G., Allard, J.L., Hunt, J.A., Gorman, N., Walton-Strong, B.W., et al. (2007) Role of CYP3A and CYP2E1 in Alcohol-Mediated Increases in Acetaminophen Hepato-Toxicity: Comparison of Wild-Type and Cyp2e1(-/-) Mice. Drug Metabolism and Disposition, 35, 1223-1231.
http://dx.doi.org/10.1124/dmd.107.014738 - 22. Yao, J.-C., Rao, J., Zeng, L.-G., Zhang, L.-Y., Jiang, Z.-Z., He, L., Hu, L. and Zhao, X.-Y. (2010) Metabolism and Enzyme Kinetics of Triptolide in Rat Liver Microsomes. China Pharmacy, 7, 577-580.
- 23. Hui, L.Q., Gao, S.R., Liu, T., Cao, C.Y., Guo, J., Hao, R., Yi, Y., Li, C.Y., Zhao, Y. and Liang, A.H. (2011) Evaluation of Vitro Hepatotoxicity of Monocrotaline by Precision-Cut Liver Slice Technique. China Journal of Chinese Materia Medica, 36, 628-632.
- 24. Hui, L.Q., Gao, S.R., Liu, T., Li, C.Y., Hao, R., Yi, Y., Guo, J., He, R., Cao, C.Y., Zhao, Y., Liang, A.H. and Zhang, Y. (2012) Hepatotoxicity on Water Extracts and the Total Alkaloid of Farfarae Flos. Chinese Journal of Experimental Traditional Medical Formulae, 18, 238-241.
- 25. Pu, S.B., Xu, D.R., Zhang, M., Zhou, H.H., Wang, Z.T. and Yu, G.D. (2004) The Detection of Hepatotoxic Pyrrolizidine Alkaloids in Flos Farfarae by LC/MSn. Chinese Journal of Natural Medicines, 2, 293-297.
- 26. Pessayre, D. (1995) Role of Reactive Metabolites in Drug-Induced Hepatitis. Journal of Hepatology, 23, 16-24.
- 27. Ma, K.F., Xie, X.J., Liu, Y. and Jia, H.Y. (2013) Progress in Genetic Polymorphism of Cytochrome P450 Enzymes and Drug-Induced Liver Injury. Chinese Journal of Pharmacology and Toxicology, 27, 889-892.
- 28. Bozina, N., Bradamante, V. and Lovric, M. (2009) Genetic Polymorghism of Metabolic Enzymes P450(CYP) as a Susceptibility Factor for Drug Response, Toxicity, and Cancer Risk. Archives of Industrial Hygiene and Toxicology, 60, 217-242.
http://dx.doi.org/10.2478/10004-1254-60-2009-1885 - 29. Yan, M.F., Hao, F.R., Xu, L.M., Tong, S.G., Ji, H.J., Shen, Z.F. and Jin, Y.Z. (2005) Effects of Mitomycin(MMC) and γ-Ray Irradiation on Cytochome p450 Content and Activities of CYP2B1 and CYP2E1 in Rats. Journal of Radiation Research and Radiation Processing, 23, 246-250.
- 30. Graaf, I.A., Groothuis, G.M. and Olinga, P. (2007) Precision-Cut Tissue Slices as a Tool to Predict Metabolism of Novel Drugs. Expert Opinion on Drug Metabolism & Toxicology, 3, 879-898.
http://dx.doi.org/10.1517/17425255.3.6.879 - 31. Olinga, P., Groen, K., Hof, I.H., De Kanter, R., Koster, H.J., Leeman, W.R., Rutten, A.A., Van Twillert, K. and Groothuis, G.M. (1997) Comparison of Five Incubation Systems for Rat Liver Slices Using Functional and Viability Parameters. Journal of Pharmacological and Toxicological Methods, 38, 59-69.
http://dx.doi.org/10.1016/s1056-8719(97)00060-9 - 32. Vanden Branden, M., Wrighton, S.A., Ekins, S., Gillespie, J.S., Binkley, S.N., Ring, B.J., Gadberry, M.G., Mullins, D.C., Strom, S.C. and Jensen, C.B. (1998) Alterations of the Catalytic Activities of Drug-Metabolizing Enzymes in Cultures of Human Liver Slices. Drug Metabolism and Disposition, 26, 1063-1068.
- 33. Xu, L.M. (2005) Understanding and Prevention of Liver Damage Caused by Chinese Herbal Medicine. Journal of Modern Medicine & Health, 21, 379-380.
NOTES
*These authors contributed equally to this work and should be considered co-first authors.
#Corresponding author.