Journal of Modern Physics
Vol.06 No.07(2015), Article ID:57672,6 pages
10.4236/jmp.2015.67099
On Universality of Laws of Ideal Gas
Vladimir G. Kirtskhalia
I. Vekua Sukhumi Institute of Physics and Technology (SIPT), Tbilisi, Georgia
Email: v.kirtskhalia@gmail.com
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 27 February 2015; accepted 27 June 2015; published 30 June 2015
ABSTRACT
It is shown that in the Earth’s atmosphere, due to influence of the gravitational field, coefficient of thermal expansion depends on altitude. The altitude intervals for individual gases for which the laws of ideal gases can be applied have been determined and it has been established that they are dependent on the adiabatic index and molar mass of these gases.
Keywords:
Ideal Gases, Inhomogeneous Medium, The Earth’s Atmosphere

1. Introduction
One of the fundamental laws of physics states that all characteristic properties of inhomogeneous (nonisotropic) medium must be inhomogeneous, in other words, they must depend on space coordinates. Until recently this principle was violated in relation speed of sound in the Earth’s atmosphere, which is one of most important thermodynamic parameter of medium. It was thought that the Earth’s gravitational field did not affect the processes of generating and propagation of sound waves and that is why the Earth’s atmosphere could be considered as a homogeneous medium in relation to sound waves. Proceeding from this assumption, sound spreads with adiabatic velocity, which is dependent only on temperature., i.e. . This circumstance can be mostly explained by the absence of experimental data concerning speed of sound measurement on different altitudes. It is proved by the fact that this kind of data is not given in a monograph-guide [1] , which is the official document of well-known American organizations like National Oceanic and Atmospheric Administration., National Aeronautics and Space Administration, United States Air Force. In the work [2] , it was shown that accounting of the inhomogeneity of the Earth’s atmosphere leads to arising of one more isobaric mechanism of generation and propagation of sound, and consequently, together with adiabatic, speed of sound possesses also isobaric speed
. This circumstance can be mostly explained by the absence of experimental data concerning speed of sound measurement on different altitudes. It is proved by the fact that this kind of data is not given in a monograph-guide [1] , which is the official document of well-known American organizations like National Oceanic and Atmospheric Administration., National Aeronautics and Space Administration, United States Air Force. In the work [2] , it was shown that accounting of the inhomogeneity of the Earth’s atmosphere leads to arising of one more isobaric mechanism of generation and propagation of sound, and consequently, together with adiabatic, speed of sound possesses also isobaric speed 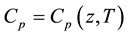 and real sound speed is defined by these two speeds
and real sound speed is defined by these two speeds 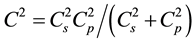 and thus
and thus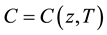 .
.
The situation is absolutely similar in relation of thermal expansion coefficient for ideal gases . The first experiments that were supposed to determine this coefficient were conducted by G. Amontons (1661-1703) at the end of XVII century. Then this problem was studied by the outstanding scientists like Robert Boyle, Alessandro Volta, John Dalton, Joseph Priestley, Nicolas-Theodore de Saussure, Jacques Charles and others. The considerable contribution in the determination of coefficient was done by the great French scientist Joseph Luis Gay-Lussac (1778-1850). The results of experiments conducted by these scholars can be briefly defined as follows:
. The first experiments that were supposed to determine this coefficient were conducted by G. Amontons (1661-1703) at the end of XVII century. Then this problem was studied by the outstanding scientists like Robert Boyle, Alessandro Volta, John Dalton, Joseph Priestley, Nicolas-Theodore de Saussure, Jacques Charles and others. The considerable contribution in the determination of coefficient was done by the great French scientist Joseph Luis Gay-Lussac (1778-1850). The results of experiments conducted by these scholars can be briefly defined as follows:
*At identical increasing of temperature, all the ideal gases are expanded identically.
*At constant pressure, volume of ideal gas linearly depends on temperature t˚C.
 (1.1)
(1.1)
where  is gas volume at which
is gas volume at which 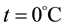 and
and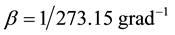 ―is the coefficient of thermal expansion. After Kelvin had introduced absolute temperature scale
―is the coefficient of thermal expansion. After Kelvin had introduced absolute temperature scale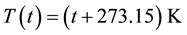 , volume’s dependence on temperature in the case of isobaric process looks like:
, volume’s dependence on temperature in the case of isobaric process looks like:
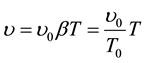 (1.2)
(1.2)
and it is called Gay-Lussac’s Law.
It is obvious that those experiments were conducted in the normal conditions, in other words, at a temperature of 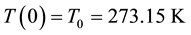 and under a pressure of
and under a pressure of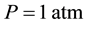 . Experiments, for defining the dependence
. Experiments, for defining the dependence 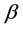 on altitude, had not been conducted. Consequently, according to Gay-Lussac’s law
on altitude, had not been conducted. Consequently, according to Gay-Lussac’s law 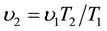 it is accepted that
it is accepted that  at any altitude of the earth’s atmosphere, where
at any altitude of the earth’s atmosphere, where 


2. Adiabaticity of Atmosphere of the Earth
It is known that the Earth’s atmosphere represents multilayered structure and in each layer, dependences of physical parameters on geometrical altitude z are different. The chart in Pic. 1 which is given in the monograph- guide [1] , and can be found in internet [6] as well as in the scientific literature [7] [8] shows that in the interval of altitudes from 








Such sharp deviation from dynamics of temperature change in the tropopause and stratosphere means that some anomalous process develops there as a result of which thermal energy is absorbed and thus medium is not adiabatic. So, we suppose that the atmosphere is adiabatic only in the troposphere, where temperature decreases according strictly to the linear law and in which entropy s satisfy the adiabatic equation.

Here t is time, 
adiabatic speed―
isobaric speed―
Here, 




from (2.2) for adiabatic speed of sound the following expression is derived:

Here, 







in expression (2.3) for isobaric speed of sound we obtain

Let’s define the altitude 


If we insert values of constant 







3. The Coefficient of Thermal Expansion in the Earth’s Atmosphere
Let’s show that isobaric sound speed (2.3) can be expressed by coefficient of thermal expansion

where

If we compare the formula (3.2) with the formula (2.7) for the coefficient of air expansion we can find
Figure 1. Molecular-scale temperature as a function of geopotencial altitude.

From (3.3), it is clear that actually







The change 







Let’s average now 



Simple calculations for average meaning 

According to Gay-Lussac’s Law it must be demanded:

The expression (3.8) has an important physical meaning, for it shows that for different gases the parameter 


In Table 1, there are presented values of 




Analysis of Table 1 permits us to make the following conclusions:
Table 1. Dependence z0 and z1 on adiabatic index and molar mass M.
1) For the gases whose number of atoms



2) For the gases whose number of atoms 



3) As the molar mass of gas increases, altitude 
It is interesting to determine an average value of thermal expansion coefficient for the case 2) in the interval

As we can see, the influence of the gravitational field on polyatomic gases 



4. Conclusions
The paper demonstrates that the commonly held opinion on universality of the laws of ideal gas that has existed in science for more than two centuries is erroneous. These laws were discovered by means of experiments carried out at the sea level where the Earth atmosphere can be considered homogeneous medium [2] [9] . The effect of gravitational field of the Earth, which making notable the influence on thermodynamic properties of gases depending on their molar mass and indicator of adiabatic index, becomes more evident with increase of altitude. Our results are based on the assumption that all gases discussed in this work at sufficient approximation can be considered as ideal ones. Practically, all the gases are ideal especially in the upper layers of the atmosphere, since the formula (3.9) has meaning only when

We have shown that for the air this altitude coincides with the upper border of troposphere 



At higher altitude 





The results of the work and considerations expressed herein are of fundamental importance for theoretical and applied physics and therefore require experimental verification. If the experiments prove correctness of our theoretical conclusions, this will lay the foundation to qualitatively new research in gasdynamics on the whole, as well as in physics of atmosphere in particular.
References
- US Standard Atmosphere, National Aeronautics and Space Administration (1976).
- Kirtskhalia, V.G. (2012) Open Journal of Acoustics, 2, 80-85. http://dx.doi.org/10.4236/oja.2012.22009
- Landau, L.D. and Lifshitz, E.M. (1964) Statistical Physics. Publishing House “MIR”, Moscow.
- Levich, V.G. (1962) Course of Theoretical Physics. Vol. I, Publishing House “FIZMATGIS”, Moscow.
- http://www.engineeringtoolbox.com/air-properties-d_156.html
- http://www.digitaldutch.com/atmoscalc
- Gossard, E.E. and Hooke, W.H. (1975) Waves in the Atmosphere. Elsevier, New York.
- Campos, L.M.B.C. (1983) Wave Motion, 5, 1-14. http://dx.doi.org/10.1016/0165-2125(83)90002-1
- Kirtskhalia, V.G. (2013) Journal of Modern Physics, 4, 1075-1079. http://dx.doi.org/10.4236/jmp.2013.48144





