Advances in Chemical Engineering and Science
Vol. 2 No. 1 (2012) , Article ID: 16710 , 7 pages DOI:10.4236/aces.2012.21011
Study on the Effect of a Magnetic Field on Pb(II) Removal Using Modified Chitosan
1Institute of Polymer Science, Sun Yat-sen University, Guangzhou, China
2Light Industry & Chemical Engineering Research Institute, South China University of Technology, Guangzhou, China
3Guangdong Jiangmen Supervision Testing Institute of Quality & Metrology, Jiangmen, China
Email: *maria.duan@gmail.com, *lfsyguo@scut.edu.cn
Received September 13, 2011; revised October 25, 2011; accepted November 6, 2011
Keywords: Magnetic Field; Chitosan; Pb(II); Removal
ABSTRACT
This work examined the removal of Pb(II) using a chitosan derivative (SB, synthesized from benzaldehyde) assisted by a magnetic field. The adsorption capacity for Pb(II) was investigated. It was found that 1) the pH and concentration of the ion solution, as well as exposure time and strength of magnetic field, affected the degree of adsorption; and 2) studies of the adsorption isotherms and kinetics of ions onto SB revealed that SB showed enhanced adsorption capacity towards Pb(II) ions in a magnetic field compared with magnetically untreated samples. The Langmuir and Freundlich isotherm were applied to describe the experimental adsorption, and the maximum adsorption capacity of SB for Pb(II) was 2.5040 mg/g, when assisted by a magnetic field of 480 kA/m.
1. Introduction
Lead, an element belonging to non-degradable environmental pollutants, is widely distributed in nature. Lead ions and soluble lead salts are toxic and can cause harm on human health and plant growth. It can poison the nervous and blood system, cause convulsions, neurological retardation and anemia, affect child development in particular. Molecular mechanism indicates that the free state Pb(II) is toxic form, if a stable complex of the ligand can form, the concentration of free state Pb(II) in the human body will reduce the free state, thereby decrease the toxicity of lead on human [1].
Traditional methods for eliminating dissolved heavy metal ions include oxidation-reduction, chemical precipitation, filtration, ion exchange, electrochemical treatment, application of membrane separation and evaporation recovery. However, these processes have considerable disadvantages including incomplete metal elimination, high reagent or energy consumption, expensive equipment and monitoring system requirements and the generation of toxic sludge or other waste products that require disposal [2-5].
In this paper, a magnetic field was applied to strengthen the adsorption of Pb(II) ions by the adsorbent. It has been demonstrated in recent years that magnetic fields influence the physicochemical properties of water and aqueous solutions and suspensions [6-24]. Different hypotheses for the influence of magnetic fields on water and aqueous solutions have been extensively studied [25- 27]. For example, Colic and Morse [25] indicated that electromagnetic fields exerted their effects through a perturbation of the gas/liquid interface and that these effects disappeared after the system was degassed. Furthermore, Madsen [27] investigated the influence of a magnetic field on the precipitation of some inorganic salts.
Chitosan (CS) is a basic polysaccharides in which the -NH2 groups can form -NH3+ under acidic conditions. In addition, these -NH2 groups cannot entirely take part in the coordination of metal ions, limiting its adsorption capabilities and being disadvantageous for regeneration. Recently, there has been a growing interest in the chemical modification of CS to improve its water solubility and strengthen its adsorption capabilities [28-32]. For example, the -NH2 groups of CS can react with aldehydes or ketones to form imines (Schiff base). It was reported that chitosan Schiff base (SB) could have excellent chelation ability with heavy metal ions [33].
The purpose of this study was to investigate the adsorption behavior of Pb(II) on SB in a magnetic field. The influence of experimental conditions, such as exposure time, strength of magnetic field and the pH and concentration of Pb(II) ion solution, on the adsorption were studied. This information might be useful for further application in the treatment of waste effluents.
2. Experimental Procedure
2.1. Materials and Apparatus
Schiff base prepared by the reaction of the -NH2 groups of CS and aldehydes of benzaldehyde (SB [34]); acetic acid and other chemicals used were analytical reagent.
The setup for generating the magnetic field, shown in Figure 1 and Table 1, was provided by the Light Industry and Chemical Engineering Research Institute at the South China University of Technology.
2.2. Measurement of Pb(II) Ions
A stock solution (1000 mg/L) of Pb(II) ions was prepared from ultra-pure Pb(NO3)2. The stock solution was then diluted to give standard solutions of appropriate concentrations. The pH of the solutions was adjusted with HNO3 and citrate-sodium sulfite reducing solution. Then, the reaction medium was extracted with chloroform and measured using a spectrophotometer (Shimadzu UV2102PC) at 510 nm with diphenylhydantoin as the complexing agent.

Figure 1. Adjusted magnetic field equipment. 1. Voltage transformer; 2. Regulator; 3. Device for recording the strength of the field; 4. Ammeter; 5. Voltmeter; 6. Coil; 7. Iron core; 8. Shell; 9. Handlebar; 10. Stand.
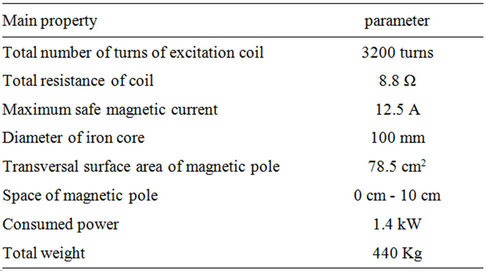
Table 1. The main performance parameters of the magnetic field.
All these batch tests were conducted in 100 ml stoppered flasks in a water bath kept at 298 K. The agitation rate was held constant at 120 rpm.
2.3. Adsorption Experiments
All adsorption tests, including kinetics, isotherms and magnetic field treatment, were performed as batch experiments with duplicates. Adsorption kinetics was generated with a fixed absorbent mass and constant conditions while the adsorption of Pb(II) ions for different times was investigated. The pH of the metal solution in these tests was adjusted to 4. In addition, when the adsorptions were observed in a magnetic field, the magnetic field strength was fixed at 400 kA/m.
Adsorption isotherms are typically generated either with a fixed absorbent mass and varying initial Pb(II) ion concentration (C0) or with varying absorbent mass and a fixed C0 level. The latter approach was used in this study. At least nine samples of 10 ml each with different Pb(II) ion concentrations ranging from 5 mg/L to 30 mg/L were placed in 50 ml conical bottles with 0.100 g of absorbent. The initial Pb(II) ion solutions were prepared by serial dilution of standard 1000 mg/L stock solutions.
When the effect of magnetic field treatment was studied, the magnetic field strength was varied from 160 kA/m to 800 kA/m. Meanwhile, the pH of the solution and the exposure time were fixed at 4 min and 30 min, respectively.
3. Results and Discussion
Adsorption kinetics and adsorption isotherms play important roles in research on the adsorption capacity of an absorbent. Therefore, the exposure time and pH of metal solutions were studied in this report. Meanwhile, the effect of field strength and concentration of Pb(II) solution on the adsorption was also investigated. Finally, adsorption isotherms were also assessed.
3.1. Effect of Exposure Time
The adsorption kinetics curves of CS and SB untreated by magnetic field are shown in Figure 2. The exposure time varied in the range of 0 h - 180 h, and the initial metal concentration was fixed at 30 mg/L. According to Figure 2, the trend in adsorption quantities and adsorption rates over time was similar for CS and SB. Specifically, adsorption increased with increased exposure time but then remained almost constant after a certain value, eventually achieving equilibrium. The time to equilibrium was 120 min for CS and approximately 60 min for SB. This phenomenon indicated that the adsorption rate of SB was higher than that of CS. It also could be seen that the adsorption quantity and adsorption rate of SB were higher than those of CS at any given time period. The adsorption quantity and adsorption rate of SB corresponded to at least 0.8907 mg Pb(II)/g SB and 29.68% for Pb(II).
Figure 3 shows the adsorption kinetics curves of CS and SB in a magnetic field of 400 kA/m. As can be seen, the trend was similar to the adsorption kinetics curves without magnetic field treatment. However, the periods required for adsorption equilibrium of CS and SB were both shorter (i.e., the equilibrium periods of CS and SB were 90 min and 30 min, respectively). Comparing Figures 2 and 3, the adsorption quantity and adsorption rate of SB assisted by a magnetic field of 400 kA/m were at least 0.1003 mg Pb(II)/g SB and 3.34% higher than that of SB without assistance of magnetic field at any given time point for Pb(II). Furthermore, the adsorption quantity and adsorption rate of SB were at least 0.6555 mg Pb(II)/g SB and 21.85% more than those of CS at any corresponding adsorption period in the magnetic field of 400 kA/m. Therefore, the adsorption capabilities of CS and SB were enhanced in the presence of a magnetic field.
3.2. Effect of Magnetic Field Strength
The effect of magnetic field strength on adsorption is shown in Figure 4. In light of the data presented in Figure 4, the adsorption capability of SB in a magnetic field was enhanced in the magnetic field strength range of 160 kA/m - 800 kA/m after 30 min. Furthermore, from 160 to 480 kA/m, the adsorption quantity and adsorption rate increased dramatically. However, the change became much less outside of this field strength range. Taking into account energy conservation, the magnetic field strength of 480 kA/m was recommended for further adsorption enhancement applications.
3.3. Effect of PH
The pH of the metal ion solution is an important parameter in the adsorption process as a whole and on the adsorption capacity of SB in particular. Figure 5 shows the effect of pH on the adsorption capacity of SB for Pb(II) after exposure in a 480 kA/m magnetic field for 45 min. It can be seen that the amount of Pb(II) adsorbed by SB slowly increased when the pH of the metal solution increased from 1 to 5. The maximum adsorptions of Pb(II) ions on SB were found at pH 4.0 in a 480 kA/m magnetic field.
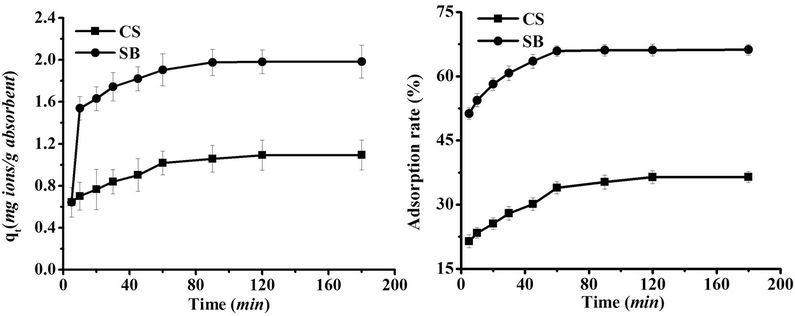
Figure 2. Effect of exposure time on adsorption of Pb(II) on CS and SB (Metal concentration: 30 mg/L; Dose: 0.0100 g/ml; pH 7.4).
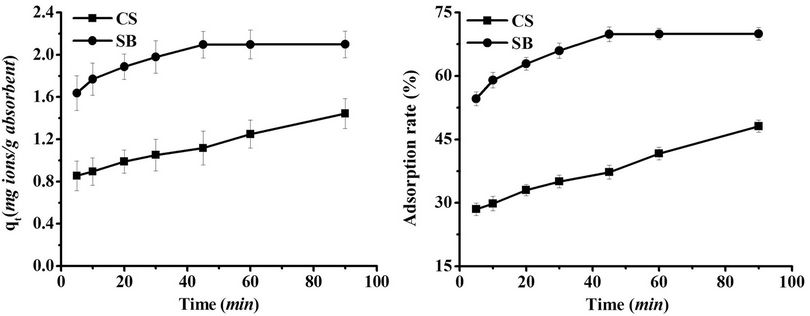
Figure 3. Effect of exposure time on adsorption of Pb(II) onto CS and SB in a magnetic field (Metal concentration: 30 mg/L; Dose: 0.0100 g/ml; pH 7.4; Strength of magnetic field: 400 kA/m).
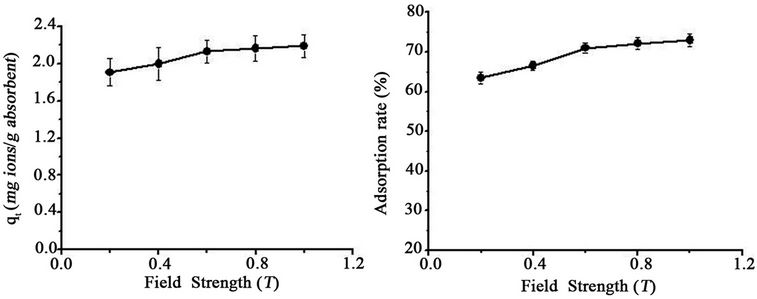
Figure 4. Effect of field strength on adsorption of Pb(II) onto SB in a magnetic field (Metal concentration: 30 mg/L; Dose: 0.0100 g/ml; pH 7.4; Exposure time: 45 min).

Figure 5. Effect of pH on adsorption of Pb(II) onto SB in a magnetic field (Metal concentration: 30 mg/L; Dose: 0.0100 g/ml; Exposure time: 45 min; Strength of magnetic field: 480 kA/m).
3.4. Adsorption Isotherms
To investigate the adsorption mechanism, the kinetics data obtained were analyzed using the Langmuir equation (Equation (1)) and the Freundlich equation (Equation (2)).
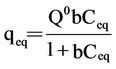 (1)
(1)
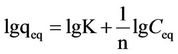 (2)
(2)
where Q0 is the maximum amount of metal ion per unit weight of cell to form a complete monolayer on the surface bound at high Ceq (mg/g), and b is a constant representing the affinity of the binding sites. Q0 stands for a practical limiting adsorption capacity when the surface is fully covered with metal ions and assists in the compareson of adsorption performance, particularly in cases where the sorbents do not reach their full saturation. Values of Q0 (mg/g), b (L/mg), and K, and 1/n were calculated from the linear plot of 1/qeq vs 1/Ceq, the intercept of linear regression, and the plot of lgqeq – lgK vs lgCeq, respectively. The metal ion adsorption experiments were carried out at different initial ion concentrations ranging from 5 mg/L to 30 mg/L at the optimum pH of 4 under agitation at 130 rpm. In order to develop an equation that could accurately describe the experimental results and could be used for design purposes, analysis of isotherm data was important [2,35]. In addition, adsorption isotherms can be used to represent how a solute interacts with an adsorbent and to optimize the use of an adsorbent.
Figure 6 displays the Langmuir and Freundlich models, respectively, of Pb(II) adsorption onto SB. The correlation coefficient and linear regression values are summarized in Table 2. The data indicated that the Langmuir and Freundlich models were useful for describing the experimental data.
3.5. The Effect of Initial Concentration of Metal Ions
During the adsorption process of a solute from a solution onto a solid surface, there is a dynamic equilibrium between the solute adsorbed on the solid surface and the solute remaining in solution. Figure 7 reveals that the adsorption capacity of SB for Pb(II) ions increased with increasing metal concentration. The maximum adsorption capacity of 2.5040 mg Pb(II)/g SB was reached at 40 mg/L metal ion solution concentrations.
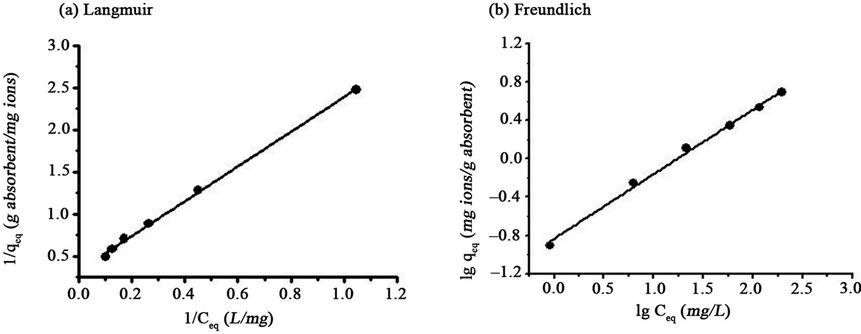
Figure 6. Effect of pH on adsorption of Pb(II) onto SB in a magnetic field (Metal concentration: 30 mg/L; Dose: 0.0100 g/ml; Exposure time: 45 min; Strength of magnetic field: 480 kA/m).
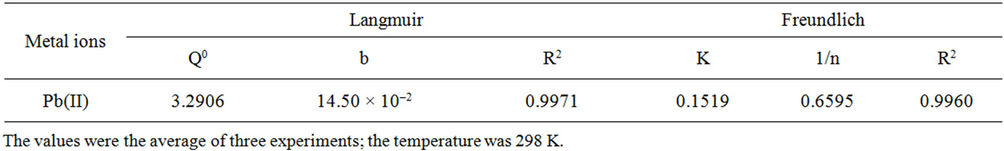
Table 2. Parameters for the Langmuir and Freundlich equations.
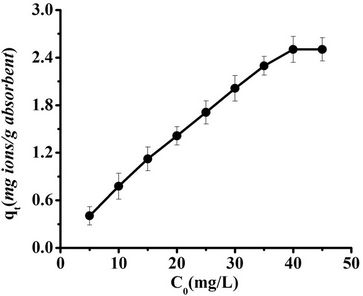
Figure 7. Effect of metal concentration on adsorption of Pb(II) onto SB in a magnetic field (Dose: 0.0100 g/ml; Exposure time: 45 min; Strength of magnetic field: 480 kA/m; pH 4).
3.6. Discussion of the Mechanism for Magnetic Treatment of Adsorption Systems
It was previously reported [36] that magnetic treatment could reduce the viscosity and surface tension of solutions, an effect possibly concerned with resonance. When the magnetic field and the oscillation frequency of the molecules were in tune, resonance could occur. This resonance would then impart energy to the system for activation, changing the structure and nature of the material in the magnetic field. Magnetic treatment could thus promote the adsorption of metal ions. However, the mechanism of magnetic treatment remains to be clarified in an in-depth study.
4. Conclusions
The adsorption of chitosan (CS) could be improved by condensation with benzaldehyde to form a Schiff base. The chitosan-benzaldehyde Schiff base (SB) had an excellent adsorption capacity for Pb(II). This SB could adsorb twice as much Pb(II) as CS in half the time. This adsorption could be expressed with the Langmuir and Freundlich isotherm equation.
The adsorption ability, including adsorption amount and adsorption rate, could be enhanced with a suitable magnetic field. For example, upon treatment with a field strength of 480 kA/m, the adsorption equilibrium period for metal ions on SB shortened from 60 min to 30 min. In the future, the mechanism of magnetic treatment on adsorption will have to be studied deeply.
5. Acknowledgements
The authors are thankful for financial support from the Doctoral Discipline Special Foundation of the Education Ministry of China (20050561014) and the National “863” Science & Technology Project of China (2007AA100405).
REFERENCES
- T. L. Liao, G. L. Sun and H. P. Dong, “Research on the Toxicity of Lead Ion in Mimetic Membrane,” Guangdong Chemical Industry, No. 1, 2010, pp. 53-55. http://202.116. 64.102 :81/asp/Detail.asp
- Z. Aksu, F. Gönen and Z. Demircan, “Biosorption of Chromium(VI) Ions by Mowital B3OH Resin Immobilized Activated Sludge in a Packed Bed: Comparison with Granular Activated Carbon,” Process Biochemistry, Vol. 38, No. 2, 2002, pp. 175-186. doi:10.1016/S0032-9592(02) 00053-5
- R. S. Bai and E. Abraham, “Studies on Chromium (VI) Adsorption Desorption Using Immobilized Fungal Biomoss,” Bioresource Technology, Vol. 87, No. 1, 2003, pp. 17-26. doi:10.1016/S0960-8524(02)00222-5
- B. Benguella and H. Benaissa, “Effects of Competing Cations on Cadmium Biosorption by Chitin,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 201, No. 1-3, 2002, pp. 143-150. doi:10.1016/S0927- 7757(01)00899-8
- F. Veglio and F. Beolchini, “Removal of Met also by Biosorption: A Review,” Hydrometallurgy, Vol. 44, No. 3, 1997, pp. 301-316. doi:10.1016/S0304-386X(96)00059-X
- K. Higashitani, A. Kage, S. Katamura, K. Imai and S. Hatade, “Effects of a Magnetic Field on the Formation of CaCO3 Particles,” Journal of Colloid and Interface Science, Vol. 156, No. 1, 1993, pp. 90-95. doi:10.1006/ jcis.1993. 1085
- S. Kobe, G. Dražić, A. C. Cefalas, E. Sarantopoulou and J. Stražišar, “Nucleation and Crystallization of CaCO3 in Applied Magnetic Fields,” Crystal Engineering, Vol. 5, No. 3-4, 2002, pp. 243-253. doi:10.1016/S1463-0184(02) 00035-7
- S. Kobe, G. Dražić, P. J. McGuiness, T. Meden, E. Sarantopoulou, Z. Kollia and A. C. Cefalas, “Control over Nanocrystalization in Turbulent Flow in the Presence of Magnetic Fields,” Materials Science and Engineering, Vol. 23, No. 6-8, 2003, pp. 811-815. doi:10.1016/j.msec. 2003.09.136
- R. Gher, Z. A. Zhai, J. A. Finch and S. R. Rao, “Reduction of Soluble Mineral Concentrations in CaSO4 Saturated Water Using a Magnetic Field,” Water Research, Vol. 29, No. 3, 1995, pp. 933-940. doi:10.1016/0043-1354(94) 002 14-R
- E. Chibowski, L. Hołysz and A. Szcześ, “Time Dependent Changes in Zeta Potential of Freshly Precipitated Calcium Carbonate,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 222, No. 1-3, 2003, pp. 41-54. doi:10.1016/S0927-7757(03)00232-2
- L. Hołysz, E. Chibowski and A. Szcześ, “Influence of Impurity Ions and Magnetic Field on the Properties of Freshly Precipitated Calcium Carbonate,” Water Research, Vol. 37, No. 14, 2003, pp. 3351-3360. doi:10.1016/S0043-1354 (03) 00159-3
- L. Hołysz, A. Szcześ and E. Chibowski, “Effects of a Static Magnetic Field on Water and Electrolyte Solutions,” Journal of Colloid and Interface Science, Vol. 316, No. 2, 2007, pp. 996-1002. doi:10.1016/j.jcis.2007.08.026
- J. M. D. Coey and S. Cass, “Magnetic Water Treatment,” Journal of Magnetism and Magnetic Materials, Vol. 209, No. 1-3, 2000, pp. 71-74. doi:10.1016/S0304-8853(99) 00648-4
- C. Gabrielli, R. Jaouhari, G. Maurin and M. Keddam, “Magnetic Water Treatment for Scale Prevention,” Water Research, Vol. 35, No. 13, 2001, pp. 3249-3259. doi:10. 1016/S0043-1354(01)00010-0
- E. Chibowski, A. Szcześ and L. Hołysz, “Influence of Sodium Dodecyl Sulfate and Static Magnetic Field on the Properties of Freshly Precipitated Calcium Carbonate,” Langmuir, Vol. 21, No. 18, 2005, pp. 8114-8122.
- S. A. Parsons, B. L. Wang, S. J. Judd and T. Stephenson, “Magnetic Treatment of Calcium Carbonate Scale-Effect of pH Control,” Water Research, Vol. 31, No. 2, 1997, pp. 339-342. doi:10.1016/S0043-1354(96)00238-2
- J. S. Backer and S. J. Judd, “Magnetic Amelioration of Scale Formation,” Water Research, Vol. 30, No. 2, 1996, pp. 247-260. doi:10.1016/0043-1354(95)00184-0
- H. Al-Qahtani, “Effect of Magnetic Treatment on Gulf Seawater,” Desalination, Vol. 107, No. 1, 1996, pp. 75- 81.
- F. Alimi, M. Tlili, C. Gabrielli, M. Georges and M. B. Amor, “Effect of a Magnetic Water Treatment on Homogeneous and Heterogeneous Precipitation of Calcium Carbonate,” Water Research, Vol. 40, No. 10, 2006, pp. 1941-1950. doi:10.1016/j.watres.2006.03.013
- K. Higashitani, K. Okuhara and S. Hatade, “Effects of Magnetic Fields on Stability of Nonmagnetic Ultrafine Colloidal Particles,” Journal of Colloid and Interface Science, Vol. 152, No. 1, 1992, pp. 125-131. doi:10.1016/ 0021-9797(92)90013-C
- K. Higashitani, H. Iseri, K. Okuhara, A. Kage and S. Hatade, “Magnetic Effects on Zeta Potential and Diffusivity of Nonmagnetic Colloidal Particles,” Journal of Colloid and Interface Science, Vol. 172, No. 2, 1995, pp. 383-388. doi:10.1006/jcis.1995.1268
- J. Oshitani, R. Uehara and K. Higashitani, “Magnetic Effects on Electrolyte Solutions in Pulse and Alternating Fields,” Journal of Colloid and Interface Science, Vol. 209, No. 2, 1999, pp. 374-379. doi:10.1006/jcis.1998.5898
- P. Vallée, J. Lafait, L. Legrand, P. Mentré, M. O. Monod and Y. Thomas, “Effects of Pulsed Low Frequency Electromagnetic Fields on Water Characterized by Light Scattering Techniques: Role of Bubble,” Langmuir, Vol. 21, No. 6, 2005, pp. 2293-2299. doi:10.1021/la047916u
- M. C. Amiri and A. A. Dadkhah, “On Reduction in the Surface Tension of Water Due to Magnetic Treatment,” Colloids Surfaces A: Physicochemical and Engineering Aspects, Vol. 278, No. 1-3, 2006, pp. 252-255. doi:10.1016/j.colsurfa.2005.12.046
- H. Madsen, “Influence of Magnetic Field on the Precipitation of Some Inorganic Salts,” Journal of Crystal Growth, Vol. 152, No. 1-2, 1995, pp. 94-100. doi:10.1016/0022-0248(95)00103-4
- M. Colic and D. Morse, “The Elusive Mechanism of the Magnetic ‘Memory’ of Water,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 154, No. 1-2, 1999, pp. 167-174. doi:10.1016/S0927-7757(98)00894-2
- K. Higashitani and J. Oshitani, “Magnetic Effects on Thickness of Adsorbed Layer in Aqueous Solutions Evaluated Directly by Atomic Force Microscope,” Journal of Colloid and Interface Science, Vol. 204, No. 2, 1998, pp. 363-368. doi:10.1006/jcis.1998.5590
- P. Wanvimol and C. Suwabun, “Conjugation of Gallic Acid onto Chitosan: An Approach for green and Water-Based Antioxidant,” Carbohydrate Polymers, Vol. 72, No. 1, 2008, pp. 169-177. doi:10.1016/j.carbpol.2007.08.002
- G. Ma, D.-Z. Yang, Y.-S. Z., X. Ming, J. F. Kennedy and N. Jun, “Preparation and Characterization of WaterSoluble N-Alkylated Chitosan,” Carbohydrate Polymers, Vol. 74, No. 1, 2008, pp. 121-126. doi:10.1016/j.carbpol.2008.01.028
- C. Peggy, K. Motoichi, E. C. Joo, Y.-Y. Yang, “Synthesis and Characterization of Chitosan-G-Poly(Ethylene Glycol)-Folate as a Non-Viral Carrier for Tumor-Targeted Gene Delivery,” Biomaterials, Vol. 28, No. 3, 2007, pp. 540-549. doi:10.1016/j.biomaterials.2006.08.046
- N. Zerrouk, G. Corti, S. Ancillotti, F. Maestrelli, M. Cirri and P. Mura, “Influence of Cyclodextrins and Chitosan, Separately or in Combination, on Glyburide Solubility and Permeability,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 62, No. 3, 2006, pp. 241-246. doi:10.1016/j.ejpb.2005.08.010
- V. M. Ramos, N. M. Rodríguez, M. S. Rodríguez, A. Heras and E. Agulló, “Modified Chitosan Carrying Phosphonic and Alkyl Groups,” Carbohydrate Polymers, Vol. 51, No. 4, 2003, pp. 425-429. doi:10.1016/S0144-8617(02)00211-4
- G. Manuel, S. N. Antonio, E. M. Maria, et al., “A Derivative of Chitosan and 2,4-Pentanedione with Strong Chelating Properties,” Carbohydrate Research, Vol. 233, No. 2, 1992, pp. 255-259. doi:10.1016/S0008-6215(00)90939-X
- L.-H. Duan, J.-Q. Yang and S.-Y. Guo, “Study on Property of Modified Chitosan for Absorbing Cr (Ⅵ) in Magnetic Field,” Journal of Shanxi University of Science and Technology, Vol. 28, No. 6, 2010, pp. 1-6.
- F. C. Wu, R. L. Tseng and R. S. Juang, “Comparative Adsorption of Metal and Dye on Flakeand Bead-Types of Chitosans Prepared from Fishery Wastes,” Journal of Hazardous Materials, Vol. 73, No. 1, 2000, pp. 63-75. doi:10.1016/S0304-3894(99)00168-5
- B. S. Zheng, S. Y. Guo, L. Li, et al., “Studies on the Strengthening Evaporation of Solution by Magnetic Treatment,” Journal of South China University Technology (Natural Science), Vol. 23, No. 7, 1995, pp. 20-25.
NOTES
*Corresponding authors.

