Applied Mathematics
Vol.07 No.06(2016), Article ID:65167,13 pages
10.4236/am.2016.76051
Modeling Rift Valley Fever with Treatment and Trapping Control Strategies
Jonnes Lugoye1, Josephine Wairimu2, C. B. Alphonce1, Marilyn Ronoh2
1Univeristy of Dar es Salaam, Dar es Salaam, Tanzania
2School of Mathematics, University of Nairobi, Nairobi, Kenya

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 December 2015; accepted 27 March 2016; published 30 March 2016
ABSTRACT
We consider a rift valley fever model with treatment in human and livestock populations and trapping in the vector (mosquito) population. The basic reproduction number 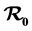 is established and used to determine whether the disease dies out or is established in the three populations. When
is established and used to determine whether the disease dies out or is established in the three populations. When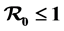 , the disease-free equilibrium is shown to be globally asymptotically stable and the disease does not spread and when
, the disease-free equilibrium is shown to be globally asymptotically stable and the disease does not spread and when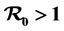 , a unique endemic equilibrium exists which is globally stable and the disease will spread. The mathematical model is analyzed analytically and numerically to obtain insight of the impact of intervention in reducing the burden of rift valley fever disease’s spread or epidemic and also to determine factors influencing the outcome of the epidemic. Sensitivity analysis for key parameters is also done.
, a unique endemic equilibrium exists which is globally stable and the disease will spread. The mathematical model is analyzed analytically and numerically to obtain insight of the impact of intervention in reducing the burden of rift valley fever disease’s spread or epidemic and also to determine factors influencing the outcome of the epidemic. Sensitivity analysis for key parameters is also done.
Keywords:
Rift Valley Fever, Mosquito Trapping, Treatment, Rift Valley Fever Control

1. Introduction
Rift Valley Fever (RVF) is an infectious disease caused by the RVF virus of the genus Phlebovirus and family Bunyaviridae. It is transmitted between animal species, including cattle, sheep, goats, and camels, primarily through the bite of the female mosquito, usually Aedes or Culex [1] . Gaff [2] formulated an epidemiological model of RVFV determining how to reduce egg classes of mosquitoes. Clements [3] modeled the distribution of two species of mosquitoes (Aedes aegypti and Culex pipiens complex) and showed that distribution of vectors had biological and epidemiological significance in relation to disease outbreak hotspots, and provided guidance for the selection of sampling areas for RVF vectors during inter-epidemic periods. Fischer in [4] studied the transmission potential of Rift Valley Fever virus in Netherlands by developing a mathematical model to determine the initial growth and Floquet ratios which were indicators of the probability of an outbreak and persistence in a periodic changing environment caused by seasonality. Their result showed that several areas of Netherlands had a high transmission potential and risk persistence of the infection [2] . The key result is that RVF virus can persist in a closed system for 10 years if the contact rate between hosts and vectors is high [5] . Meshe [6] formulated and analysed a mathematical model described by a system of non-linear ordinary differential equations to gain insight on the dynamics of RVF in mosquito, livestock and human hosts. The disease’s threshold was computed and used to investigate the local stability of the equilibria and infer the behaviour of the disease. Tianchan et al. [7] developed a mathematical model incorporating the effect of space into the mathematical model of RVF to study the effect of the virus spread as affected by the movements of livestock, human and mosquitoes. The simulated results showed that different geographic spaces have a great effect on the spread of the pathogen and the disease in general. [8] presented the mathematical model for Rift Valley fever (RVF) transmission in cattle and mosquitoes by extending the existing models for vector-borne diseases to include an asymptomatic host class and vertical transmission in vectors. RVF remains a threat to livestock keepers and nations where the disease is occurring due to its major economic implications through the costs of the measures taken at individual, collective and international levels to prevent or control infections and disease outbreaks [9] . In this study we extend the work of [6] by incorporating the aspect of control in the modelling transmission dynamics of RVF in humans and animals, by answering the question: How does trapping of mosquitoes and/or treatment of humans and animals or both affect the spread of the disease?
The rest of the paper is arranged as follows. In Section 2, we formulate the mathematical model and establish the basic properties of the model. In Section 3, we compute the basic reproduction number herein referred to as the effective reproduction number, and determine the local and global stability of the Disease Free equilibrium. In Section 4, we establish the existence and stability of the Endemic Equilibrium. In Section 5, we have sensitivity analysis with its interpretation. Section 6 has numerical simulation and Section 7 is the conclusion.
2. Model Formulation
In this model we divide the three populations into the susceptible, 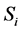 and infected,
and infected, 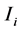 classes, for
classes, for 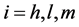 for, human (h), livestock (l) and mosquitoes (m), respectively. The three susceptible populations become infected via an infectious mosquito bite at per capita rates
for, human (h), livestock (l) and mosquitoes (m), respectively. The three susceptible populations become infected via an infectious mosquito bite at per capita rates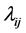 . The newborns in each category are recruited at the per capita birth rate of
. The newborns in each category are recruited at the per capita birth rate of 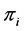 and hosts either die naturally or owing to the disease at per capita rates
and hosts either die naturally or owing to the disease at per capita rates 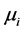 and
and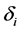 , respectively. Treatment in livestock is introduced at a constant rate
, respectively. Treatment in livestock is introduced at a constant rate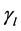 ; treatment in humans at a constant rate
; treatment in humans at a constant rate 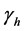 and trapping in mosquito at a constant rate
and trapping in mosquito at a constant rate 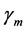 resulting in the classes of treated livestock
resulting in the classes of treated livestock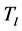 , treated humans
, treated humans 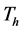 and trapped mosquito
and trapped mosquito



with initial conditions, 
are given by 
mission rates. Adding equations system 1, we have

2.1. Model Analysis
In this section, we carry out stability analysis of the model (1). The model properties are employed to establish criteria for positivity of solutions and well-possessedness of the system.
2.1.1. Invariant Region
In this section a region in which solutions of the model system (4.1) are uniformly bounded in a proper subset


so
where 


Hence, 



Lemma
The region 

2.1.2. Positivity of Solutions
Lemma
Let the initial data be


Proof From the first equation of the model system 1

that is
integrating by the equation above gives,

Then

Similarly, it can be shown that the remaining eight equations of system (4.1) are also positive
3. Steady State Solutions
In this section the model system (4.1) is qualitatively analysed by determining the equilibria, carrying out their corresponding stability analysis and interpreting the results. Let

From the second, fourth and sixth equations of (4), we write 


defining

Equation (2) reduces to (3).
3.1. Disease Free Equilibrium
This solution 


3.2. The Effective Reproductive Number, Reff
In this section, the threshold parameter that governs the spread of a disease referred to as the effective reproduction number is determined. Mathematically, it is the spectral radius of the next generation matrix [10] . The equations of the system (1) are re-written starting with infective classes, to obtain

From the system (6), 

substituting 


The partial derivatives of 





The eigenvalues of 

The effective reproduction number 




Local Stability of the Disease Free-Equilibrium
The disease-free equilibrium point is 

system (1) is computed by differentiating each equation in the system with respect to the state variables

where



Using Birkhoff and Rota's theorem on the differential inequality (3) we obtain

From the matrix (7) we note that the first, third, fourth, fifth and sixth have diagonal entries. Therefore their corresponding eigenvalues are;


With the help of mathematical software, the following characteristic equation is obtained


and

If


Theorem
The disease-free equilibrium point is locally asymptotically stable if 

4. The Endemic Equilibrium, E3
In the presence s of infection, that is, 




where 






We let 


Adding the last two equations of the system and making some simplifications we obtain

where

The equation, 

1) If 


2) If


3) Otherwise, there is none.
However it is important to note that A is always positive if 

Theorem 5 The rift valley fever basic model has,
1) Precisely one unique endemic equilibrium if
2) Precisely one unique endemic equilibrium if 

3) Precisely two endemic equilibrium if

4) None, otherwise.
From (iii) it is observed that backward bifurcation is possible if the discriminant is set 

where backward bifurcation occurs for values of 


Theorem 5 The endemic equilibrium point, 

5. Sensitivity Analysis
Sensitivity analysis determines parameters that have a high impact on 

The indices are crucial and will help us determine the importance of each individual parameter in transmission dynamics and prevalence of the Rift Valley Fever Virus.
Definition 1 The normalized forward sensitivity index of a variable, u, that depends differentiably on index
on a parameter, p is defined as;
The analytical expression for the sensitivity of 

volved in
Interpretation of Sensitivity Analysis
From Table 1, it shows that when the parameters 








6. Numerical Simulation
We carry out numerical simulations for mathematical model of rift valley fever for the set of parameters from literature as shown in Table 1. The parameter values that changed the value of 


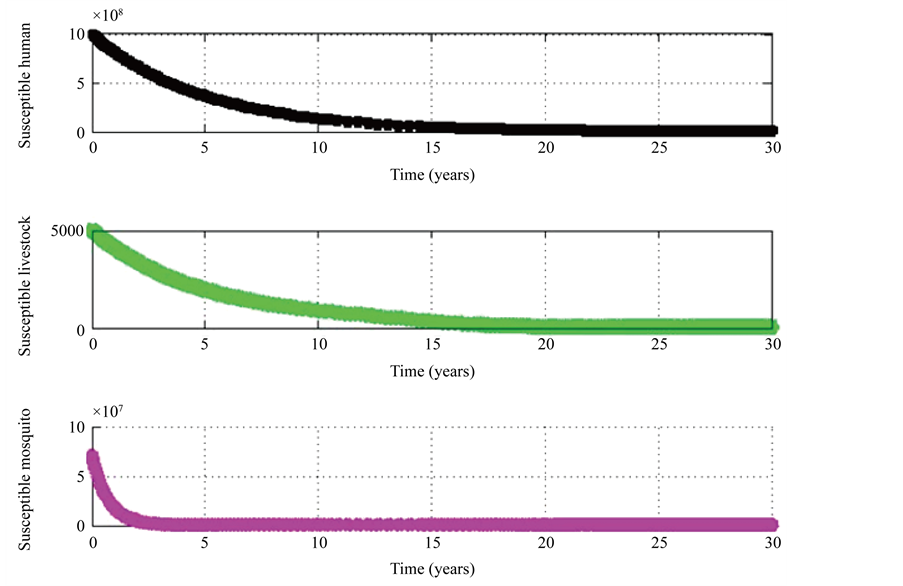
We have the following simulation results (Figures 1-6). Figure 1 shows variation of the different populations for specified parameter values. As treatment rates 











Table 1. Parameter values and the calculated sensitivity indices.
Figure 1. Schematic diagram for Rift Valley Fever Model with interventions.
Figure 2. Population Dynamics of the rift valley fever without intervention model.
Figure 3. Effects of treatment of livestock on mosquitoes population.
Figure 4. Effects of treatment of human on mosquitoes population.
Figure 5. Effects of trapping of mosquitoes on human population.
Figure 6. Effects of trapping of mosquitoes on human population.
6.1. Variation of Different Parameters on the Dynamics of Rift Valley Fever Model with Treatment and Trapping
In this section parameters, 



6.2. Discussion
The Rift Valley Model formulated in this study is well posed and exists in a feasible region where disease free and endemic equilibrium points are obtained and their stability investigated. The model has two interventions; treatment for human and livestock and trapping for mosquitoes. We have shown that disease free equilibrium exist and is locally asymptotically stable whenever its associated effective reproduction number 






In the absence of treatment of human or livestock and trapping for mosquitoes: 

With human or livestock and trapping for mosquitoes

7. Conclusion
In this paper, the rift valley fever model with interventions was formulated and analysed. Using the theory of differential equations, the invariant set in which the solutions of the model are biologically meaningful was derived. Boundedness of solutions was also proved. Analysis of the model showed that there exist two possible solutions, namely the disease-free point and the endemic equilibrium point. Further analysis showed that the disease-free point is locally stable implying that small perturbations and fluctuations on the disease state will always result in the clearance disease if
Cite this paper
Jonnes Lugoye,Josephine Wairimu,C. B. Alphonce,Marilyn Ronoh, (2016) Modeling Rift Valley Fever with Treatment and Trapping Control Strategies. Applied Mathematics,07,556-568. doi: 10.4236/am.2016.76051
References
- 1. Pepin, M., Bouloy, M., Bird, M., Kempand, B.H. and Paweska, A. (2010) Rift Valley Fever Virus (Bunyaviridae: Phlebovirus): An Update on Pathogenesis, Molecular Epidemiology, Vectors, Diagnostics and Prevention. Veterinary Research, 41, 61.
http://dx.doi.org/10.1051/vetres/2010033 - 2. Gaff, H.D., Hartley, D.M and Leahy, N.P. (2007) An Epidemiological Model of Rift Valley Fever. Electronic Journal of Di-Erential Equations, 1, 12.
- 3. Clements, A.C., Pfeifer, D.U., Martin, V. and Otte, M.J. (2007) A Rift Valley Fever Atlas for Africa. Preventive Veterinary Medicine, 2, 72-78.
http://dx.doi.org/10.1016/j.prevetmed.2007.05.006 - 4. Fischer, E., Boender, G.J. and De Koeijer, A.A. (2013) The Transmission Potential of Rift Valley Fever Virus among Livestock in the Netherlands: A Modeling Study. Veterinary Research, 44, 58.
http://dx.doi.org/10.1186/1297-9716-44-58 - 5. Métras, R., Collins, L.M., White, R.G., Alonso, S. and Chevalier, V. (2011) Rift Valley Fever Epidemiology, Surveillance, and Control: What Have Models Contributed? Vector Borne and Zoonotic Diseases, 11, 761-771.
http://dx.doi.org/10.1089/vbz.2010.0200 - 6. Mpeshe, S.C., Haario, H. and Tchuenche, J.M. (2011) A Mathematical Model of Rift Valley Fever with Human Host. Acta Biotheoretica, 59, 231-250.
http://dx.doi.org/10.1007/s10441-011-9132-2 - 7. Niu, T.C., Gaff, H.D., and Papelis, Y.E. and Hartley, D.M. (2012) An Epidemiological Model of Rift Valley Fever with Spatial Dynamics. Computational and Mathematical Methods in Medicine, 2012, Article ID: 138757.
- 8. Chitnis, N., James, M.H. and Carrie, A.M. (2013) Modelling Vertical Transmission in Vectorborne Diseases with Applications to Rift Valley Fever. Journal of Biological Dynamics, 7, 11-40.
http://dx.doi.org/10.1080/17513758.2012.733427 - 9. Otte, J.M., Nugent, R. and McLeod, A., Eds. (2004) Transboundary Animal Diseases: Assessment of Socio-Economic Impacts and Institutional Response. Volume 9, FAO, Livestock Policy Discussion Paper 9.
http://www.fao.org/ag/againfo/resources/en/publications/ - 10. Van Den, D.P. and Watmough, J. (2002) Reproduction Numbers and Sub-Threshold Endemic Equilibria for Compartmental Models of Disease Transmission. Mathematical Biosciences, 80, 29.
http://dx.doi.org/10.1016/S0025-5564(02)00108-6 - 11. Chitnis, N., Hyman, J.M. and Cushing, J.M. (2008) Determining Important Parameters in the Spread of Malaria through the Sensitivity Analysis of a Mathematical Model. Bulletin of Mathematical Biology, 70, 1272-1296.
http://dx.doi.org/10.1007/s11538-008-9299-0