Advances in Chemical Engineering and Science
Vol.4 No.3(2014), Article
ID:48132,15
pages
DOI:10.4236/aces.2014.43041
Synthesis, Structural and Photophysical Properties of Gd2O3:Eu3+ Nanostructures Prepared by a Microwave Sintering Process
Ana P. de Moura1, Larissa H. Oliveira1, Içamira C. Nogueira2, Paula F. S. Pereira1, Máximo S. Li3, Elson Longo1, José A. Varela1, Ieda L. V. Rosa4*
1Chemistry Institute, State University of Sao Paulo-UNESP, Araraquara, Brazil
2Department of Engineering Materials, Federal University of Sao Carlos, São Carlos, Brazil
3Institute of Physics of São Carlos, USP, São Carlos, Brazil
4Department of Chemistry, Federal University of Sao Carlos, São Carlos, Brazil
Email: *ilvrosa@ufscar.br
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 June 2014; revised 7 July 2014; accepted 17 July 2014
ABSTRACT
In this paper, we report the obtention of gadolinium oxide doped with europium (Gd2O3:Eu+3) by thermal decomposition of the Gd(OH)3:Eu3+ precursor prepared by the microwave assisted hydrothermal method. These systems were analyzed by thermalgravimetric analyses (TGA/DTA), X-ray diffraction (XRD), structural Rietveld refinement method, fourrier transmission infrared absorbance spectroscopy (FT-IR), field emission scanning electron microscopy (FE-SEM) and photoluminescence (PL) measurement. XRD patterns, Rietveld refinement analysis and FT-IR confirmed that the Gd(OH)3:Eu3+ precursor crystallize in a hexagonal structure and space group P6/m, while the Gd2O3:Eu3+ powders annealed in range of 500˚C and 700˚C crystallized in a cubic structure with space group Ia-3. FE-SEM images showed that Gd(OH)3:Eu3+ precursor and Gd2O3:Eu3+ are composed by aggregated and polydispersed particles structured as nanorods-like morphology. The excitation spectra consisted of an intense broad band with a maximum at 263 nm and the Eu3+ ions can be excitated via matrix. The emission spectra presented the characteristics 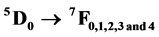 ket# transitions of the Eu3+ ion, whose main emission,
ket# transitions of the Eu3+ ion, whose main emission, 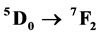 , is observed at 612 nm. The photophysical properties indicated that the microwave sintering treatment favored the Eu3+ ions connected to the O-Gd linkages in the Gd2O3 matrix. Also, the emission in the Gd2O3:Eu3+ comes from the energy transfered from the Gd-O linkages to the
, is observed at 612 nm. The photophysical properties indicated that the microwave sintering treatment favored the Eu3+ ions connected to the O-Gd linkages in the Gd2O3 matrix. Also, the emission in the Gd2O3:Eu3+ comes from the energy transfered from the Gd-O linkages to the 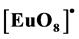 clusters in the crystalline structure.
clusters in the crystalline structure.
Keywords:Gadolinuim Oxide, Europium Luminescence, Nanorods

1. Introduction
One-dimensional nanomaterials, such as nanotubes, nanowires, nanobelts or nanoribbons have attracted much interest in the past decade due to their physical properties and potential applications in nanotechnology fields [1] -[8] . Moreover, these materials can be applied as displays, catalysts, biological sensing, and other optoelectronic devices [9] -[11] .
The demand for efficiency and high resolution waveguides, lamps and other optical devices has also stimulated the discovery of new luminescent materials with superior properties. Thus, there has been a tremendous interest in the subject of materials science for the development of new luminescent materials. The improved performance of display requires high-quality phosphors for sufficient brightness and long-term stability. To enhance the luminescent characteristics of phosphors, extensive research has been carried out on rare-earth activated oxide phosphors due to their superiority in color purity, chemical and thermal stabilities [12] -[14] . In this context, lanthanide hydroxides and oxides have actively been investigated for its application in multilayered capacitors, luminescent lamps and displays, solid-laser devices, optoelectronic data storages, waveguides, and heterogeneous catalysts. Their composition, structure and particle size depend on the synthesis method. Moreover, the chemical homogeneity and morphology of the synthesized products determine the effectiveness of their properties [11] [15] [16] . When they are applied for a fluorescent labeling, for instance, there are several advantages such as sharp emission spectra, long lifetimes, and high resistance against photobleaching in comparison with conventional organic fluorophores and quantum dots [17] -[19] .
In particular, the gadolinium oxide doped with Eu3+ (Gd2O3:Eu3+) exhibits a strong paramagnetic behavior (S 1/4 72) as well as strong UV and cathode-rays have also been observed in the lanthanide (Sm3+, Er3+) doped Gd2O3 excited luminescence, which are useful in biological fluorescent label, contrast agent, and display applications [20] -[22] . In addition, Gd2O3:Eu3+ is a very efficient X-ray and thermo-luminescent phosphor [23] .
Europium ion in a trivalent state is one of the most studied rare earth element because of the simplicity of its emission spectra and due to the wide application as red phosphor in color TV screens. Eu3+ f-f transitions are sensitive to its local environment. The monitoring of different concentrations of the Eu3+ content into a ceramic material is very interesting in understanding the nature of the lattice modifiers as well as the degree of order-disorder into its crystalline structure. The most intense f-f transition is the 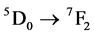 transition at 616 nm. When this ion is presented in a non-centrosymmetric site, it can be used as an activator ion with red emission which has been used in the most commercial red phosphor. Moreover, the intensity of Eu3+ excitations at around 394 and 465 nm is improved in these materials as compared with most other Eu3+ doped phosphors [24] [25] . Because of it, this ion is able to be applied as biological sensors, phosphors, electroluminescent devices, optical amplifiers or lasers when it is used as a dopant in a variety of ceramic materials [26] -[28] .
transition at 616 nm. When this ion is presented in a non-centrosymmetric site, it can be used as an activator ion with red emission which has been used in the most commercial red phosphor. Moreover, the intensity of Eu3+ excitations at around 394 and 465 nm is improved in these materials as compared with most other Eu3+ doped phosphors [24] [25] . Because of it, this ion is able to be applied as biological sensors, phosphors, electroluminescent devices, optical amplifiers or lasers when it is used as a dopant in a variety of ceramic materials [26] -[28] .
A variety of preparation methods have been developed to reduce the reaction temperature and achieve a small particle size of high quality Gd2O3:Eu3+ phosphors [11] [29] -[32] .
Microwave heat processing has been successfully applied for the preparation of micro or nanosized inorganic materials [33] -[38] . The microwave-assisted heating is a greener approach to synthesize materials in a shorter time (from several minutes to a few hours) and with lower power consumption (hundreds of Watts) compared to the conventional heating at the same temperatures [39] -[43] . This is a consequence of directly and uniformly heating of the components, and exchange in the reaction selectivity, which can increase the reactional rates (microwave catalysis). Consequently, microwave synthesis is becoming quite common in several material sciences areas, nanotechnology, inorganic, organic, biochemical, or pharmaceutical laboratories [44] -[50] .
In the present work, we investigated the photo-physical properties of Gd2O3: Eu3+ phosphors obtained by the thermal decomposition in range of 500˚C and 700˚C of the Gd(OH)3:Eu3+ precursor prepared by the microwave assisted hydrothermal method. These materials were structured and microstructurally analyzed by means of X-ray diffraction (XRD), Rietveld refinement method, fourrier transmission infrared absorbance spectroscopy (FT-IR), field emission scanning electron microscopy (FE-SEM). The photo-physical properties were investigated through the excitation and emission spectra of the Eu3+ ion as well as lifetime measurements.
2. Experimental Procedure
2.1. Synthesis of the Precursors
The synthesis of the precursors was performed using the following procedure: In a typical synthesis, 1.8 g of Gd2O3 and 0.018 g of Eu2O3 were dissolved in 3.0 mL of the HNO3 solution. After the formation of a clear solution, this solution was kept under constant heating until complete evaporation of the acid. Then 80 mL of distilled water were added to the solution and stirred for 30 min at room temperature. After that, an aqueous KOH (2.0 M) solution was added until the pH of solution was adjusted to be in the range of 12 giving rise to a colloidal precipitates. After stirring for about 30 min, the resultant solution was transferred to a Teflon lined stainless autoclave. This autoclave was then sealed and placed into a microwave system (MH) using 2.45 GHz microwave radiation with maximum power of 800 W. The MH conditions were kept at 140˚C for 1 minute. The white powders obtained (Gd(OH)3:Eu3+) were collected, washed with water and ethanol, and then dried at 60˚C for 8 h under atmospheric air in a conventional furnace.
2.2. Synthesis of Gd2O3:Eu3+ Powders
The Gd2O3:Eu3+ powders were obtained from thermal decomposition of the Gd(OH)3:Eu3+ precursors. These precursor powders were placed in ceramic crucibles and heated in a microwave sintering furnace at 500˚C, 550˚C, 600˚C, 650˚C and 700˚C for 5 min under an ambient atmosphere using a heating rate of 5˚C/min producing white powders denoted as Gd2O3:Eu3+.
2.3. Characterization
The Gd(OH)3:Eu3+ and Gd2O3:Eu3+ powders were structurally characterized by X-ray diffraction (XRD) in normal routine and Rietveld routine using a Rigaku-DMax/2500PC (Japan) with Cu-Kα radiation (λ = 1.5406 Å) and in the 2θ range from 10˚ to 130˚ with a scanning rate of 0.02˚/min. Fourier Transmission Infrared absorbance spectroscopy (FT-IR) analysis were taken in a FT-IR Bruker model EQUINOX spectrophotometer in range of 500 and 4000 cm−1. Crystals morphologies were verified using a Scanning Electron Microscope (Jeol JSM-6460LV microscope). Photoluminescence (PL) was measured with a Thermal Jarrel-Ash Monospec 27 monochromator and a Hamamatsu R446 photomultiplier. The 350.7 nm exciting wavelength of a krypton ion laser (Coherent Innova) was used, with the nominal output power of the laser power kept at 200 mW. All the measurements were taken at room temperature. The excitation and emission spectra of the Gd2O3:Eu3+ powders were measured in a Jobin Yvon-Fluorolog 3 spectrofluorometer at room temperature using a 450 W xenon lamp as excitation energy source. Lifetime data of the Eu3+ 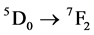 (lexc = 394 nm, lem = 612 nm) transition in the Gd2O3:Eu3+ samples were evaluated from the decay curves using the emission wavelength set at 612 nm and excitation wavelength set at 393 nm.
(lexc = 394 nm, lem = 612 nm) transition in the Gd2O3:Eu3+ samples were evaluated from the decay curves using the emission wavelength set at 612 nm and excitation wavelength set at 393 nm.
3. Conclusion
In summary, the obtained results showed that the Gd(OH)3:Eu3+ (precursor) was synthesized by the microwave assisted hydrothermal method in a short period of time (30 minutes). After heated treated from 500˚C to 700˚C,
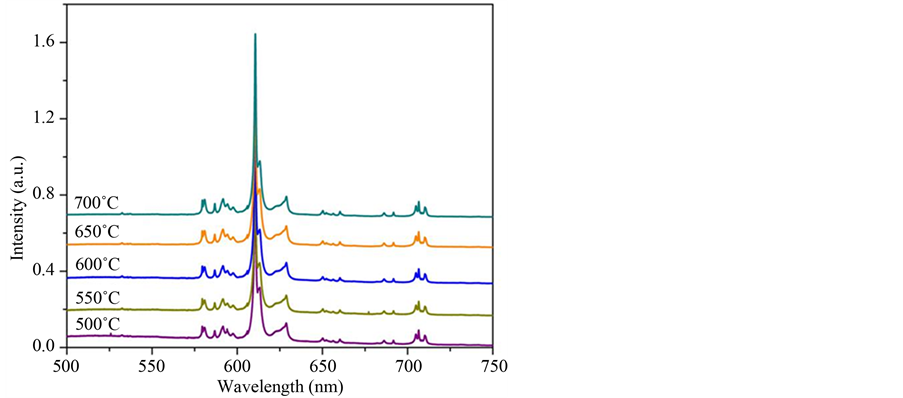
Figure 10. Emission spectra of Gd2O3:Eu3+ samples calcined at 500˚C, 550˚C, 600˚C, 650˚C and 700˚C, lex = 263 nm.
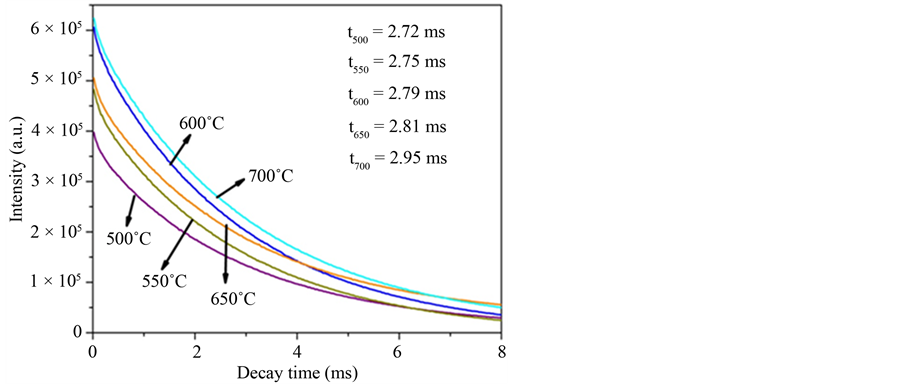
Figure 11. Decay curves and lifetime of the 5D0 → 7F2 transition characteristic of the Eu3+ of the Gd2O3:Eu3+ nanorods heat treated at 500˚C, 550˚C, 600˚C, 650˚C and 700˚C (lex = 612 nm and lem = 612 nm).
the XRD patterns and Rietveld refinement and FT-IR analyses indicated the formation of Gd2O3:Eu3+ powders which crystallizes in a cubic structure of crystalline Gd2O3 and space group Ia-3. No secondary phases related to the Eu3+ ions were detected indicating that these ions were incorporated to the hydroxide and oxide matrixes in the analyzed powders. FE-SEM images indicated that the Gd(OH)3:Eu3+ precursor and Gd2O3:Eu3+ powders are composed by several aggregated particles with nanorods-like morphology, which sizes are in the range of 8 and 20 nm. Eu3+ emission and excitation spectra pointed out that the emission in the Gd2O3:Eu3+ powders comes from the energy transfer from the Gd-O and 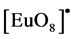 clusters in the crystalline structure. Moreover, these are in accordance to the lifetime values, which presented an increase as the temperature increases. This method is very simple and effective, and can be extended to synthesize some other rare earth and metal oxide nanorods.
clusters in the crystalline structure. Moreover, these are in accordance to the lifetime values, which presented an increase as the temperature increases. This method is very simple and effective, and can be extended to synthesize some other rare earth and metal oxide nanorods.
Acknowledgements
The authors acknowledge the financial support of the Brazilian research financing institutions: CNPq (INCTMN), CAPES and FAPESP (CEPID). A special thanks for Maria Fernanda Cgnin de Abreu.
References
- Iijima, S. (1991) Synthesis of Carbon Nanotubes. Nature, 354, 56-58.
http://dx.doi.org/10.1038/354056a0 - Ajayan, P.M. (1999) Nanotubes from Carbon. Chemical Reviews, 99, 1787-1800.
http://dx.doi.org/10.1021/cr970102g - Hu, J.T., Odom, T.W. and Lieber, C.M. (1999) Chemistry and Physics in one Dimension: Synthesis and Properties of Nanowires and Nanotubes. Accounts of Chemical Research, 32, 435-445.
http://dx.doi.org/10.1021/ar9700365 - Xia, Y.N., Yang, P.D., Sun, Y.G., Wu, Y.Y., Mayers, B., Gates, B., Yin, Y.D., Kim, F. and Yan, H.Q. (2003) One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Advanced Materials, 15, 353-389. http://dx.doi.org/10.1002/adma.200390087
- Rao, C.N.R., Deepak, F.L., Gundiah, G. and Govindaraj, A. (2003) Inorganic Nanowires. Progress in Solid State Chemistry, 31, 5-147. http://dx.doi.org/10.1016/j.progsolidstchem.2003.08.001
- Huang, M.H., Mao, S., Feick, H., Yan, H.Q., Wu, Y.Y., Kind, H., Weber, E., Russo, R., Yang, P.D. (2001) Room-Temperature Ultraviolet Nanowire Nanolasers. Science, 292, 1897-1899.
http://dx.doi.org/10.1126/science.1060367 - Pan, Z.W., Dai, Z.R. and Wang, Z.L. (2001) Nanobelts of Semiconducting Oxides. Science, 291, 1947-1949. http://dx.doi.org/10.1126/science.1058120
- Shi, W.S., Peng, H., Wang, N., Li, C.P., Xu, L., Lee, C.S., Kalish, R. and Lee, S.T. (2001) Free-Standing Single Crystal Silicon Nanoribbons. Journal of the American Chemical Society, 123, 11095-11096. http://dx.doi.org/10.1021/ja0162966
- Hu, J., Odom, T.W. and Lieber, C.M. (1999) Chemistry and Physics in One Dimension: Synthesis and Properties of Nanowires and Nanotubes. Accounts of Chemical Research, 32, 435-445.
http://dx.doi.org/10.1021/ar9700365 - Kazes, M., Lewis, D.Y., Ebenstein, Y., Mokari, T. and Banin, U. (2002) Lasing from Semiconductor Quantum Rods in a Cylindrical Microcavity. Advanced Materials, 14, 317-321.
http://dx.doi.org/10.1002/1521-4095(20020219)14:4<317::AID-ADMA317>3.0.CO;2-U - Lee, K.-H., Bae, Y.-J. and Byeon, S.-H. (2008) pH Dependent Hydrothermal Synthesis and Photoluminescence of Gd2O3:Eu Nanostructures. Bulletin of the Korean Chemical Society, 29, 2161-2168. http://dx.doi.org/10.5012/bkcs.2008.29.11.2161
- Ropp, R.C. (1993) The Chemistry of Artificial Lighting Devices: Lamps, Phosphors, and Cathode Ray Tubes. Elsevier, New York.
- Blasse, G. and Grabmaier, B.C. (1994) Luminescent Materials. Springer, New York.
ttp://dx.doi.org/10.1007/978-3-642-79017-1 - Wan, J., Wang, Z., Chen, X., Mu, L. and Qian, Y. (2005) Shape-Tailored Photoluminescent Intensity of Red Phosphor Y2O3:Eu3+. Journal of Crystal Growth, 284, 538-543.
http://dx.doi.org/10.1016/j.jcrysgro.2005.07.040 - Xu, G.X. and Xiao, J.M. (1985) New Frontiers Rare Earth Science and Application. Academic Press, New York.
- Cuif, J.P., Rohart, E., Macaudiere, P., Bauregard, C., Suda, E., Pacaud, B., Imanaka, N., Masui, T. and Tamura, S. (2004) Binary Rare Earth Oxides. Kluwer Academic Publishers, Dordrecht.
- Bae, Y.J., Lee, K.H. and Byeon, S.H. (2009) Synthesis and Eu3+ Concentration-Dependent Photoluminescence of Gd2-xEuxO3 Nanowires. Journal of Luminescence, 129, 81-85.
http://dx.doi.org/10.1016/j.jlumin.2008.08.004 - Beaurepaire, E., Buissette, V., Sauviat, M.P., Mercuri, A., Martin, J.L., Lahlil, K., Aume, D., Huignard, A., Gacoin, T., Boilot, J.P. and Alexandrou, A. (2004) Functionalized Fluorescent Oxide Nanoparticles: Artificial Toxins for Sodium Channel Targeting and Imaging at the Single-Molecule Level. Nano Letters, 4, 2079-2083. http://dx.doi.org/10.1021/nl049105g
- Louis, C., Bazzi, R., Marquette, C.A., Bridot, J.L., Roux, S., Ledoux, G., Mercier, B., Blum, L., Perriat, P. and Tille-ment, O. (2005) Nanosized Hybrid Particles with Double Luminescence for Biological Labeling. Chemistry of Materials, 17, 1673-1682. http://dx.doi.org/10.1021/cm0480162
- Nichkova, M., Dosev, D., Gee, S.J., Hammock, B.D. and Kennedy, I.M. (2005) Quantum Dots as Reporters in Multiplexed Immunoassays for Biomarkers of Exposure to Agrochemicals. Analytical Letters, 40, 1423-1433.
- Goldys, E.M., Tomsia, K.D., Jinjun, S., Dosev, D., Kennedy, I.M., Yatsunenko, S. and Godlewski, M. (2006) Optical Characterization of Eu-Doped and Undoped Gd2O3 Nanoparticles Synthesized by the Hydrogen Flame Pyrolysis Method. Journal of the American Chemical Society, 128, 14498-14505. http://dx.doi.org/10.1021/ja0621602
- Zhou, Y., Lin, J. and Wang, S. (2003) Energy Transfer and Up-Conversion Luminescence Properties of Y2O3:Sm and Gd2O3:Sm Phosphors. Journal of Solid State Chemistry, 171, 391-395.
http://dx.doi.org/10.1016/S0022-4596(02)00219-0 - Rossner, W. and Grabmaier, B.C. (1991) Phosphors for X-Ray Detectors in Computed Tomography. Journal of Luminescence, 48-49, 29-36. http://dx.doi.org/10.1016/0022-2313(91)90072-4
- Guo, C., Chen, T., Luan, L., Zhang, W. and Huang, D. (2008) Luminescent Properties of R2(MoO4)3:Eu3+ (R = La, Y, Gd) Phosphors Prepared by Sol-Gel Process. Journal of Physics and Chemistry of Solids, 69, 1905-1911. http://dx.doi.org/10.1016/j.jpcs.2008.01.021
- Pereira, P.F.S., de Moura, A.P., Nogueira, I.C., Lima, M.V.S., Longo, E., de Sousa Filho, P.C., Serra, O.A., Nassar, E.J. and Rosa, I.L.V. (2012) Study of the Annealing Temperature Effect on the Structural and Luminescent Properties of SrWO4:Eu Phosphors Prepared by a Non-Hydrolytic Sol-Gel Process. Journal of Alloys and Compounds, 526, 11-21. http://dx.doi.org/10.1016/j.jallcom.2012.02.083
- Rosa, I.L.V., Oliveira, L.H., Suzuki, C.K., Varela, J.A., Leite, E.R. and Longo, E. (2008) SiO2-GeO2 Soot Perform as a Core for Eu2O3 Nanocoating: Synthesis and Photophysical Study. Journal of Fluorescence, 18, 541-545. http://dx.doi.org/10.1007/s10895-007-0297-7
- Morais, E.A., Scalvi, L.V.A., Tabata, A., De Oliveira, J.B.B. and Ribeiro, S.J.L. (2008) Photoluminescence of Eu3+ Ion in SnO2 Obtained by Sol-Gel. Journal of Materials Science, 43, 345-349.
http://dx.doi.org/10.1007/s10853-007-1610-1 - Marques, A.P.A., Tanaka, M.T.S., Longo, E., Leite, E.R. and Rosa, I.L.V. (2011) The Role of the Eu3+ Concentration on the SrMoO4:Eu Phosphor Properties: Synthesis, Characterization and Photophysi-
cal Studies. Journal of Fluorescence, 21, 893-899. http://dx.doi.org/10.1007/s10895-010-0604-6 - Yan, M.F., Huo, T.C.D. and Ling, H.C.J. (1987) Preparation of Y3Al5O12-Based Phosphor Powders. Journal of the Electrochemical Society, 134, 493-498. http://dx.doi.org/10.1149/1.2100487
- Shea, L.E., McKittrick, J., Lopez, O.A. and Sluzky, E. (1996) Synthesis of Red-Emitting, Small Particle Size Luminescent Oxides Using an Optimized Combustion Process. Journal of the American Ceramic Society, 79, 3257-3265. http://dx.doi.org/10.1111/j.1151-2916.1996.tb08103.x
- Ravichandran, D., Roy, R., White, W.B. and Erdei, S. (1997) Synthesis and Characterization of Sol-Gel Derived HexaAluminate Phosphor. Journal of Materials Research, 12, 819-824.
http://dx.doi.org/10.1557/JMR.1997.0119 - Erdei, S., Roy, R., Harshe, G., Juwhari, S., Agrawal, H.D., Ainger, F.W. and White, W.B. (1995) The Effect of Powder Preparation Processes on the Luminescent Properties of Yttrium Oxide Based Phosphor Materials. Materials Research Bulletin, 30, 745-753. http://dx.doi.org/10.1016/0025-5408(95)00052-6
- Santos, M.L., Lima, R.C., Riccardi, C.S., Tranquilin, R.L., Bueno, P.R., Varela, J.A. and Longo, E. (2008) Preparation and Characterization of Ceria Nanospheres by Microwave-Hydrothermal Method. Materials Letters, 62, 4509-4511. http://dx.doi.org/10.1016/j.matlet.2008.08.011
- Lima, R.C., Macario, L.R., Espinosa, J.W.M., Longo, V.M., Erlo, R., Marana, N.L., Sambrano, J.R., Santos, M.L.D., Moura, A.P., Pizani, P.S., Andres, J., Longo, E. and Varela, J.A. (2008) Toward an Understanding of Intermediateand Short-Range Defects in ZnO Single Crystals. A Combined Experimental and Theoretical Study. The Journal of Physical Chemistry A, 112, 8970-8978. http://dx.doi.org/10.1021/jp8022474
- Moura, A.P., Cavalcante, L.S., Sczancoski, J.C., Stroppa, D.G., Paris, E.C., Ramirez, A.J., Varela, J.A. and Longo, E. (2010) Structure and Growth Mechanism of CuO Plates Obtained by Microwave-Hydrothermal without Surfactants. Advanced Powder Technology, 21, 197-202.
http://dx.doi.org/10.1016/j.apt.2009.11.007 - de Moura, A.P., Lima, R.C., Moreira, M.L., Volanti, D.P., Espinosa, J.W.M., Orlandi, M.O., Pizani, P.S., Varela, J.A. and Longo, E. (2010) ZnO Architectures Synthesized by a Microwave-Assisted Hydrothermal Method and Their Photoluminescence Properties. Solid State Ionics, 181, 775-780. http://dx.doi.org/10.1016/j.ssi.2010.03.013
- Motta, F.V., Lima, R.C., Marques, A.P.A., Li, M.S., Leite, E.R., Varela, J.A. and Longo, E. (2010) Indium Hydroxide Nanocubes and Microcubes Obtained by Microwave-Assisted Hydrothermal Method. Journal of Alloys and Compounds, 497, L25-L28. http://dx.doi.org/10.1016/j.jallcom.2010.03.069
- de Moura, A.P., Lima, R.C., Paris, E.C., Li, M.S., Varela, J.A. and Longo, E. (2011) Formation of β-Nickel Hydroxide Plate-Like Structures under Mild Conditions and Their Optical Properties. Journal of Solid State Chemistry, 184, 2818-2823.
- Bohr, H. and Bohr, J. (2000) Microwave-Enhanced Folding and Denaturation of Globular Proteins. Physical Review E, 61, 4310-4314. http://dx.doi.org/10.1103/PhysRevE.61.4310
- Blanco, C. and Auerbach, S.M. (2002) Microwave-Driven Zeolite-Guest Systems Show Athermal Effects from None-quilibrium Molecular Dynamics. Journal of the American Chemical Society, 124, 6250-6251. http://dx.doi.org/10.1021/ja017839e
- Favretto, L., Nugent, W.A. and Licini, G. (2002) Highly Regioselective Microwave-Assisted Synthesis of Enantiopure C3-Symmetric Trialkanolamines. Tetrahedron Letters, 43, 2581-2584.
http://dx.doi.org/10.1016/S0040-4039(02)00306-4 - Hoz, A.D.L., Diaz-Ortiz, A. and Moreno, A. (2004) Selectivity in Organic Synthesis under Microwave Irradiation. Current Organic Chemistry, 8, 903-918. http://dx.doi.org/10.2174/1385272043370429
- Bren, M., Janezic, D. and Bren, U. (2010) Microwave Catalysis Revisited: An Analytical Solution. The Journal of Physical Chemistry A, 114, 4197-4202. http://dx.doi.org/10.1021/jp100374x
- Sun, L.D., Yao, J., Liu, C., Liao, C. and Yan, C.H. (2000) Rare Earth Activated Nanosized Oxide Phosphors: Synthesis and Optical Properties. Journal of Luminescence, 87-89, 447-450.
http://dx.doi.org/10.1016/S0022-2313(99)00471-8 - Kappe, C.O., Stadler, A. and Dallinger, D. (2012) Microwaves in Organic and Medicinal Chemistry. 2nd Edition, Vol. 52, Wiley-VCH, Weinheim. http://dx.doi.org/10.1002/9783527647828
- Obermayer, D., Gutmann, B. and Kappe, C.O. (2009) Microwave Chemistry in Silicon Carbide Reaction Vials: Separating Thermal from Nonthermal Effect. Angewandte Chemie International Edition, 48, 8321-8324. http://dx.doi.org/10.1002/anie.200904185
- Yao, B.D. and Wang, N. (2001) Carbon Nanotube Arrays Prepared by MWCVD. The Journal of Physical Chemistry B, 105, 11395-11398. http://dx.doi.org/10.1021/jp011849k
- Zhu, Y.J., Wang, W.W., Qi, R.J. and Hu, X.L. (2004) Microwave-Assisted Synthesis of Single-Crystalline Tellurium Nanorods and Nanowires in Ionic Liquids. Angewandte Chemie International Edition, 43, 1410-1414.
- Tompsett, G.A., Conner, W.C. and Yngvesson, K.S. (2006) Microwave Synthesis of Nanoporous Materials. Chem-PhysChem, 7, 296-319. http://dx.doi.org/10.1002/cphc.200500449
- de Moura, A.P., de Oliveira, L.H., Paris, E.C., Li, M.S., Andrés, J., Varela, J.A., Longo, E. and Rosa, I.L.V. (2011) Photolumiscent Properties of Nanorods and Nanoplates Y2O3:Eu3+. Journal of Fluorescence, 21, 1431-1438. http://dx.doi.org/10.1007/s10895-010-0827-6
- Chang, C. and Mao, D. (2007) Thermal Dehydration Kinetics of a Rare Earth Hydroxide, Gd(OH)3. International Journal of Chemical Kinetics, 39, 75-81. http://dx.doi.org/10.1002/kin.20221
- Rietveld, H.M. (1969) A Profile Refinement Method for Nuclear and Magnetic Structures. Journal of Applied Crystal-lography, 2, 65-71. http://dx.doi.org/10.1107/S0021889869006558
- Larson, C.A. and Von Dreele, R.B. (2001) The Regents of the University of California, Copyright 1985-2000, Los Alamos National Laboratory, Los Alamos, EUA.
- Thompson, P., Cox, D.E. and Hastings, J.B. (1987) Rietveld Refinement of Debye-Scherrer Synchrontron X-Ray Data from Al2O3. Journal of Applied Crystallography, 20, 79-83.
http://dx.doi.org/10.1107/S0021889887087090 - Finger, L.W., Cox, D.E. and Jephcoat, A.P. (1994) A Correction for Powder Diffraction Peak Asymmetry Due to Axial Divergence. Journal of Applied Crystallography, 27, 892-900.
http://dx.doi.org/10.1107/S0021889894004218 - Stephens, P.W. (1999) Phenomenological Model of Anisotropic Peak Broadening in Powder Diffraction. Journal of Applied Crystallography, 32, 281-289.
http://dx.doi.org/10.1107/S0021889898006001 - Momma, K. and Izumi, F. (2008) VESTA: A Three-Dimensional Visualization System for Electronic and Structural Analysis. Journal of Applied Crystallography, 41, 653-658.
http://dx.doi.org/10.1107/S0021889808012016 - Buijs, M., Meyerink, A. and Blasse, G. (1987) Energy Transfer between Eu3+ Ions in a Lattice with Two Different Crystallographic Sites: Y2O3:Eu3+, Gd2O3:Eu3+ and Eu2O3. Journal of Luminescence, 37, 9-20. http://dx.doi.org/10.1016/0022-2313(87)90177-3
- Kevorkov, A.M., Karyagin, V.F., Munchaev, A.I., Uyukin, E.M., Bolotina, N.B., Chernaya, T.S., Bagdasarov, K.S. and Simonov, V.I. (1995) Y2O3 Single Crystals: Growth, Structure and Photoinduc-
ed Effects. Crystallography Reports, 40, 23. - Godinho, M., Ribeiro, C., Longo, E. and Leite, E.R. (2008) Influence of Microwave Heating on the Growth of Gadolinium-Doped Cerium Oxide Nanorods. Crystal Growth Design, 8, 384-386.
http://dx.doi.org/10.1021/cg700872b - Baraldi, P. and Davolio, G. (1989) An Electrochemical and Spectral Study of the Nickel Oxide Electrode. Materials Chemistry and Physics, 21, 143-154.
http://dx.doi.org/10.1016/0254-0584(89)90109-0 - Guo, H., Yang, X., Xiao, T., Zhang, W., Lou, L. and Mugnier, J. (2004) Structure and Optical Properties of Sol-Gel Derived Gd2O3 Waveguide Films. Applied Surface Science, 230, 215-221.
http://dx.doi.org/10.1016/j.apsusc.2004.02.032 - Jayasimhadri, M., Ratnam, B.V., Jang, K., Lee, H.S., Chen, B., Yi, S.S., Jeong, J.H. and Moorthy, L.R. (2011) Combustion Synthesis and Luminescent Properties of Nano and Submicrometer-Size Gd2O3:Dy3+ Phosphors for White LEDs. International Journal of Applied Ceramic Technology, 8, 709-717. http://dx.doi.org/10.1111/j.1744-7402.2010.02499.x
- Liu, G., Hong, G., Wang, J. and Dong, X. (2007) Hydrothermal Synthesis of Spherical and Hollow Gd2O3:Eu3+ Phosphors. Journal of Alloys and Compounds, 432, 200-204.
http://dx.doi.org/10.1016/j.jallcom.2006.05.127 - Liu, G., Hong, G., Dong, X. and Wang, J. (2008) Preparation and Characterization of Gd2O3:Eu3+ Luminescence Nanotubes. Journal of Alloys and Compounds, 466, 512-516.
http://dx.doi.org/10.1016/j.jallcom.2007.11.108 - Schmechel, R., Kennedy, M., von Seggerm, H., Winkler, H., Kolbe, M., Fischer, R.A., et al. (2001) Luminescence Properties of Nanocrystalline Y2O3:Eu3+ in Different Host Materials. Journal of Applied Physics, 89, 1679-1686. http://dx.doi.org/10.1063/1.1333033
- Pang, M.L., Lin, J., Fu, J., Xing, R.B., Luo, C.X. and Han, Y.C. (2003) Preparation, Patterning and Luminescent Properties of Nanocrystalline Gd2O3:A (A = Eu3+, Dy3+, Sm3+, Er3+) Phosphor Films via Pechini Sol-Gel Soft Lithography. Optical Materials, 23, 547-558. http://dx.doi.org/10.1016/S0925-3467(03)00020-X
- Teotonio, E.E.S., Felinto, M.C.F.C., Brito, H.F., Malta, O.L., Najjar, A.C.R. and Strek, W. (2004) Synthesis, Crystal-line Structure and Photoluminescence Investigations of the New Trivalent Rare Earth Complexes (Sm3+, Eu3+ and Tb3+) Containing 2-Thiophenecarboxylate as Sensitizer. Inorganica Chimica Acta, 357, 451-460. http://dx.doi.org/10.1016/j.ica.2003.08.009
- Rosa, I.L.V., Maciel, A.P., Longo, E., Leite, E.R. and Varela, J.A. (2006) Synthesis and Photoluminescence Study of La1.8Eu0.2O3 Coating on Nanometric α-Al2O3. Materials Research Bulletin, 41, 1791-1797. http://dx.doi.org/10.1016/j.materresbull.2006.03.026
NOTES

*Corresponding author.

