Materials Sciences and Applications
Vol. 3 No. 8 (2012) , Article ID: 21805 , 9 pages DOI:10.4236/msa.2012.38081
Alginate Reinforced Chitosan and Starch Beads in Slow Release Formulation of Imazaquin Herbicide—Preparation and Characterization
![]()
1Department of Chemistry, University of Agriculture, Makurdi, Nigeria; 2Department of Chemistry, Nnamdi Azikiwe University, Awka, Nigeria.
Email: *lami.nnamonu@gmail.com
Received May 7th, 2012; revised June 10th, 2012; accepted July 8th, 2012
Keywords: alginate; chitosan; starch; encapsulation; imazaquin
ABSTRACT
In a bid to make slow release formulations of imazaquin, the herbicide was encapsulated in starch and chitosan beads reinforced with alginate. The beads were characterized using SEM, DSC and FTIR. Two types of formulations were made by extrusion into 0.25 M calcium chloride solution: chitosan/alginate (LNCI) and starch/alginate (LNSI) beads, and the third was by gelatinization of starch at 75˚C (LNSI2). Findings showed highly porous spherical beads, the starch/alginate beads bigger and less porous than the chitosan/alginate beads with diameters of 2.53 ± 0.01 and 2.31 ± 0.01 mm; porosity of 57.58% ± 0.2% and 81.28% ± 0.2% and swelling of 34.91% ± 0.2% and 80.35% ± 0.2%, respectively. FTIR revealed a reduction in intensity of the carboxylate peaks of alginate and the peak at 1058 cm−1, present in the FTIR of the matrices, is shifted to lower wave-numbers in the formulations, signifying interactions between the formulation components that make for good slow release. The DSC thermograms of all formulations showed evidence of interaction of imazaquin carboxylate group with the N-atoms of the macromolecules, which is indicative of reduced crystallinity of imazaquin.
1. Introduction
Reconciling the need to produce enough food with the need to preserve the environment, particularly in developing countries, has become one of the biggest challenges of the modern era. Achieving the Millennium Development Goals puts sustainable agriculture at the centre of the development agenda. Slow release (SR) technology improves pesticide delivery by optimizing pesticide activity profile while limiting its contact with the environment. In this setting, the amount of pesticide immediately available for undesirable losses is minimized through its application as a material adsorbed on a matrix or carrier.
Imazaquin is a broad-spectrum imidazolinone herbicide used for the control of annual grasses and broad leaf weeds in wheat, barley, soy beans and other legume crops. It may be applied either pre-plant incorporated, pre-plant surface, preor early post-emergence [1]. Imazaquin is a mobile, persistent herbicide with very high leaching potential and it controls weeds best if applied several times over in the course of the growing season [2]. It is therefore a good choice for slow release formulation since the technology eliminates repeated application of pesticides.
Chitosan, a cationic polysaccharide, is the deacetylated derivative of chitin (N-acetyl-d-glucosamine), a water insoluble polymer present in insect and crustacean exoskeletons and fungal cell walls.
Chitosan has received enormous interest for medical and pharmaceutical applications due to its nontoxic odourless nature, biodegradable properties and biocompatibility in animal tissues [3]. Blending of chitosan with other polymers is a convenient and effective method of improving its physical and mechanical properties for practical applications [4]. For agricultural applications, chitosan has been used for controlled release of N-P-K compound fertilizer [5] and of urea and atrazine [6].
Alginate gels are used as matrices for SR in agricultural applications because of their biodegradability and the ease of incorporation of pesticides using an aqueous system at ambient temperatures. The leaching of pesticides during the preparation of alginate beads has been improved by synthesizing bi-polymeric beads of alginate with other natural polysaccharides. Alginate-clay complexes have been used for various pesticides: diquat bromide [7], 2,6-dichlorobenzonitrile [8], carbofuran [9], thiobencarb [10]; metribuzin and alachlor [11,12], diuron [13]. Starch has been incorporated into alginate-clay formulations of thiram [14,15].
In this work, imazaquin is encapsulated in chitosan and starch modified by cross-linking with alginate, and the SR beads characterized. The use of alginate helps bead formation and strengthens the structure of the matrices. SEM was used to determine bead shape, diameter, overall morphology and distribution of the imazaquin herbicide in the formulations. DSC was used to ascertain the state of crystallinity of the herbicide. In addition to spectroscopic characterization of the SR formulations, information on intermolecular interactions was obtained using FTIR.
2. Experimental Procedure
2.1. Materials
Chitosan (high molecular weight, Brookfield viscosity 800,000 cps, deacetylation degree minimum 85.0%), imazaquin, calcium chloride dihydrate, corn starch amylopectin, corn starch amylose (both practical grade) and sodium alginate (algin) were obtained from Sigma Aldrich UK and used as received. Sodium hexametaphosphate was purchased from VWR Prolabo (BDH) International N/America and Europe. Glacial acetic and sodium hydroxide are the other chemicals used. High purity water used all through this work was obtained from a purification unit by Millipore® Corporation.
2.2. Methods
The formulations, except that of gelatinized starch, were prepared by external gelation achieved by extrusion through a silicone tubing (internal diameter 3 mm) with the aid of a peristaltic pump (Pharmacia LKB pump P-1), at a flow rate of 48 droplets/min, into a 200 ml gellant solution of 0.25 M CaCl2∙2H2O [16]. The distance from the orifice to the surface of the gellant solution was 240 mm to allow good droplet penetration. The bipolymeric beads formed immediately the slurry droplets hit the calcium chloride solution. The beads were allowed to set for 25 minutes, suction-filtered through a coarse frit Buchner funnel, washed twice with distilled water and air-dried. Blank beads were prepared in the same way but without herbicide.
2.2.1. Starch Beads
The starch beads were prepared using an aqueous dispersion containing 3% (w/w) of sodium alginate, 2% (w/w) of imazaquin and 15% (w/w) starch. The ungelatinized starch formulation, code named LNSI, was formed by dropping the slurry into the gellant solution, while LNSI2 (the gelatinized starch formulation) was prepared by gelatinization of the starch slurry at 75˚C. Stirring speed was increased to 500 rpm for 10 minutes to keep the gelatinized suspension homogeneous. This was allowed to cool to room temperature, cut into pieces of approximately 5 mm diameter and air-dried. Starch as used in this work refers to a 20:80 mixture of pure corn amylose: amylopectin starch.
2.2.2. Algin Core/Chitosan Beads (LNCI)
The chitosan SR formulation was prepared having a sodium alginate core in chitosan coating. An aqueous dispersion of 1.5 g (3%) sodium alginate and 2% imazaquin in 5 ml methanol was added drop wise, into 100 ml aqueous solution of 0.5% (v/v) acetic acid, 4% (w/w) CaCl2·2H2O and chitosan (100 mg) with pH adjusted to 5.0 using 0.5 M NaOH solution. The colorless glassy alginate-chitosan beads were washed with milli-Q water followed by 4% (w/v) sodium hexametaphosphate to harden the chitosan coat and prevent adhesion of beads during drying.
2.2.3. Swelling
Swelling studies of the beads were conducted by the water evaporation method straight after separation from the gellant solution. A sample of the beads was blotted with Kimberly-Clark® professional medical wipes to remove excess water and weighed immediately. The sample was air-dried in the fume cupboard and weighed until constant dry mass, which took between 24 to 48 hrs. The percent equilibrium swelling of the beads was computed from the mass changes.
2.2.4. Porosity
Porosity of beads was determined by the volume/density method. A 10 ml measuring cylinder was weighed empty, beads poured in and the sides of the cylinder gently tapped until the beads were well packed. The total volume (Vt) was noted and the cylinder and contents weighed. The volume occupied by the beads, Vb was calculated using the density of the matrix (ρ)
Volume of beads, Vb = mass/ρ
Pore volume and percent porosity were deduced from these.
Pore volume, (Vp) = Total volume (Vt)
– Volume of beads (Vb)
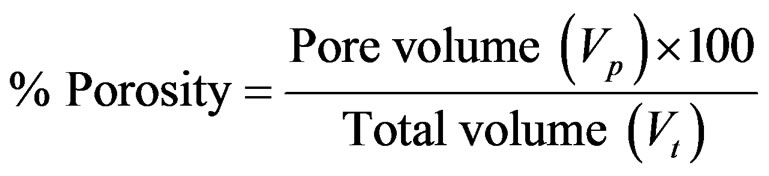
Density (g/ml) of matrices: Algin 1.73; Chitosan 0.6 - 0.8 (high density).
2.2.5. Scanning Electron Microscopy
The shape, external morphology and internal structure of the SR formulations were examined by scanning electron microscopy (SEM). Images (at different magnifications) of dry whole beads and their cross sections were captured on an FEI/PHILIPS XL 30 ESEM microscope after the samples were deposited on brass stubs (using double sided carbon tapes) and sputter coated with gold (20 nm) on an Edwards Pirani 501 Scan Coat Six sputter coater. The samples were exposed to accelerated voltage beam strength of 20.0 KV.
2.2.6. Differential Scanning Calorimetry
To evaluate crystallinity of herbicide, differential scanning calorimetry of pure herbicide, blank formulation and herbicide-loaded formulations were done on a PerkinElmer Pyris 1 model differential scanning calorimeter using Helium purge gas at 20 ml/min. A different machine, TA DSC Q1000 at 20 ml/min using Nitrogen purge gas and an internal coolant (intercooler IP) was used for chitosan. The specimen powder was passed through mesh 100 sieve and accurately weighed samples were heated in sealed aluminium pans at a rate of 10.00˚C/min from 25˚C to 250˚C temperature range. Starch was heated at 300.00˚C/min, while chitosan was heated from 0˚C to 600˚C on the DSC Q1000.
3. Results and Discussion
3.1. Encapsulation
In this work, it was necessary to modify chitosan with alginate because it has been reported that the mechanical strength of chitosan beads is low, thus limiting its use as a pharmaceutical dosage form [17]. The alginate matrixwhich consists of an open lattice structure, forms porous beads [18] and it was used to modify chitosan in a polyelectrolyte complex [18]. This approach was therefore employed here. The structure of chitosan is modified by cross linking with alginate via ionic interaction between the carboxyl residues of alginate and the amino terminals of chitosan [16]. A conceptual representation of this is depicted in Figure 1. This complexation reduces the porosity of the alginate beads and decreases the leakage of the encapsulated substances. In addition, chitosan acquires a higher level of mechanical strength with the support of the alginate gel mass. The interaction could also be via intermolecular hydrogen bonding which is the mechanism of starch—alginate cross linking [19].
3.2. Formulation Characteristics
All formulations had the same active ingredient loading, gelatinization not affecting loading since the melting point of imazaquin (224˚C) is much higher than gelatinization temperature (75˚C). The procedure gave good encapsulation efficiency of between 64% - 85% with total solid recovery above 95% (Table 1) for all formulations. The chitosan beads were smaller and had higher swelling and porosity than the starch beads. These could therefore also absorb water and preserve soil moisture on the field, in addition to releasing herbicide in an ecofriendly manner.
3.3. Scanning Electron Microscopy
The starch/alginate beads were spherical with visible micro particles (Plate 1(b)) while the chitosan/alginate beads were rougher and irregular. The alginate-chitosan formulation has an outer coat of chitosan (appearing like ordered cubes) on the inner algin skeleton. A higher magnification (×1500) showed cracks in the algin skeleton (Plate 1(a)). A cross section of the chitosan bead is
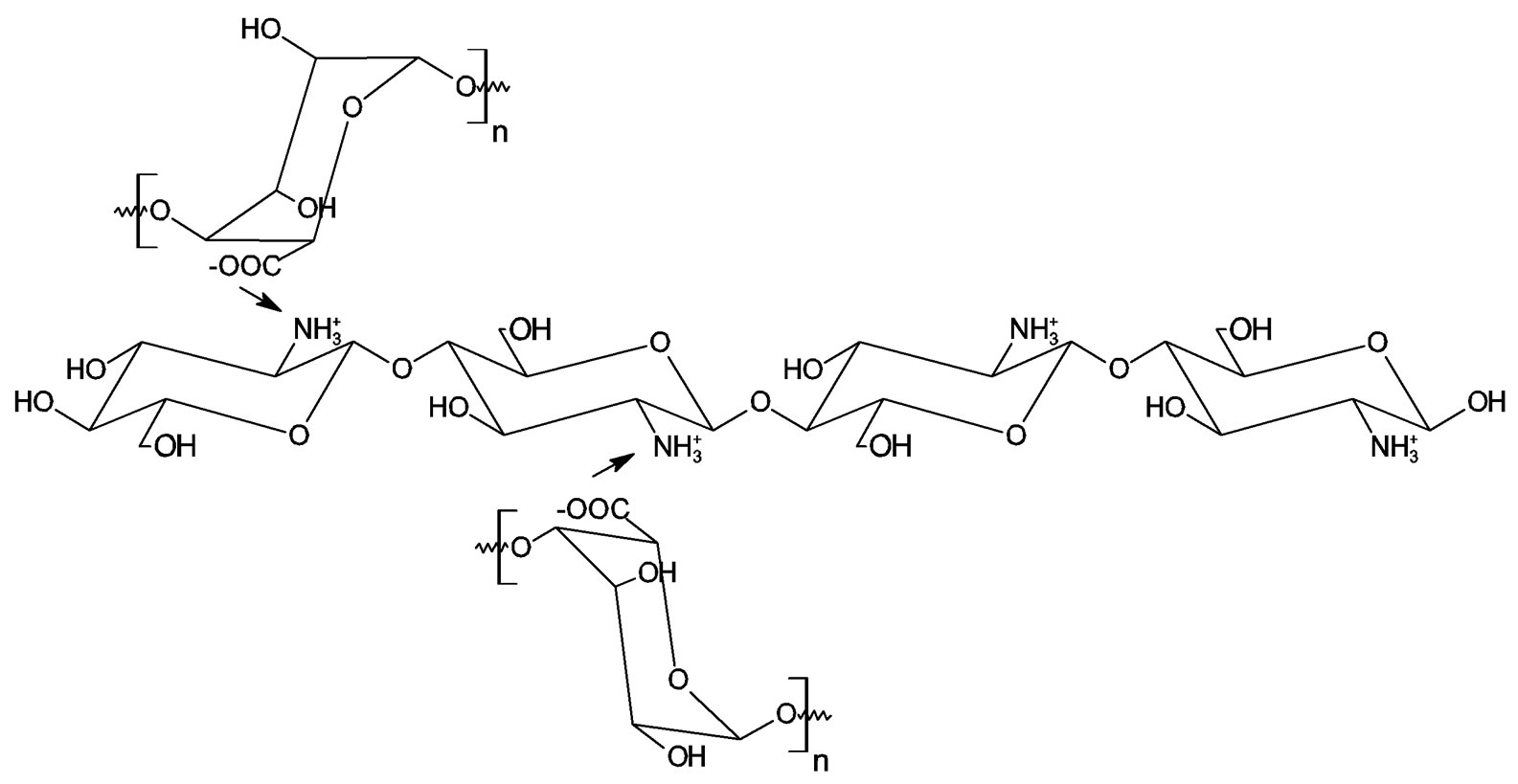
Figure 1. Chitosan-alginate cross linking interaction.
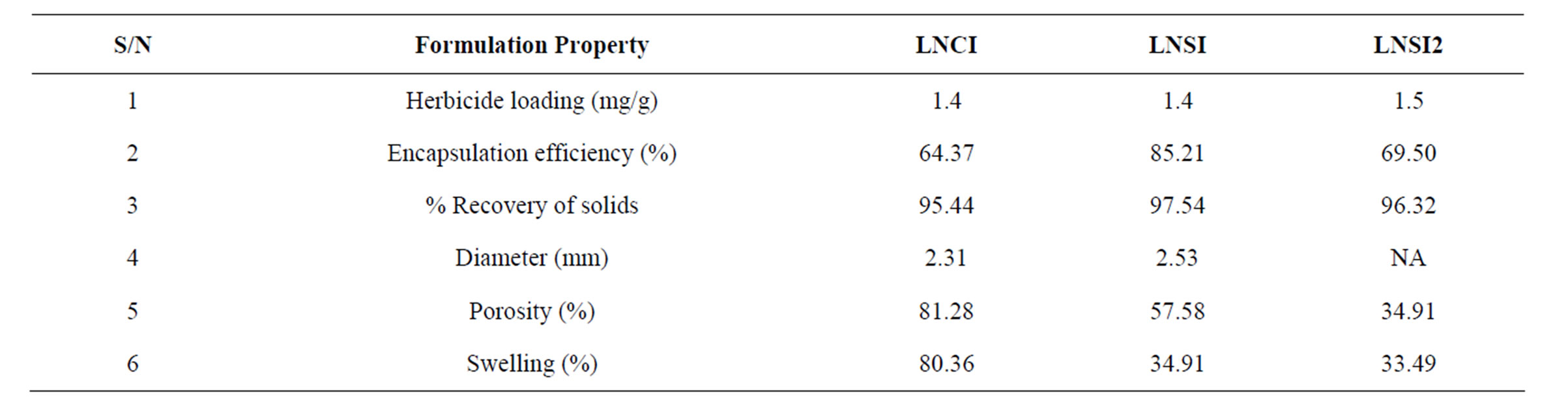
Table 1. Formulation characteristics.
shown in Plate 1b with tiny imazaquin particles at higher magnification. Small depressions, without visible pores were seen on the surfaces of the gelatinized formulation (Plate 2(a)). Gelatinization seems to expose the imazaquin crystals to the surface of the thin slices.
The porosity of the outer chitosan coat belies the dense internal structure of the algin core. Even though the cross section of the starch/alginate bead (Plate 2(b)) reveals a more “grainy” internal structure than that of the chitosan/ alginate formulation, the overall higher porosity of the latter must be as a result of two factors: the observed cracks in the algin skeleton and the intrinsic natural porosity of chitosan in the coat.
3.4. Differential Scanning Calorimetry
Imazaquin DSC thermograms reveal a sharp endothermic peak from 238˚C - 250˚C (Tp = 245.72˚C, peak height 31.3978 mW; peak area = 432.307 mJ, ∆H = 153.1917 J/g). Since the melting point of imazaquin is 224˚C, the wide range of this endothermic peak is probably suggestive of low purity of the technical grade imazaquin sample. There is also a sharp exothermic peak at 268˚C (Figure 2). Thermogravimetry associates this with a 100% loss of weight of the 6.258 mg sample. This means that decomposition occurred at this point. Chitosan powder exhibited exothermic peaks at 270˚C and 350˚C with Tp = 306.84˚C, ∆H = 1012 J/g). This process (accompanied by a 59% loss of weight as revealed by thermogravimetry) is indicative of polymer decomposition. There is an exothermic peak centered at about 20˚C in the DSC of chitosan (not shown), which is evident of a volume phase transition. Endothermic peaks occur over a large temperature range of about 35˚C to 180˚C (with peak temperature, Tp = 137.43˚C). This peak (∆H = 842.4 J/g), is attributable to water loss, the value of ∆H representing the energy required to vapourize water present in the powder samples. It has been reported that chitosan films were degraded at <280˚C - 300˚C [20], which agrees well with the results of this study. The absence of other endotherms in the DSC thermograms, besides the one at 35˚C - 180˚C, implies that chitosan is amorphous. The chitosan sample was heated from 30˚C to 500˚C at 10.00˚C/min for the first cycle and cooled from 500˚C to 30˚C at 200.00˚C/min for the second cycle.
When the starch sample was heated at 10.00˚C/min, no Tg was detected on the DSC thermogram. However, on increasing the heating rate to 500.00˚C/min, Tg of 54.35˚C (ΔCp = 0.017 J/g∙˚C) was recorded. Starch was heated from 0˚C to 30˚C at 500.00˚C/min; cooled from 300˚C to 0˚C at 500.00˚C/min and for the third cycle, heating was from 0˚C to 300˚C at 500.00˚C/min. In the thermogram, there was an exothermic peak (∆Cp = 0.704 J/g·˚C) between 50˚C and 57˚C (Figure 3). Also, at 281.35˚C, an endothermic peak was observed (δY = 10.294%). Thermogravimetry shows a loss of weight of 93.407%.
For sodium alginate, a 14.766 mg sample was heated from 30˚C to 350˚C recording an 86.52% loss of weight. A broad exothermic peak starting from 219˚C to 262˚C (Tp = 240˚C) was observed with δY = 52.060% and ΔCp = 1.259 J/g∙˚C.
Stacked thermograms of neat active ingredient, blank matrices and herbicide-loaded SR formulations show evidence of interaction of COO− group of imazaquin with the protonated N-atom of the macromolecule. The DSC parameters are summarized in Table 2.
3.5. Fourier Transform Infra Red Spectroscopy
The strong and broad absorption band between 3600 and 3200 cm−1 is observed in most of the spectra recorded in this work due to the hydroxyl group O−H stretching vibrations which is characteristic of natural polysaccharides. The strong asymmetric stretching absorption band at 1650 cm−1 and weaker symmetric stretching band near 1430 cm−1 are due to the presence of carboxylate anions [15]. The band at 1993 cm−1 is also assigned to asymmetric stretching of −COO− groups. The peak at 1058 cm−1 is present in the FTIR of the matrices, and shifted to lower wave-numbers in the formulations. In Figure 4, the bands at 1092 and 1043 cm−1 are characteristic of the



Plate 1. SEM images of (a) Algin core-chitosan/imazaquin SR bead; (b) its cross section.
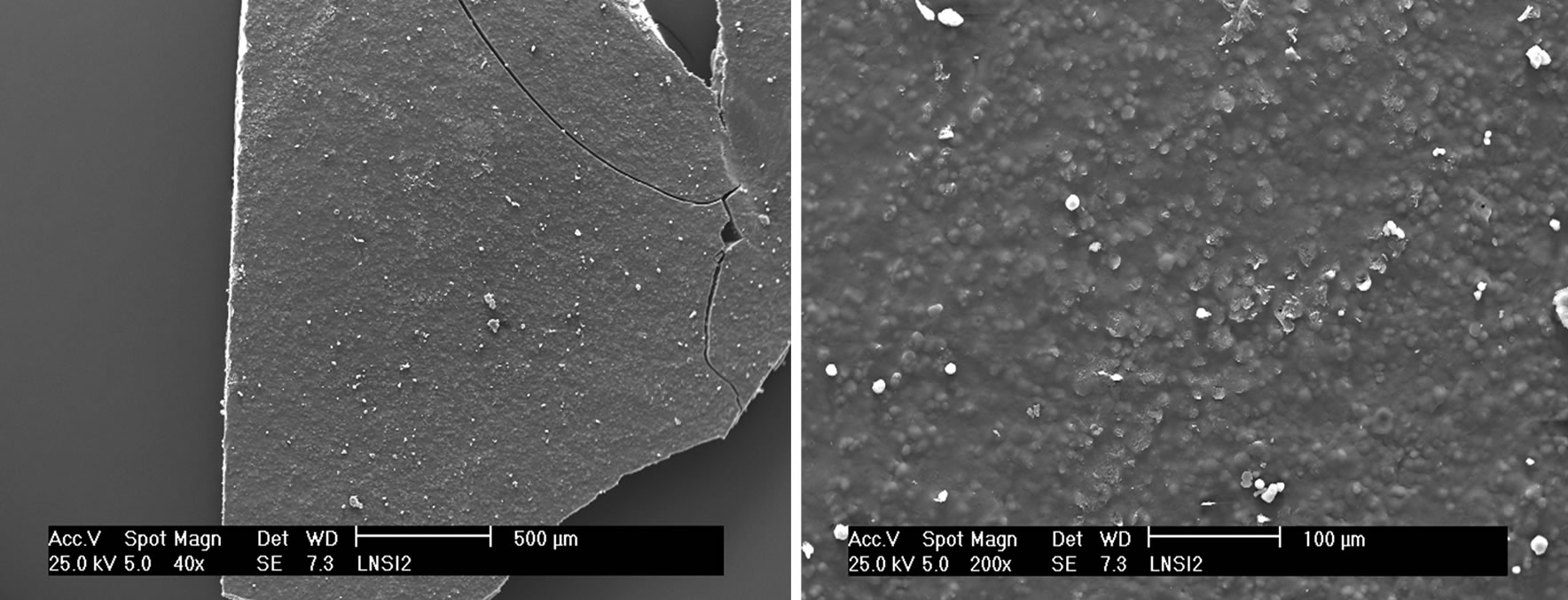
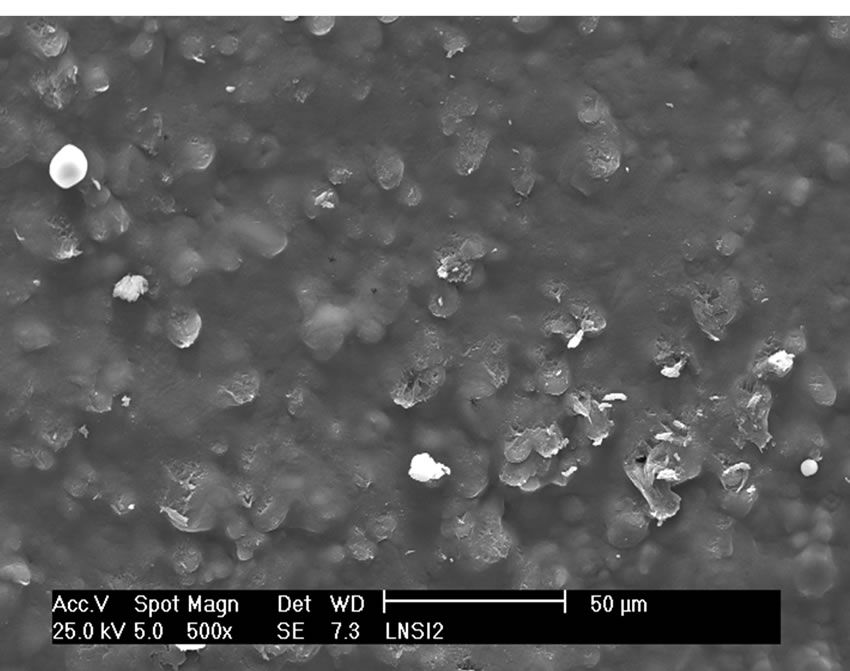

Plate 2. SEM images of (a) Gelatinized starch/imazaquin formulation; (b) Starch-alginate/imazaquin bead.
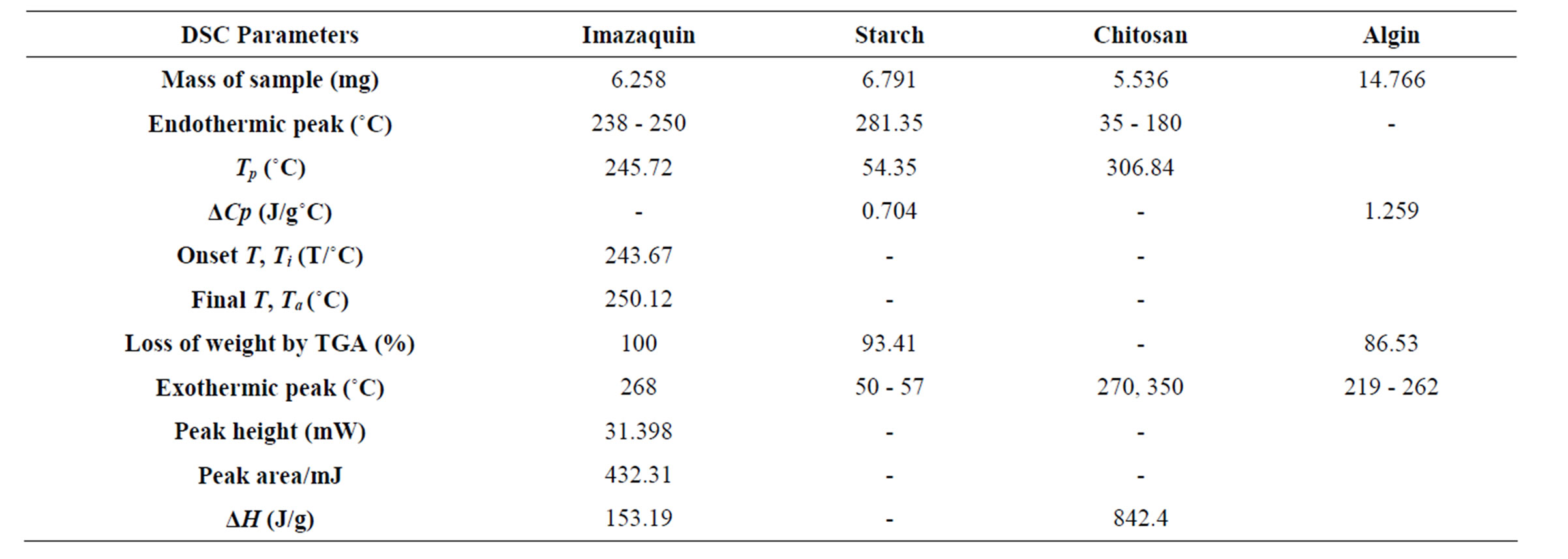
Table 2. DSC parameters of herbicide and matrices.
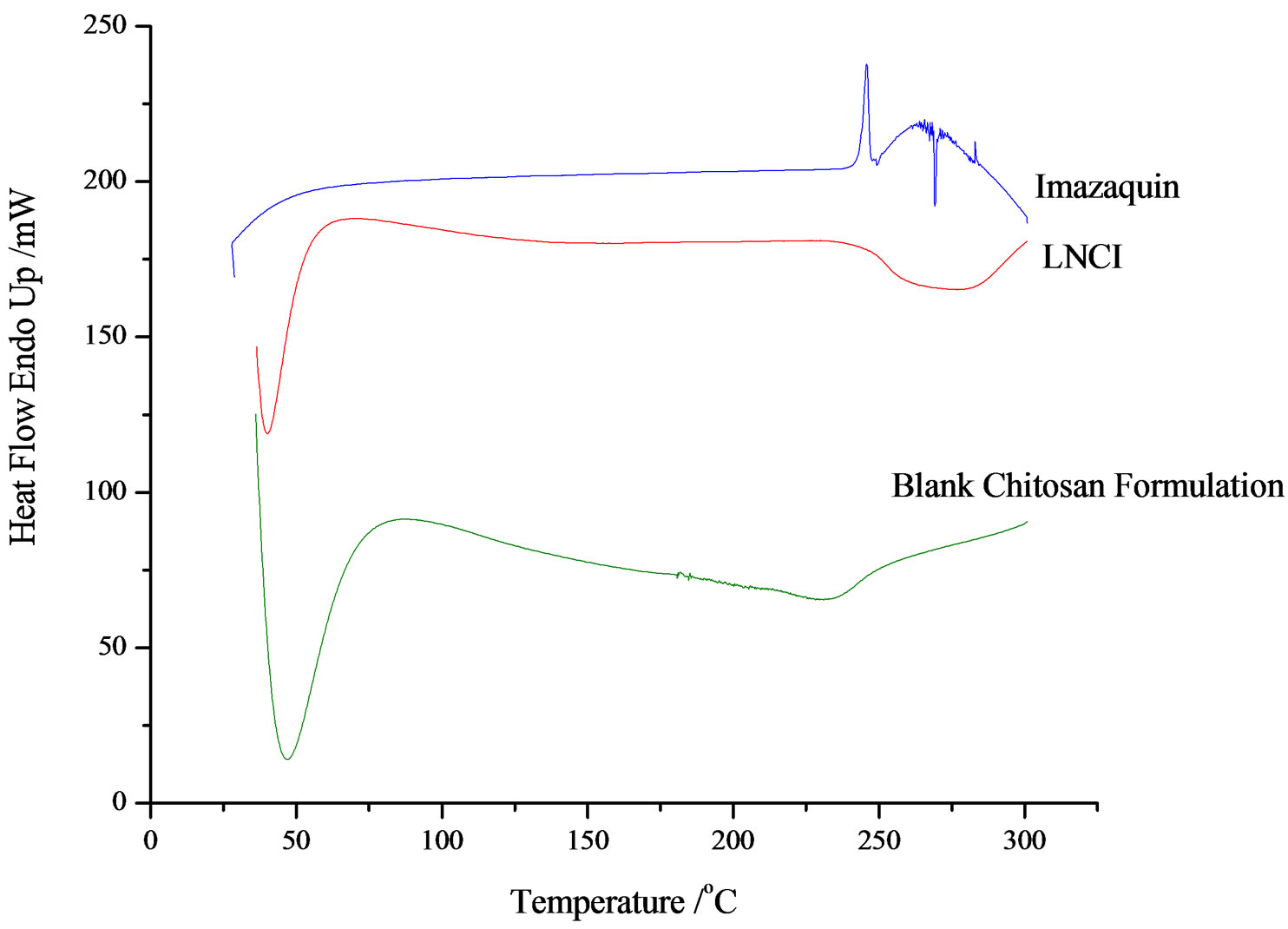
Figure 2. DSC thermograms of imazaquin, chitosan/imazaquin SR bead and blank chitosan formulation.
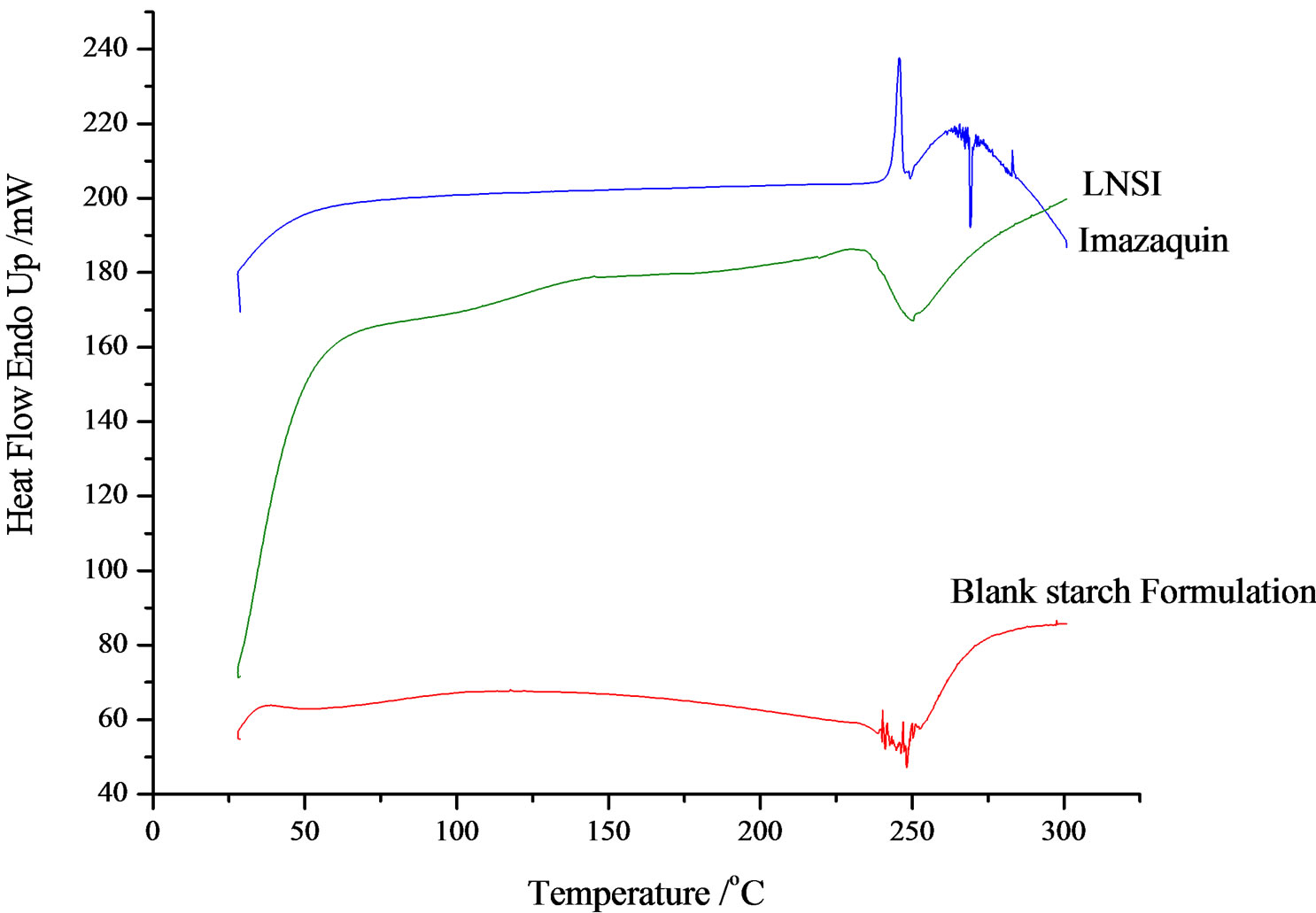
Figure 3. DSC thermograms of imazaquin, starch/imazaquin SR bead and blank starch formulation.
C−O−C link. There are two weak peaks observed between 2380 and 2350 cm−1 in the chitosan FTIR spectrum. These were shifted to 2875 cm−1 in the chitosan SR formulation. The medium intensity broad band at 4000 - 3500 cm−1 is characteristic of chitosans.
Imazaquin is uniquely a weak acid, possessing the carboxylic acid group, –COOH, which should be largely ionized at pH 5. When the imazaquin ion comes in contact with chitosan, the –COO– group interacts strongly with the protonated N-atoms of the macromolecule.
4. Conclusion
Starch and chitosan were reinforced with alginate and employed in compounding slow release formulations of imazaquin herbicide. The addition of alginate improved matrix strength and prevented leakage of encapsulated herbicide. DSC and FTIR of these SRFs revealed interaction between the macromolecules and imazaquin which makes for good slow release. The high porosity and swelling of chitosan SR beads is indicative that it would also serve water retentive functions and conserve soil moisture in the field.
5. Acknowledgements
Analytical services, Chemistry Department, Durham University, England is acknowledged. The corresponding
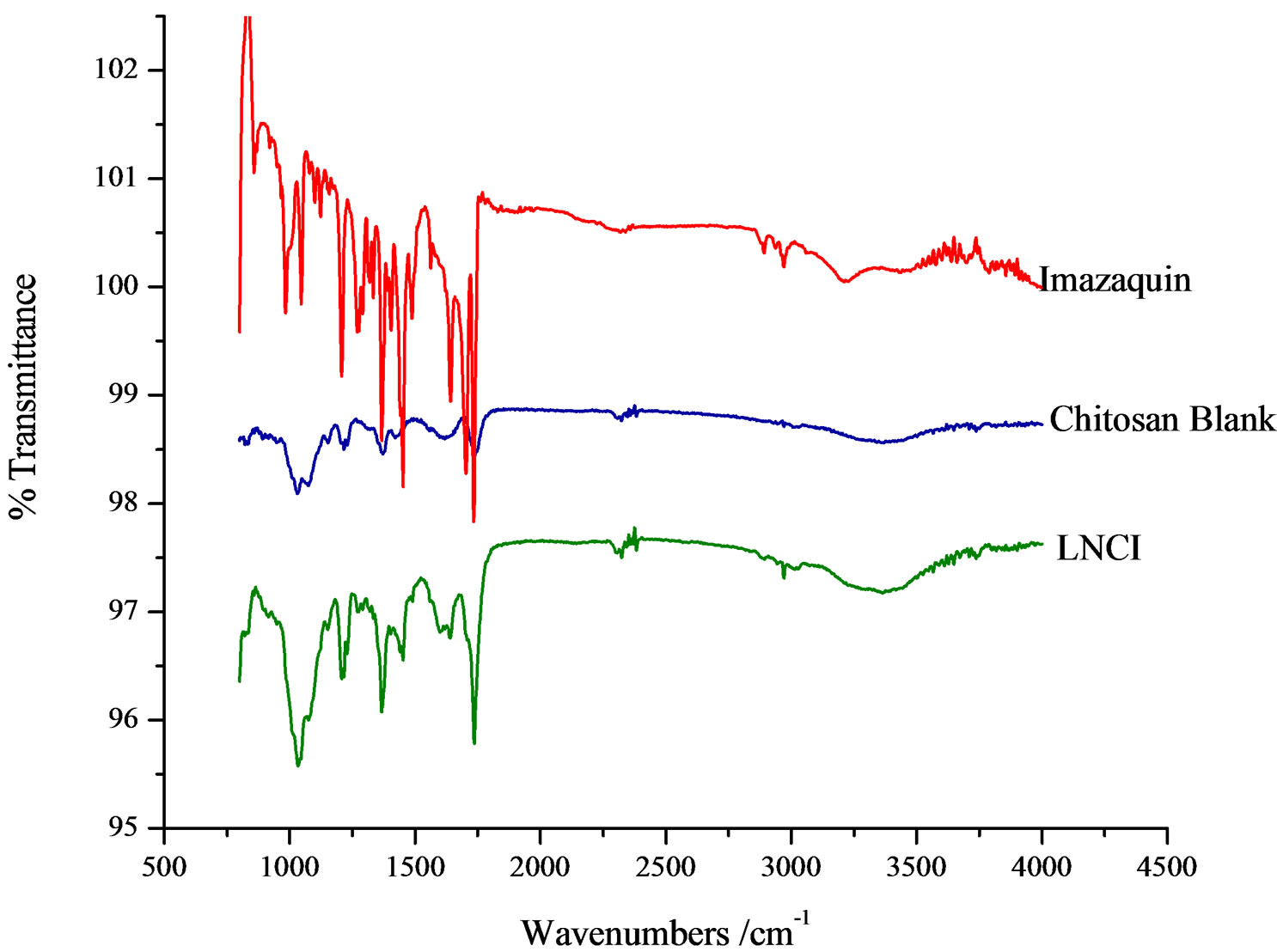
Figure 4. FTIR spectra of imazaquin, chitosan blank and chitosan/imazaquin formulation.
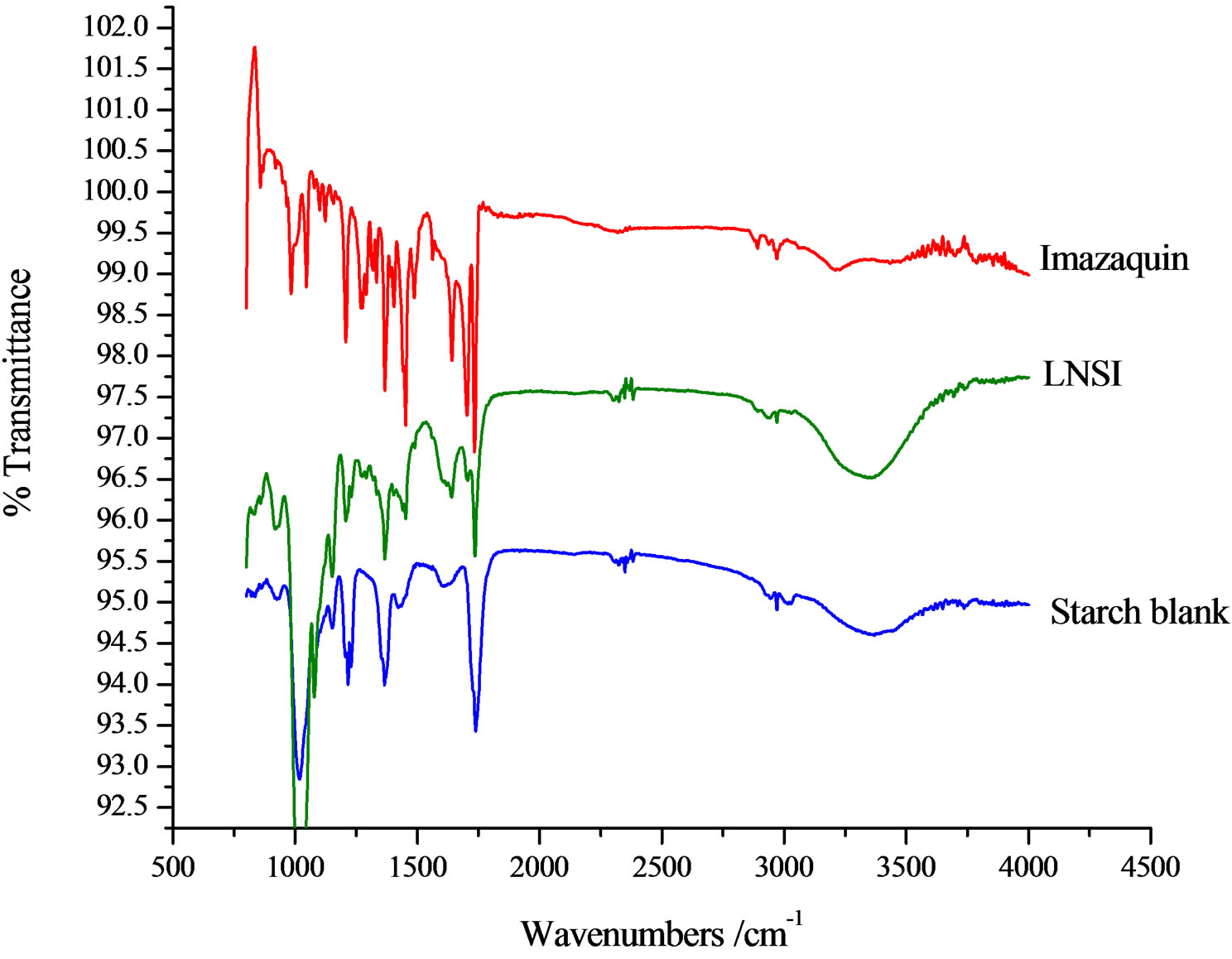
Figure 5. FTIR spectra of imazaquin, starch blank and starch/imazaquin formulation.
author is grateful to the Association of Commonwealth Universities for scholarship that made this work possible.
REFERENCES
- T. Roberts, “Metabolic Pathways of Agrochemicals Part 1: Herbicides and Plant Growth Regulators,” The RSC, Cambridge, 1998.
- H. E. Quicke, E. Hoien and L. Beaudrot, “Imazaquin, Imazapic and Pendimethalin for Weed Control in Hybrid Poplar: The 1998 Clatskanie, Oregon Study,” American Cyanamid Forestry Technical Service Research Report, 1999, pp. 99-101.
- A. Borzacchielo, L. Ambrosias, P. Netter, L. Nicholais, A. Balard and P. Sam, “Chitosan—Based Hydrogels: Synthesis and Characterization,” Journal of Material Sciences: Materials in Medicine, Vol. 12, No. 10-12, 2001, pp. 861-864. doi:10.1023/A:1012851402759
- X. Z. Shu and K. J. Zhu, “A Novel Approach to Prepare Tripolyphosphate/Chitosan Complex Beads for Controlled Release Drug Delivery,” International Journal of Pharmaceutics, Vol. 201, No. 1, 2000, pp. 51-58. doi:10.1016/S0378-5173(00)00403-8
- L. Wu and M. Lin, “Preparation and Properties of Chitosan-Coated NPK Compound Fertilizer with Controlled Release and Water Retention,” Carbohydrate Polymers, Vol. 72, No. 2, 2008, pp. 240-247. doi:10.1016/j.carbpol.2007.08.020
- M. A. Teixeira, W. J. Paterson, Q. Li, E. J. Dum, B. K. Hunter and M. F. A. Goosen, “Assessment of Chitosan Gels for the Controlled Release of Agrochemicals,” Industrial & Engineering Chemistry Research, Vol. 29, No. 7, 1990, pp. 1205-1209. doi:10.1021/ie00103a019
- P. R. F. Barret, “Some Studies on the Use of Alginates for the Placement and Controlled Release of Diquat on Submerged Aquatic Plants,” Pesticide Science, Vol. 9, No. 5, 1978, pp. 425-433. doi:10.1002/ps.2780090507
- W. J. Connick Jr., “Controlled Release of the Herbicides 2,4-D and Dichlobenil from Alginate Gels,” Journal of Applied Polymer Science, Vol. 27, No. 9, 1982, pp. 3341- 3348. doi:10.1002/app.1982.070270912
- L. Vollner, “Agrochemical Formulations for Improved Efficacy and Reduced Environmental Impacts: Slow Release Formulations with Natural Polymers,” In: M. Mansour, Ed., Study and Prediction of Pesticides Behaviour in Soils, Plants and Aquatic Systems, Neuherberg GSF, 1990, pp. 136-145.
- J. Gan, M. Hussaina and M. N. Rathor, “Behaviour of Alginate Kaolin Based Controlled-Release Formulations of the Herbicide Thiobencarb in a Simulated Ecosystem,” Pesticide Science, Vol. 42, No. 4, 1994, pp. 261-272. doi:10.1002/ps.2780420403
- A. B. Pepperman and J. W. Kuan, “Slow Release Formulations of Metribuzin Based on Alginate-Kaolin-Linseed Oil,” Journal of Controlled Release, Vol. 26, No. 1, 1993, pp. 21-30. doi:10.1016/0168-3659(93)90205-J
- A. B. Pepperman, J. W. Kuan, “Controlled Release of Alachlor Based on Calcium Alginate,” Journal of Controlled Release, Vol. 34, No. 1, 1995, pp. 17-23. doi:10.1016/0168-3659(94)00111-7
- M. Fernandez-Perez, M. Villa-Franca-Sanchez, E. Gonzalez-Pradas and F. Flores-Cespedes, “Controlled Release of Diuron from an Alginate-Bentonite Formulation: Water Release Kinetics and Soil Mobility Study,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 2, 1999, pp. 791-798. doi:10.1021/jf980878y
- F. Cespedes, M. Villafranca Sanchez, S. Perez Garcia and M. Fernandez Perez, “Modifying Sorbents in Controlled Release Formulations to Prevent Herbicides Pollution,” Chemosphere, Vol. 69, No. 5, 2007, pp. 785-794. doi:10.1016/j.chemosphere.2007.05.005
- B. Singh, D. K. Sharma, R. Kumar and A. Gupta, “Controlled Release of the Fungicide Thiram from StarchAlginate-Clay Based Formulation,” Applied Clay Science, Vol. 45, No. 1-2, 2009, pp. 76-82. doi:10.1016/j.clay.2009.03.001
- T. W. Wong, L. W. Chan, S. B. Kho and P. W. S. Heng, “Design of Controlled-Release Solid Dosage Forms of Alginate and Chitosan Using Microwave,” Journal of Controlled Release, Vol. 84, No. 3, 2002, pp. 99-114. doi:10.1016/S0168-3659(02)00237-7
- X. Z. Shu and K. J. Zhu, “A Novel Approach to Prepare Tripolyphosphate/Chitosan Complex Beads for Controlled Release Drug Delivery,” International Journal of Pharmaceutics, Vol. 201, No. 1, 2000, pp. 51-58. doi:10.1016/S0378-5173(00)00403-8
- Y. Maeda, T. Miyamoto and E. Kawashima, “Preparation of Chitosan-Reinforced Alginate Gel Beads—Effects of Chitosan on Gel Matrix Erosion,” International Journal of Pharmaceutics, Vol. 96, No. 1-3, 1993, pp. 139-145. doi:10.1016/0378-5173(93)90221-Z
- W. S. Wan-Ngah, A. Kamari and Y. J. Koay, “Equilibrium and Kinetic Studies of Adsorption of Copper (II) on Chitosan and Chitosan/PVA Beads,” International Journal of Biological Macromolecules, Vol. 34, No. 3, 2004, pp. 155-161. doi:10.1016/j.ijbiomac.2004.03.001
- M. F. Cervera, J. Heinamaki, K. Krogans, A. C. Jorgensen, M. Karjaleinen, A. Colarte and J. Yliruusi, “Solid State and Mechanical Properties of Aqueous ChitosanAmylose Starch Films Plasticized with Polyols,” AAPS PharmSciTech, Vol. 5, No. 1, 2004, pp. 109-114.
NOTES
*Corresponding author.

