World Journal of Condensed Matter Physics
Vol.3 No.1(2013), Article ID:28325,5 pages DOI:10.4236/wjcmp.2013.31011
The Role of Replacing CdO by Fe2O3 on the Fast Neutron Removal Cross Sections in Cd-Boro Phosphate Glass Shield
![]()
Physics Department, Faculty of Science, Al-Azhar University, Cairo, Egypt.
Email: heba_saudi@yahoo.com
Received September 20th, 2012; revised November 6th, 2012; accepted November 19th, 2012
Keywords: Iron Phosphate Glasses; Fast Neutron; Removal Cross-Section
ABSTRACT
This work deals with the application of [MERCSF-N] computer program in calculating the macroscopic effective removal cross-section of fast neutrons, ΣR (cm−1), for two different boro phosphate glass systems: (0.5 - x) CdO-x Fe2O3-0.4 P2O5-0.1 B2O3 and (0.5 - x) B2O3-x Fe2O3-0.1 CdO-0.4 P2O5 (with 0.05 ≤ x ≤ 0.5 by mole), to realize from the role of iron in the attenuation process and hence the usefulness of the glass containing iron as neutrons shielding material. The effect of replacing cadmium and boron oxides by iron oxide has been analyzed which proved that iron is more efficient than cadmium in attenuating and removing fast neutrons and that the presence of small amounts of B2O3 at least 0.1 mole fraction, with iron is needed to aid improving the removal cross-section of iron phosphate glasses. Experimental IR results are developed and used to trace the structural change and confirm the role of iron in the removal cross section.
1. Introduction
Phosphate glasses are technologically important materials because they generally have high thermal expansion coefficients, low transition temperatures and low preparation temperatures [1,2]. These properties have made them ideal materials for fundamental studies. However, their poor chemical durability limits their diverse uses. Addition of different types of metal oxides like Fe2O3, to binary phosphate glasses [3,4] has been found to improve the chemical durability and the attenuation of fast neutron [5,6].
An essential step towards the solution to the neutronshielding problem is the development of accurate database for effective fast neutrons removal cross-sections of glass shield. The macroscopic effective fast neutrons removal cross-section, for simplicity removal cross-section, ΣR (cm−1), is the probability that a fast or fission-energy neutron undergoes a first collision, which removes it from the group of penetrating, uncollided neutrons [7]. The removal cross-section ΣR of a given material behaves formally as a macroscopic cross-section in determining neutron attenuation, but it is not a cross-section in the sense of representing the probability of a particular neutron-nucleus interaction. Since all interacttions tend to remove energy from the fast neutron beam, so as a result ΣR value is not too different from the total (scattering and capture) macroscopic cross-section ΣT (ΣT = NσnT) of the material, but is generally lower [8].
It was found that addition of iron to concrete is more effective than concrete alone and neutron dose attenuation can be achieved with thinner shielding [9]. So the main objective of this work is to investigate the role of: 1) replacing CdO by Fe2O3 in the glass system: (0.5 - x) CdO-x Fe2O3-0.4 P2O5-0.1 B2O3, (where x = 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5 by mole); 2) replacing B2O3 by Fe2O3 in the glass system: (0.5 - x) B2O3-x Fe2O3-0.1 CdO-0.4 P2O5 glass system, (where x = 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5 by mole) on the fast neutron attenuation.
Although ΣR (cm−1) is usually determined empirically, it can be derived from theoretical consideration. In this work, the MERCSF-N program has been computerized based on the required physical data of all periodic table elements and mass removal cross-sections ΣR/ρ (cm2∙g−1) are calculated for the glasses of interest.
IR (infrared) measurements of some iron concentrations in the first glass system are preformed to trace the structural changes which led to an improvement of the neutron removal cross section by iron.
2. Theory
The removal cross-section for compounds may be calculated from the value of ΣR or ΣR/ρ for various elements in the compounds or mixtures by the general formula [9,10].
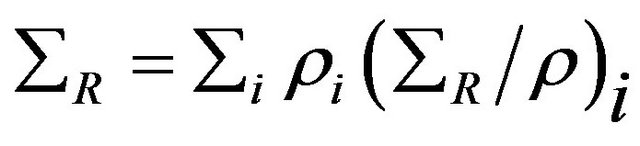 (1)
(1)
where ρi and (ΣR/ρ)i are the partial density (the density as it appears in the mixture) and mass removal crosssection of the ith constituent, respectively. Based on Equation (1), the removal cross-section for any compound or composite can be found by the following technique: if a chemical compound C having, for instance, the chemical formula Xi Yj Zk, where X, Y and Z are the chemical symbols of its elements and i, j and k are their fixed proportions by mass, then the compound molar mass MC will given [8] by:
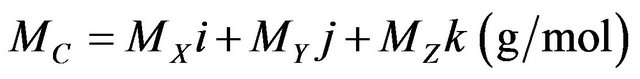 (2)
(2)
where MX, MY and MZ are the atomic masses of the elements X, Y and Z respectively. Hence the mass removal cross-section of the compound C, in cm2∙g−1, can be calculated [8,9] by the following Equation (8):
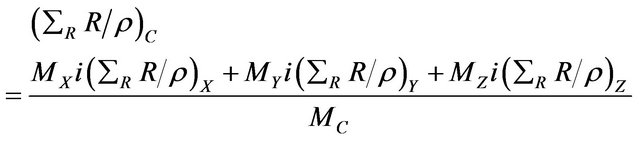 (2)
(2)
The required physical constant data were compiled from the recommended information in the literature and stored as a data base file. The calculation can be achieved by accounting for the contribution of all composite elements.
3. Experimental
Analytically pure grade chemicals were used to prepare the following glass samples according to the formula ((0.5 - x) CdO-x Fe2O3-0.4 P2O5-0.1 B2O3 by mole where x = 0, 0.05, 0.2). The batch mixtures were melted in porcelain crucibles at 1100˚C for two hours until homogeneous glasses were obtained and then annealed in a separate annealing furnace at 250˚C and then slowly cooled to the room temperature to remove any internal stresses. The infrared absorption spectra of the glass system were measured at room temperature in the range 2000 - 400 cm−1 by a Fourier Transform infrared spectrometer (type Perken Elmer spec-trometer, model RTX, FTIR) using the KBr disc technique.
4. Results and Discussion
4.1. Infrared Analysis (IR)
The infrared absorption spectra of the studied glass samples show the presence of two broad bands. The broad bands as shown in Figure 1 and Table 1 are an overlapping of some individual bands with each other. Each individual band is related to some type of vibration of a specific structural group. Therefore, it was necessary to use a deconvolution process [11] for separating these individual bands. Each individual band has its characteristic parameters such as its center (C), which is related to some type of vibration of a specific structural group, and its relative area (A), which is proportional to the concentration of this structural group. The deconvolution parameters of the bands for the investigated glasses are given in Table 1.
The two broad bands at 536 and 1018 cm−1 that appeared for the glass containing no Fe2O3 are assigned to the deformation vibrations of Phosphate groups [12,13]. The sub bands at 448, 551 cm−1, which appear in most binary and ternary phosphate glasses [14] are due to overlapping of CdO, harmonics of bending vibration of P-O linkages [15,16]. The absorption sub bands in the region 600 - 670 cm−1 may be due to the bending mode of PO4 units [17]. The sub bands in the region 810 - 920 cm−1 is overlapping the asymmetric stretching vibration of P-O-P linkages and the symmetrical stretching vibrations of BO4 units [16,17]. The sub band around 1037 cm−1 is attributed to the symmetric stretching vibration of the 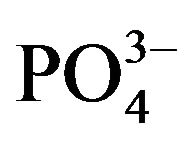 tetrahedral (P-O ionic group) [18] and BO4 units. The sub band at 1198 cm−1 is assigned to PO2 asymmetric stretching vibration [17]. The sub bands at 1345, 1451 cm−1 are assigned to the stretching vibrations of the B-O of trigonal (BO3)3− units. The addition of
tetrahedral (P-O ionic group) [18] and BO4 units. The sub band at 1198 cm−1 is assigned to PO2 asymmetric stretching vibration [17]. The sub bands at 1345, 1451 cm−1 are assigned to the stretching vibrations of the B-O of trigonal (BO3)3− units. The addition of
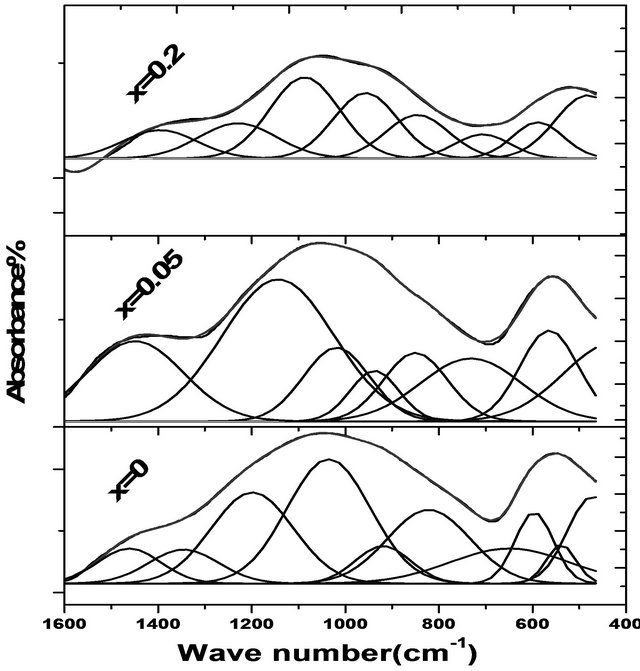
Figure 1. Infrared absorption spectra of the glass system ((0.5 - x) CdO-x Fe2O3-0.4 P2O5-0.1 B2O3 by mole where x = 0, 0.05, 0.2).
Table 1. Deconvolution parameters of the IR spectra of the glass system (0.5 - x) CdO-x Fe2O3-0.4 P2O5-0.1 B2O3 with x = 0, 0.05, 0.2 by mole). C is the component band center and A is the relative area (%) of the component band.
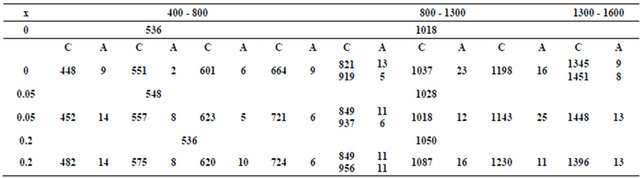
Fe2O3 resulted in an increase in the bending vibrations of B-O linkages and stretching vibrations of the B-O of trigonal (BO3)3− that increases ionic character, as observed from the appearance of sub bands around 720 cm−1 and hence caused a shift of the band around 1018 cm−1 to higher wave number at 1028 cm−1. This was attributed to [17] the entrance of Fe2O3 in interstitial position between P-O-P layers. The area of sub band around 458 cm−1 increased on the addition of Fe2O3 on the expense of CdO, which can be attributed to the presence of highly distorted FeO6 overlapping with CdO. This means that iron and cadmium ions act manly as a glass modifier where the Fe and Cd atoms are located in octahedral sites and they occupy the positions between P-O-P layers creating non-bridging oxygen ions (NBOs) without change of the number of P=O bonds [16]. On the other hand the appearance of a sub band around 620 cm−1 associated with FeO4 in the glass containing Fe2O3 indicated that some Fe enter the glass network as a glass former. The appearance of the band at 1230 cm−1 corresponding to the asymmetric stretching of the double bonded oxygen vibrations for the glass containing 0.2 mole % Fe2O3, indicates that there is a chance for Fe to cause more P=O breakage and the formation of the P-O-Fe bonds [19, 20].
4.2. The Mass Removal Cross-Section, ΣR/ρ, for the Two Glass Systems
By aiding of the previously mentioned mathematical treatment, the MERCSF-N program was modified to calculate the mass removal cross-sections of the mentioned glass systems. .The description and validation, as well as, the use of the MERCSF-N program have been described [21]. The definition of the ΣR in accordance with Equation (1) is dependent on the elemental composition of a shielding material. The calculated values of ΣR/ρ for the glass containing (0.5 - x) CdO-x Fe2O3-0.4 P2O5-0.1 B2O3, are found to increase with increasing Fe2O3 as shown in Figure 2 and Table 2. This indicates that the mass removal cross-section of Fe2O3 is higher than each of the removal cross-section of CdO as well as the increase in the value of the total molar mass of the glass on increasing Fe2O3 mole fraction on expense of CdO. This increase in the neutron removal cross section as Fe2O3 increase is consistent with the IR results which indicate that as Fe2O3 increase the amount of iron which inter the glass network as glass former increase. This iron glass former was shown [6] to improve the removal cross section for fast neutrons by the breakage of P-O-P bonds and formation of P-O-Fe bonds. So Fe2O3 play an important role for improving the ΣR/ρ values and requires a lower size in the shield design.
For the glass system: (0.5 - x) B2O3-x Fe2O3-0.4 P2O5- 0.1 CdO, the ΣR/ρ values showed, to some extend, similar behavior as the first system; i.e. it increased as shown in Figure 3 and Table 2, which proves once more the capability of iron for attenuating neutrons. This behavior can be attributed to that the increase in the value of the ΣR/ρ of Fe2O3 is greater than each of the value of the ΣR/ρ of B2O3 as well as the increase in the value of the total molar mass of the glass on increasing Fe2O3 mole fraction on expense of B2O3. The observed differences are the some what higher values of ΣR/ρ for the glasses containing up to 0.35 mol fraction of Fe2O3 than the glasses containing the same amount of Fe2O3 in the first system, and that this difference in the value of the ΣR/ρ between the two systems decrease as the B2O3 content decreased as observed in Table 2. On the other hand the glasses containing 0.45 and 0.50 Fe2O3 in this system have lower ΣR/ρ than the corresponding ones in the first system, which retain 0.1 B2O3. These results indicated that: 1) iron is more efficient than cadmium in removing fast neutrons; 2) the presence of suitable amount of B2O3 (at least 0.1 mol fraction) in phosphate glass shield containing iron is necessary for moderating the energy of the neutrons, so aids in improving the removal cross section of iron in phosphate glass shield. These results can be understand from the obtained structural properties (IR) which showed that addition of 0.05 mole % Fe2O3 aids
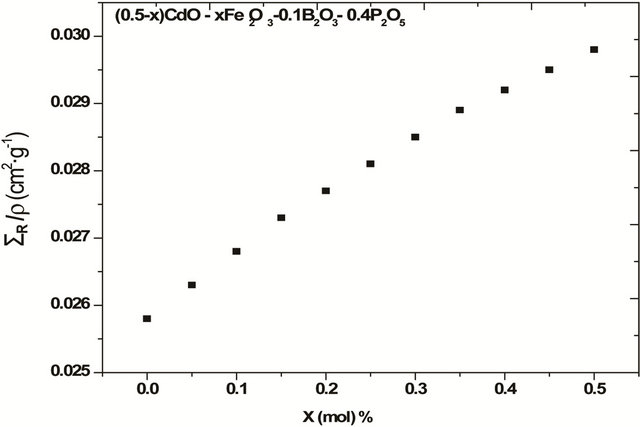
Figure 2. The mass removal cross-section ΣR/ρ for glass system (0.5 - x) CdO-x Fe2O3-0.4 P2O5-0.1 B2O3.
Table 2. The calculated values for the glass molar mass, MC, and the mass removal cross-section, ΣR/ρ, for the two glass systems.
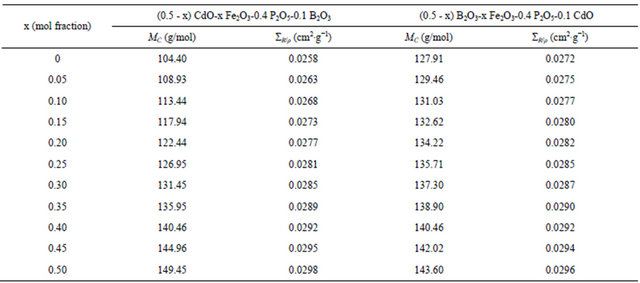
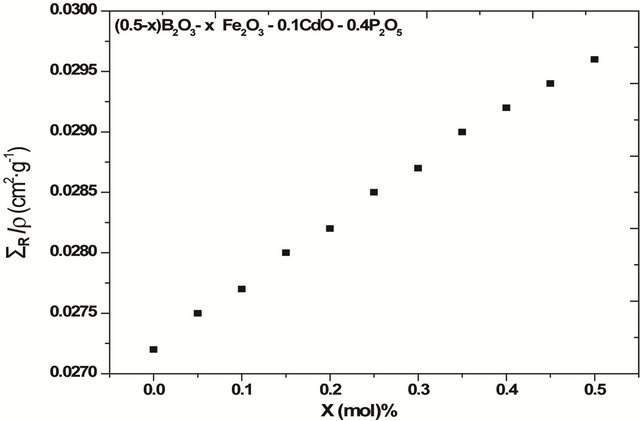
Figure 3. The mass removal cross-section ΣR/ρ for glass system (0.5 - x) B2O3-x Fe2O3-0.4 P2O5-0.1 CdO.
change in the bending vibrations of the boron atoms and hence enabled more collision and elastic scattering of neutrons by boron.
5. Conclusions
Iron is more efficient than cadmium in attenuating and removing fast neutrons. The presence of small amounts of B2O3 at least 0.10 mol fractions with iron is needed to aid and improve the removal cross-section of the iron phosphate glass, which indicates that Fe2O3 is more efficient for the lower energy range of fast neutrons than the higher energy range.
Glasses are potential candidates for neutron shielding materials. Higher values of ΣR/ρ with adding Fe2O3 to the two glass systems indicate that iron plays a very important role in the process of attenuation of neutrons. It reduces the required thickness of the shield design and therefore, permits low cost shield.
REFERENCES
- S. H. Im, Y. H. Na, N. J. Kim, D. H. Kim, C. W. Hwang and B. K. Ryu, “Structure and Properties of Zinc Bismuth Phosphate Glass,” Thin Solid Films, Vol. 518, No. 24, 2010, pp. e46-e49. doi:10.1016/j.tsf.2010.03.128
- J. A. Wilder, “Phosphate Glass Dissolution in Aqueous Solutions,” Journal of Non-Crystalline Solids, Vol. 38-39, 1984, pp. 879-884. doi:10.1016/0022-3093(80)90548-7
- X. Fang, C. S. Ray, A. Mogus-Milankovic and D. E. Day, “Iron Redox Equilibrium, Structure, and Properties of Iron Phosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 283, No. 3, 2001, pp. 162-172. doi:10.1016/S0022-3093(01)00416-1
- P. A. Bingham, R. J. Hand, S. D. Forder and A. Lavaysierre, “Vitrified Metal Finishing Wastes: II. Thermal and Structural Characterisation,” Journal of Hazardous Materials, Vol. 122, No. 1-2, 2005, pp. 129-138. doi:10.1016/j.jhazmat.2005.03.031
- M. H. Kharita, S. Yousef and M. Al Nassar, “Review on the Addition of Boron Compounds to Radiation Shielding Concrete,” Progress in Nuclear Energy, Vol. 53, No. 2, 2011, pp. 207-211. doi:10.1016/j.pnucene.2010.09.012
- H. A. Saudi, A. G. Mostafa, N. Sheta, S. U. El Kameesy and H. A. Sallam, “The Structural Properties of CdOBi2O3 Borophosphate Glass System Containing Fe2O3 and Its Role in Attenuating Neutrons and Gamma Rays,” Physica B: Condensed Matter, Vol. 406, No. 21, 2011, pp. 4001-4006.
- E. P. Blizard and L. S. Abbott, “Introduction to Nuclear Engineering,” 3rd Edition, John Wiley & Sons, Inc., Hoboken, 1962.
- J. Wood, “Computational Methods in Reactor Shielding,” Pergamon Press, Inc., New York, 1982.
- M. F. Kaplan, “Concrete Radiation Shielding: Nuclear Physics, Concrete Properties, Design and Construction,” John Wiley & Sons, New York, 1989.
- Y. Iwamoto and R. M. Ronningen, “Attenuation of Ambient Dose Equivalent from Neutrons by Thick Concrete, Cast Iron and Composite Shields for High Energy Proton, 3He, 48Ca and 238U Ions on Cu Targets for Shielding Design,” Nuclear Instruments and Methods in Physics Research Section B, Vol. 269, No. 3, 2011, pp. 353-363. doi:10.1016/j.nimb.2010.11.046
- H. S. Liu, P. Y. Shih and T. S. Chin, “High Photoluminescent Property of Low-Melting Sn-Doped Phosphate Glass,” Applied Physics Express, Vol. 3, No. 8, 2010, p. 227.
- V. V. Kushnikov, Y. I. Matyunin, T. V. Smelova and A. V. Demin, “Investigations of Plutonium Immobilization into the Vitreous,” Materials Research Society Symposium Proceedings, Vol. 465, 1997, p. 55.
- B. C. Sales and L. A. Boatner, “New Rare-Earth-Activated Phosphate Glass Scintillators,” Materials Letters, Vol. 2, No. 4, 1984, pp. 301-304. doi:10.1016/0167-577X(84)90038-7
- S. T. Reis, A. Mogusˇ-Milankovic, V. Licˇina, J. B. Yang, M. Karabulut, D. E. Day and R. K. Brow, “Iron Redox Equilibrium, Structure and Properties of Zinc Iron Phosphate Glasses,” Journal of Non-Crystalline Solids, Vol. 353, No. 2, 2007, pp. 151-158. doi:10.1016/j.jnoncrysol.2006.10.002
- A. Mogous-Milankovic and D. E. Day, “Infrared Spectra of Fe2O3-PbO-P2O5 Glasses,” Journal of Non-Crystalline Solids, Vol. 162, 1993, p. 275.
- I. W. Donald, B. L. Metcalfe and R. N. J. Taylor, “The Immobilization of High Level Radioactive Wastes Using Ceramics and Glasses,” Journal of Materials Science, Vol. 32, No. 22, 1997, pp. 5851-5887. doi:10.1023/A:1018646507438
- B. C. Sales and L. A. Boatner, In: W. Lutze and R. C. Ewing, Eds., Radioactive Waste Forms for the Future, North-Holland, Amsterdam, 1988, p. 193.
- A. P. Mukhamet-Galeyev, L. O. Magazina, K. A. Levin, N. D. Samotoin, A. V. Zotov and B. I. Omelianenko, “The Interaction of Na-Al-P-Glass (Cs, Sr-Bearing) with Water at Elevated Temperatures,” Materials Research Society Symposium Proceedings, Vol. 353, 1995, p. 79. doi:10.1557/PROC-353-79
- Y. M. Moustafa, K. El-Egili, H. Doweidar and I. Abbas, “Structure and Electrical Conduction of Fe2O3-P2O5 Glasses,” Physica B: Condensed Matter, Vol. 353, No. 1-2, 2004, pp. 82-91. doi:10.1016/j.physb.2004.09.004
- Y. M. Moustafa and A. El-Adawy, “Structural and Physical Properties of Iron Oxychloride Phosphate Glasses,” Physica Status Solidi A, Vol. 179, No. 1, 2000, pp. 83-93.
- A. M. El-Khayatt and A. E.-S. Abdo, “MERCSF-N Calculation: A Program for Fast Neutron Removal Cross Sections in Composite Shields,” Annals of Nuclear Energy, Vol. 36, No. 6, 2009, pp. 832-836. doi:10.1016/j.anucene.2009.01.013

