Journal of Signal and Information Processing
Vol.06 No.02(2015), Article ID:56609,11 pages
10.4236/jsip.2015.62015
Human Quantitative Electroencephalographic and Schumann Resonance Exhibit Real-Time Coherence of Spectral Power Densities: Implications for Interactive Information Processing
Michael A. Persinger, Kevin S. Saroka
Behavioural Neuroscience, Biomolecular Sciences, and Human Studies Programs, Sudbury, Canada
Email: mpersinger@laurentian.ca, kx_saroka@laurentian.ca
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 29 April 2015; accepted 22 May 2015; published 25 May 2015
ABSTRACT
Spectral Power Densities (SPD) within the Quantitative Electroencephalographic (QEEGs) Profiles of 41 men and women displayed repeated transient coherence with the first three modes (7 - 8 Hz, 13 - 14 Hz, and 19 - 20 Hz) of the Schumann Resonance in real time. The duration of the coherence was about 300 ms about twice per min. Topographical map clusters indicated that the domain of maximum coherence was within the right caudal hemisphere near the Parahippocampal gyrus. These clusters, associated with shifts of about 2 μV, became stable about 35 to 45 ms after the onset of the synchronizing event. During the first 10 to 20 ms, the isoelectric lines shifted from clockwise to counterclockwise rotation. The results are consistent with the congruence of the frequency, magnetic field intensity, voltage gradient, and phase shifts that are shared by the human brain and the earth-ionospheric spherical wave guide. Calculations indicated that under certain conditions interactive information processing might occur for brief periods. Natural and technology-based variables affecting the Schumann parameters might be reflected in human brain activity, including modifications of cognition and dream-related memory consolidation.
Keywords:
Schumann Resonances, Human Brain Activity, Coherence, Memory Consolidation, Pico Tesla Magnetic Fields, Photon Emissions

1. Introduction
One of the most common correlates of signal and information processing is the magnitude compatibility between the two sources. Intuitively, such congruence facilitates the transfer and transformation of information between the two loci with minimum distortion that may occur when adjusting transduction to increase fidelity. Two of the most congruent magnitude (and vector) related sources involve the electromagnetic fields generated by the human brain as inferred by quantitative electroencephalography (QEEG) and those produced within the spherical wave guide between the earth’s surface and ionosphere. The latter has been labeled the Schumann Resonance with a fundamental frequency of between 7.5 and 8 Hz. Here, we present the results of direct measurements of the phase-congruence in quantitative electroencephalographic activity of human brains and real-time fluctuations in the parameters of the Schumann Resonance that can suggest a condition conducive for the interaction of information.
Prediction of an intrinsic resonance of approximately 7 to 8 Hz (band width about 2 Hz) within the cavity between the earth’s surface and the ionosphere, based primarily upon the circumference of the earth-ionosphere shell and the velocity of light, was realized by Schumann, Koenig and their colleagues in the 1950s [1] . Since that time, the specific parameters of the harmonics of this resonance (which occur in increments of approximately 6 Hz added to the fundamental frequency), the amplitudes of the components of their electric (~mV・m−1) and magnetic fields (~2 pT), and various phase modulations have been reported by several authors [2] [3] . The recent very thorough analyses and measurements reported by Nickolaenko and Hayakawa [4] have consolidated details for both the construction of the measurement devices and novel relationships between Schumann frequencies and environmental phenomena. Their comprehensive volume extends the precocious perspective of Cherry [5] concerning the pervasive role of the Schumann Resonances in human activity. One will expect that most human activity is determined by brain function and its capacity for intrinsic signaling and information processing.
There is a remarkable congruence between essential dynamic physical properties of the human cerebrum and the electromagnetic signals within the earth-ionosphere cavity. Spectral analyses of the quantitative electroencephalographic (QEEG) profiles from approximately 100 volunteers recorded over a two-year period indicated the clear presence of the fundamental frequency as well as the first three harmonics (14 Hz, 20 Hz and 26 Hz) immersed within the power density of normal brain activity [6] . Even analogous “split” spectra (that was a bimodal peak within the 7.5 to 8 Hz band which was a less well known feature of the Schumann fundamental) and the analogue within the human QEEG data were observed. However, we could only infer that this was a correlation rather than an indicator of causality. Given the presence of the Schumann Resonance during abiogenesis [7] and the likely contribution of the conditions (lightning discharges) that produced these ringing oscillations to the formation of amino acids, the essential units of proteins [8] , these “coincidences” could reflect an unrelated evolutionary artifact rather than a contemporary “moment-to-moment” causality. More direct temporal-quantitative coherence was required.
The typical intensity of the magnetic field component of the fundamental Schumann Resonance is about 2 pT while the electric field is approximately 0.3 to 1.0 mV・m−1. The directly measured potential differences from the living human brain are in the order of 2 to 20 μV (per Hz) 10−1 m (assuming an average diameter of ~10 cm to accommodate the volume) or ~0.2 mV・m−1. The intensity of the dynamic magnetic field associated with the QEEG has been measured within the pT to femtoTesla per Hz range [9] . However, we [6] have shown by calculation that diffusivity (m2・s−1) is an important component of signal processing between the human brain and the environment. Assuming the resistivity of the whole brain’s primary constituent (physiological water) is about 2 Ω∙m, then when multiplied by magnetic susceptibility (4π × 10−7 N∙A−2) the resulting diffusivity is 1.7 × 106 m2・s−1. The median potential difference of 2 × 10−6 V (2μV) divided by 106 m2∙s−1 results in about 10−12 T (pT). In other words, the similarity between the intensity of the magnetic field component of the Schumann Resonance and human cerebral cortical activity associated with cognition will be strongly dependent upon the magnetic properties of space and the conductivity (or inverse of resistivity) of the aqueous environment in which neurons and glial cells are immersed.
Multiple subtle and previously not reported classes of congruence occur in several parameters within the Schumann Resonance and QEEG data. First, the Schumann Resonances are the aggregate phenomena generated by global lightning discharges [4] . The QEEG is the aggregate phenomena associated with action potentials. Interestingly, the magnitudes of the electric current densities for the typical lightning stroke and the action potential [10] , when scale is accommodated, are very similar (105 A∙m−2). Second, the duration required for the propagating field from a single lightning discharge to return to the source over the spherical guide is about 20 to 25 ms with a phase shift of 13 ms during the 2 Hz wave band of 7 to 9 Hz [4] . The recurrent 20 to 25 ms [“40 Hz”] propagating wave that integrates large areas of the cerebral cortices between the rostral and caudal cerebrum has been considered as a major correlate of consciousness [11] . This particular pattern occurs predominately during waking and dream sleep [12] , but not during slow wave sleep. The phase modulation, as superbly demonstrated by Llinas and his colleagues [13] years ago, is about 12.5 ms. Third, both cerebral cortical electromagnetic fields and the Schumann fields display strong trans-spatial correlations in their intensity values over the entire boundaries of their respective surfaces.
The conditions for the two similarities, from a signaling perspective, can be consistent with Lorentz’s Lemma [14] which relates any two electromagnetic fields if a) they are the same frequency, b) outside of the source, and c) in a linear isotropic medium. If we assume 1) the two fields are the Schumann Resonance generated between the surface of the earth and ionosphere by lightning, and, the cerebral resonance generated between the corona of the cortices and the multiform layer of the cerebrum by action potentials, and 2) the fields are harmonic in time, then:
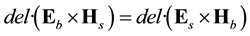 (1)
(1)
where E refers to the electric field vector component, H is the magnetic field (A∙m−1) vector component, and the subscripts refer to b (brain) and s (Schumann) sources. The aggregate is Watts per meter squared.
There are multiple examples of measurements that the magnetic field of the cognitive correlates of brain function and of the Schumann Resonance at the fundamental (7 - 8 Hz) is about 10−12 T and that the electric field component is about 0.1 to 1 mV/m. The Lorentz Lemma adds the dimension of radiant flux density. For the human brain with an average of 1 μV per 10 cm per Hz or 10−5 V∙m−1 and current gradient of 1 × 10−6 V divided 2 Ω・m or 0.5 × 10−6 A∙m−1, the flux power density would be about 5・10−12 W・m−2. This convergence of electromagnetic amplitudes and radiant power density is within the range of the photon emissions measured from the right caudal hemisphere of human brains [15] . This creates the condition that one mode of “information” exchange between the Schumann and cerebral fields will involve discrete and very small quantities of photons through non-local processes. There is strong evidence that ultraweak photon emissions among and within cells can mediate the information that controls the powerful dynamics of cellular activity [16] [17] . Non-local effects (excess correlations) between two sources of photons that share counterclockwise and rotating magnetic fields with changing angular velocities have been shown experimentally [18] .
Given these convergent similarities between human cerebral electromagnetic activity and the wave guide, the conditions are present that may permit signal and information processing by the human brain for some components of Schumann phenomena as predicted by Cherry [5] . To test this possibility, we reasoned that real-time coherence should be demonstrable between QEEG spectral profiles from human brain and direct measures of the Schumann Resonance. In a previous experiment, we [6] [19] had shown brief (less than 1 s) coherence within the first two or three Schumann harmonics recorded locally by our Schumann Resonance detector and those same frequencies within the spectra of QEEG. Here we show, with a larger population sample, the more specific quantitative features of these transient congruence periods and how they can contribute to implicit signaling and information processing.
2. Methods
2.1. Participants
Participants were 41 males and females who were recruited from a first-year psychology course offered at Laurentian University and after REB approval. All volunteers gave consent and were informed that the nature of the experiment was simply to collect baseline eyes-open and eyes-closed measurements.
2.2. Data Acquisition
Once admitted into a sound-proof acoustic chamber, all participants were invited to sit on a comfortable chair. Participants were then outfitted with a cap with 19 sensors (Electro-Cap International). Electro-Gel was applied to form a contact between the scalp and the sensor. Each sensor was arranged to be consistent with the 10 - 20 International Standard of Electrode Placement. The impedance of all sensors was maintained at less than 5 kΩ. The time-varying voltages measured from the cap were directed into a Mitsar 201 quantitative electroencephalograph. Once amplified the brain activity was then recorded using WinEEG software at a sampling rate of 250 Hz with 16-bit analog-to-digital conversion.
In addition to the 19 channels recording various locations of the brain, ultra-low atmospheric electric-field perturbations were also recorded simultaneously. The live stream was obtained from Mr. Renato Romero’s open radio observatory in Cumiana, Italy (www.vlf.it). Essentially, the recorded sound files from the Marconi antenna were streamed directly into the Mistar box using a custom-constructed audio-to-ECG cable. Spectral analysis performed on this channel verified the presence of the Schumann resonance with peaks at traditional frequencies. Ping times were measured using MS-DOS with delays from Italy to Canada not exceeding more than 50 milliseconds. The current experiment consisted only of the collection of simultaneous EEG/ELF when the subjects’ eyes were open and closed (approximately 5-minutes each).
2.3. Data Processing
Sixty (60) seconds of eyes closed 20 channel (19 EEG + 1 ELF) recordings were extracted for periods in which the Schumann resonance was clearly visible and not obscured by artifacts generated from wind, rain or other inclement weather. This was verified by checking the website www.accuweather.com for current weather conditions within the Cumiana, Italy region. These data were then imported into MATLAB software where the EEGLab toolkit [20] was used in order to re-filter the QEEG data between 1.5 - 40 Hz. Indicators of the caudal cerebrum were obtained by computing the caudal root-mean-square (T5, P3, Pz, P4, T6, O1 and O2) as has been employed in previous analyses. These data were then merged with the streamed ULF atmospheric data.
2.4. Identification of Occurrence, Duration and Topographical Properties Associated with Harmonic Synchrony
Cross-channel coherence between cRMS-ELF activities was completed for 41 participants in 30-second time bins. Selection criteria for “harmonic synchrony” included simultaneous coherence across the 7.8 - 8 Hz, 13.7 - 14.3 Hz and 19 - 20 Hz frequency bands. Any occurrences that did not meet the criteria were not extracted. The onset and offset times, as indicated by the cross-channel spectrograms, of each of the occurrences was then recorded and extraction of only these time-periods was performed. Mean occurrence and duration were calculated.
For each occurrence, 4 topographical maps were produced based upon the 19 channels of time-varying voltages associated with the 200 milliseconds before and after onset of harmonic synchrony separately using a k- means clustering algorithm within SPSS. The resultant 4 topographical maps for each baseline and onset conditions were then saved and entered into a secondary clustering procedure (N = 228 per condition) for each condition separately, again using the k-means clustering algorithm. The resultant cluster centers were then saved and interpolated onto 2-D scalp maps based upon the locations of the channels using the topoplot. m function within EEGLab software.
To compare directly the differences between averaged topographies associated with baseline and “harmonic synchrony” occurrences, within-topography z-scores for each of the 8 clusters that characterized baseline (4) and harmonic synchronies (4) were computed. Because of the high degree of similarity between the two cluster sets, similar topographies from both conditions were simply subtracted from each other.
To explore the average topological time-course associated with the onset of a harmonic synchrony event, an integrated 19-channel dataset was produced by computing the average across all events and for all participants. This dataset was then imported into MapWin software where topographical maps were produced within the time interval of −120 msec < t < 160 msec in 8-millisecond intervals.
3. Results
The 41 individuals whose caudal (root mean squared) cerebral activities were monitored and compared simultaneously with the recording of Schumann values in Italy displayed conspicuous phase coherence at 7.8 Hz, 14 Hz and 20 Hz. Of the 41 cases analyzed, 33 (80%) displayed at least one occurrence of a harmonic synchronous event within a 60-second interval for a total of 61 harmonic synchrony events. The means and standard deviations for the occurrence and durations of these harmonic synchronies were 1.72 (SD = 0.97) occurrences per minute and 349 ms (SD = 75), respectively. In other words the transient coherence between the Schumann and brain values in real time occurred once (on average) every 30 s for about one-third of a second. This is equivalent to about 3 to 5 microstates about twice per minute. An example of the 30-second coherence plot from which the averages were obtained is shown in Figure 1.
K-means clustering analysis revealed four topographies consistent between the two baseline and harmonic synchrony conditions. They were characterized by 1) bilateral prefrontal, 2) inferior temporo-posterior, 3) bilateral caudal and 4) rostral-caudal orientations (Figure 2(a)). Evident is the appearance of a polarity shift for cluster 2. One-way analyses analysis of variance for both cluster sets (baseline and event) on the averaged channel voltage across 19-channels indicated that the cluster models explained 63 and 65 percent of the variance
Figure 1. Example of a 30-second (horizontal axis) cross-coherence plot from which harmonic synchronous events were identified and extracted. Note the occurrence of increased coherence (red areas) between the brain and ELF frequencies (vertical axis) measured in Italy within ~8, 14, and 20 Hz. The simultaneous occurrence is most evident at about 6 seconds.


Figure 2. (a) Topographical maps of clusters before (top line) and during (middle line) the onset of harmonic synchronous events. NC refers to the numbers of clusters contributing to the grand mean cluster; (b) Standardized differences between the two conditions.
in classification. To ensure that the clusters were consistent between conditions (baseline versus harmonic synchrony), Spearman rank-order correlations were completed between the arrays of cluster mean centers. The results indicated significant relationships for each cluster between conditions with respective correlation coefficients of 1) Rho = 0.59, p < 0.05, 2) −0.85 (p < 0.05), 3) Rho = 0.54 (p < 0.05) and 4) Rho = 0.77 (p < 0.05), respectively.
To directly compare differences in topographical orientations between baseline conditions (200 milliseconds before the event) and during the occurrence of harmonic synchrony, each map was z-scored separately. The z- scores of topographies were then simply subtracted from each other. The results (Figure 2(b)) indicated that only the second cluster, characterized by the right inferior temporo-parietal focus, displayed z-score differences greater than 3, and are most likely the result of the polarity shift observed between conditions. This profile was similar to the one completed between the specific 8 Hz range in the QEEG profile and the “atmospheric noise” at 8 Hz measured in Italy in real time for about 10 subjects in a previous experiment [19] . Here, positive z-scores (red) indicated that the standardized voltage for the synchronous event was greater than baseline while negative z-scores (blue) indicated the opposite. This topography was associated with a 10 μV difference among conditions.
To discern the latency for the real-time coherence to occur between the Schumann fluctuations and the QEEG activity the results of 61 harmonic synchrony events from 41 different participants were mapped. The results showed that the right temporo-parieto-occipital topography became stable approximately 35 to 45 ms after the onset of the synchronizing event.
The onset of the averaged synchronizing event was associated with the ±2 μV transient emergence of a rostral-caudal dipole with the centroids located approximately over the anterior and posterior cingulate regions. Reconstruction of the isoelectric field lines in real time strongly suggested that during the approximately 10 to 20 ms of the onset the rotational component of the progressive dipole microstates reversed from clockwise (from the top of the brain) to counterclockwise rotations. These results are shown in Figure 3.
4. Discussion
To our knowledge this is the first detailed, quantitative demonstration that there is real time coherence between the specific frequency bands reliably measured within human electroencephalographic activity and comparable fluctuations in electromagnetic characteristics within the earth-ionospheric spherical wave guide. Both sources share similar fundamental frequencies, harmonics, magnetic and electric field strengths, and phase-shifts. Here, we demonstrated that the quantifications of these changes can be strongly although transiently congruent. If the Lorentz Lemma [14] is applicable, then the special condition for interactive information processing between the two sources would be at least intermittently possible. Considering the strong coherence of Schumann parameters over tens of thousands of kilometers and the fact that the brain measurements in Sudbury were powerfully correlated with spherical wave guide values measured in Italy [19] , the possibility exits that similar human brain- Schumann Resonance interactions could occur anywhere on the earth’s surface for individual brains or large aggregates of brains. The involvement of diffusivity coupled to magnetic permeability of the medium complements this suggestion [21] .
The duration of the coherence was about 300 ms or approximately the period of a protracted percept [22] , the just noticeable difference in the “stream of consciousness” that defines the boundaries between successive images and experiences. The most typical descriptions of subjective experiences when perceptual information exists for such limited durations is “a flash” or “intuition” or sudden “insight” that can be considered to be significant. This duration if it occurred once every approximately 30 s would not be sufficient to affect the “stream of consciousness” or disrupt ongoing cognition. However it could affect the direction of the thematic components of the cognition. Individuals [23] who exhibit microstates that are intrinsically much briefer, would more likely be affected by the coherence because of the resultant protraction.
The synchronization of cerebral activity within the Schumann range and the actual Schumann values required about 35 to 45 ms to become stable. This is within the range of approximately two recurrent phases of the approximately 25 ms rostral-caudal recurrent waves that are generated over the cortical manifold. As aptly articulated by Nunez [24] , with a bulk velocity of about 4.5 m・s−1 and a cerebral circumference of 60 cm, the cortical standing wave is about 7.5 Hz. The time required for this wave to move across the rostral-caudal curvature would be about 20 to 25 ms. Thus, the information from the ionospheric-brain interaction might be expected to occur between the transition of any two successive recurrences of these “40 Hz” fields.
Figure 3. Averaged time course of a synchronous event for 61 occurrences of harmonic synchrony. The topographies follow the progression from 120 milliseconds before onset and 160 milliseconds after onset in 8 millisecond intervals. Colour bar denotes microvolt polarity.
The apparent reversal of the direction of the isoelectric lines during the phase coupling between real-time Schumann values and cerebral activity for about 10 to 20 ms, the phase-shift duration noted by Llinas et al. [13] , is both novel and very relevant to the mechanisms by which signaling and information processing could occur. The specific duration of this transience is precisely the value we [18] have found to be most effective to produce non-local (excess correlations) interactions between chemoluminescent reactions separated by as far as 3 km (the furthest tested). Dotta and Persinger [18] showed that the superposition of the two loci (as indicated by doubling of photon emissions) only occurred with changes in counterclockwise angular velocities of magnetic fields with base rates of 20 ms.
That photons could be a major candidate for the atmospheric-brain interaction is supported quantitatively. Flux densities of ~10−12 W・m−2 have been measured from the right caudal hemisphere of human volunteers [15] while they engaged in imaginative tasks while sitting in hyperdark conditions. This magnitude is almost identical to the values measured from slices of hippocampal tissue [25] . This brain structure is the major correlate of information processing and is central to the initial stages of the representation of experience (memory). We have speculated that this narrow range of radiant flux power density is necessary for optimal transmission of information without significant distortion from extraneous sources within the body volume and is primarily the functional reason for the encapsulation of the brain by the skull. Because of this maintained “hyperdarkness” within the skull the potential for non-local interaction between Schumann and cerebral sources could be maintained. During local night and in very dark habitats during dream sleep when intracerebral photon emissions are most likely to increase the coupling might be enhanced.
In a series of original and innovative publications Bokkon [26] and his colleagues [27] [28] have pursued the hypothesis that imagery is the experience of fields of photons within the cerebral volume. Whereas traditional interpretations suggest that imagery is a subjective state generated by configurations of action potentials modulated by syncytiums of glial cells, Bokkon’s concept is that the visual experiences are fields of photons. His calculations [28] for the numbers of photons and their densities within cells are commensurate with potential sources from known biochemical changes within plasma cell membranes. Dotta et al. [29] demonstrated that photon emissions from a variety of cells primarily originate from the changes in small potential differences associated with the cell membrane dynamics.
The potential for “excess correlation” involving photons between earth-ionospheric wave guide and brain sources may be determined by the shared intensity of the magnetic fields for both, i.e., 2 pT. The energy associated with a fluctuating 2 pT magnetic field within the human cerebral volume can be estimated by:
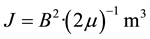 (2)
(2)
where μ is the magnetic permeability, B is the strength of the field and m3 is the volume. Assuming the human cerebral volume of 1.3 × 10−3 m3 and the transient, combined intensity from the Schumann and brain sources to be 4 × 10−12 T, the energy would be 8.3 × 10−21 J. Within the approximately half of a second involved with the interface measured here, the net quantity is very proximal to the Landauer limit. This is the energy, defined by ln2・kT (where k is Boltzmann’s constant and T is temperature in Kelvin), where a bit of information is dissipated into energy or the energy is converted to a bit of information from entropy [30] .
The shift in polarity localized to the right inferior temporo-parietal region before and after onset of a harmonic synchronous event could also be a revealing characteristic of these events with respect to mechanism. It is well known that a static magnet accelerating into or out of a coil induces a voltage with a respective polarity. Moving the magnet out of the coil induces negative voltage across the coil terminals while moving inwards produces a positive voltage. If this principle is applicable to the observed topographical orientations and a reflective process is pursued, the data might suggest that 200 milliseconds prior to brain-atmosphere synchrony the direction of the magnetic flux is from the head to the atmosphere, while 200 milliseconds during the event the direction is from the atmosphere to the head. In information technology, this would be classified as a ‘ping’.
The magnetic field strengths associated with the change in polarity measured directly by QEEG would be consistent with this model. If the ping reached the lower E-layer of the atmosphere and was reflected back towards the human brain, the total distance travelled would be about 200 km. If the ping time was 400 milliseconds to complete one full cycle, the quantum of information would be travelling at a velocity of about 5 × 105 m∙s−1. The absolute potential difference between the two conditions within the right inferior temporoparietal focus was about 10 μV. Dividing 1 × 10−5 V by 5 × 105 m∙s−1 results in 20 pico Tesla・metres. In radio science, bursts of megahertz frequencies are sent into the ionosphere and reflect back to a sensor in order to infer ionospheric ion density. For the E layer, frequencies that reflect back to the earth are between 1 and 5 MHz. If the ping associated with harmonic synchrony were a 3 MHz burst superimposed upon the Schumann waveguide, the wavelength would be about 100 meters and can be obtained by dividing c, the speed of light, by 3 × 106 Hz. Dividing 20 pico Tesla・metres by 100 m reveals a magnetic field magnitude of 0.2 pT and is within the operating intensity of the magnetic field of the brain and the approximate intensity of the Schumann resonance.
The current measurements as well as those reported [15] [31] earlier emphasized the importance of the right Parahippocampal gyrus as one central focus of coherence between the human brain and the earth-ionosphere waveguide. Neurons within the Parahippocampal gyrus show unique properties that could facilitate this coherence. The stratum stellare of Stephan (Layer II) of the entorhinal cortices of the human Parahippocampal gyrus contains star-shaped cells that are organized into small elevations (verrucae gyri hippocampi) on the cortical surface that can be recognized visually [32] . These cells exhibit intrinsic oscillations of a few mV within the 8 Hz range [33] . The energies are almost unity with the quantum for the loss or gain (Lindauer Threshold) of 1 bit of information to or from entropy. The sensitivity of the right hemisphere was demonstrated during whole body exposure to experimentally-generated, weak (~20 nT) 7 to 8 Hz magnetic fields [34] . The second order magnetic field, induced by the currents within extracellular fluid from the changes in electric field associated with these applied fields, is within the pT range [35] .
The hippocampal formation, the area involved with the representation of experiences (memory), receives its primary input from second layer neurons within the entorhinal cortices of the Parahippocampal gyrus. If there is about 1 bit of information per second associated with coherence between the ionospheric waveguide and the brain per 300 ms and about 107 neurons [32] within the human hippocampus, then there would be the potential for exchange of about one million bytes or 1 MByte once every approximately 30 s. During a 24-hr day, the cumulative maximum information processing could be as high as 3 GBytes. This would suggest that the accumulation of information would occur as successive “packets” of information, analogous to processes utilized by information transmission through the NET, that remain within a cerebral buffer until further integration into the complete “transmission” is completed. We suggest the buffer is the (right) parahippocampal region where the information remains until subsequent REM (dream) episodes during the same or following night.
The typical values associated with transmission of packets of information within modern communication networks are instructive. For point-to-point Ethernet connections the information might contain 1.49 k Bytes for a G Byte connection. For one second there would be the potential of about one to two thousand packets each with durations similar to the peak of an action potential. For about 1 M Byte per second system parameters result in 168 Bytes per packet and 0.8 ms transmission latency. For every 13 to 14 ms out of every 50 ms the server transmits at about 1120 packets per second. Interestingly, inter-packet intervals are within the range of 50 ms.
Because the parahippocampal area is considered the interface for cross-(sensory) modal integration of the entire cerebrum and receives and sends information through the cerebrum these discrete intervals could affect the entire brain function over time. This would include the patterns of proteins that are synthesized and hence the intrinsic neural pathways from which “memories” are later reconstructed. Whether or not these changes can contribute to “induced” or pseudo memories, which have been shown to be enhanced by weak, transcerebral magnetic fields [36] [37] must still be evaluated. Stimulation for about 1 s once every 30 s is within the temporal parameters, such as kindling or even long-term potentiation, that produce the shifts in microstructure that are the spatial equivalents of “memories”. Some authors have suggested that the neurons within the parahippocampal region contain the central “transform”, independent of the “line codes” of sensory pathways, which integrates neuronal information into the aggregates that comprise experiences.
The data measured in the present study suggests that the interface between the ionospheric Schumann sources and the brain’s complimentary patterns occurs within the region of the right Parahippocampal gyrus. This region has been implicated in other experiments [38] and by other authors [39] as a potential site through which subtle changes in geomagnetic activity could be mediated. If classical principles of brain organization are applicable, the predominant visual “field” to which experiences would be attributed would be the upper left peripheral visual field.
If activity within the right hemisphere is particularly sensitive to the Schumann frequencies, then the influence should be greater when the human brain’s hemispheric bias shifts towards this direction. This occurs primarily during REM or dream sleep. This state is associated with significant increases in protein synthesis and has been attributed to the “consolidation” of experiences, as memories, acquired during the previous 24 to 48 hr. The durations of this state, whose electroencephalographic pattern and 20 ms recurrent shifts are remarkably similar to the waking state [12] [13] , range from about 5 min at the beginning of the sleeper’s night to about 15 to 20 min during the last dream episode. The latter is more likely to occur just before the person awakens for the day. The contents of those dreams are more likely to be remembered and can affect the person’s disposition for the remainder of the day.
The equivalent power density for the Schumann frequency would be 0.3 × 10−3 V per m multiplied by 10−6 A per m (1 μA per m = 0.4πpT) or about 3 × 10−10 W・m−2. This radiant power density would be equivalent to about 10−20 J per second per cross-sectional area of a neuronal soma. This is an important value [40] because it constitutes the energy associated with an action potential, the binding energies between many ligands and receptors, and the quantity that emerges from the normal electrical forces between separations of potassium ions that are attributed to the resting membrane potential. It is also likely to be a fundamental value intrinsic to space and to physical processes that contribute to non-local effects and excess correlation or “entanglement” [41] .
For the equity in the Lorentz Lemma equation to occur according to the values indicated in the last paragraph, there should be (at least transient) increases in either the H or E component of the cerebrum by a factor of about 100. On the other hand equity would occur between the cerebral power levels at a distance of between 400 and 850 km above the earth’s surface where the voltages of the first peak of the Schumann Resonance are about 0.25 μV∙m−1 √Hz−1. For a 7 Hz oscillation the power density would be congruent with that we have measured from the right hemisphere. Estimated values about 100 km above the earth’s surface would be in the order of 2 μV which is typical of the values obtained within the 40 Hz (about the 6th Schumann harmonic) range for human brain activity [4] .
If the coupling occurs, through non-local mechanisms or by the special conditions of Maxwell’s equations as applied through the Lorentz Lemma, then one would predict even the slightest perturbation at that altitude should be quantitatively evident in cerebral activity spectra. About five decades ago while pursuing lunar tidal forces within the upper atmosphere, Palumbo [42] had suggested that that there must be a phase reversal between the pressure tide at ground level and that required in the dynamo region of the ionosphere. He identified this reversal, as inferred by data from photographic meteors, to occur between altitudes of 85 and 110 km. If this relationship is directly applicable, then changes in atmospheric density variations following lunar transits should display significant increases in brain-Schumann coherence. At ground level the enhancement should occur about 30 min after lunar transit. According to Palumbo’s data the peak should occur about 3 hr after transit.
There should be solar-geomagnetic interactions that might be discerned within QEEG data as well. Ondraskova et al. [43] reported a decrease in Schumann Resonance frequencies during the 2008-2009 solar cycle minimum. There are also infrequent “peculiar” events such as the overlapping transients in the vertical electric field over western Slovakia during May and June 2006 [44] . They were associated with juxtaposed transients whose onsets were separated by 130 to 150 ms. Decreases of the fundamental by about 0.15 Hz during peaks in proton penetrations concurrent with a diminished amplitude of about 0.2 pT and a decrease in resonance bandwidth (about 0.2 Hz) have also been reported [45] . Heating the ionosphere with high frequency electromagnetic waves from specialized equipment at the High Frequency Active Auroral Research Program (HAARP) in Alaska [46] initiated larger amplitude (by a factor of 2 or 3) enhancements for every 0.2 Hz between 7.4 Hz to 8.0 Hz. This was accomplished maximally when the HAARP-transmitted waves were 3.04 and 4.57 MHz.
5. Conclusion
The consistency and congruence of the fundamental, harmonics, magnetic field intensities, electric field potentials, and phase shifts between the earth-ionosphere spherical waveguide (the Schumann Resonance) and quantitative human cerebral cortical activity indicate the potential for information interaction. Direct real-time measurement verified a reliable and intermittent coherence with durations in the order of a “perception” or a brain microstate once every 30 second, the decay time for short-term memory, between Schumann and cerebral resonances. The latency required to establish the peak coherence and the transient reversal of the cerebral rotation of isoelectric lines within the 20 ms range reflect the processes associated with consciousness. The specific quantities of energy associated with both sources are sufficient to allow significant interaction of information. If the estimated radiant flux densities for ultraweak photon emissions for both sources converge, then excess correlations or non-locality could occur intermittently.
Acknowledgements
We thank Dr. Blake T. Dotta for his contribution. This manuscript is dedicated to the memory of Dr. H. L. Koenig. The technical advice of Viger M. Persinger is appreciated.
References
- Koenig, H.L., Krueger, A.P., Lang, S. and Sonning, W. (1981) Biological Effects of Environmental Electromagnetism. Springer-Verlag, New York. http://dx.doi.org/10.1007/978-1-4612-5859-9
- Polk, C. (1982) Schumann Resonance. In: Volland, H., Ed., CRC Handbook of Atmospherics, Vol. I, CRC Press, Boca Raton (Fla), 112-174.
- Campbell, W.H. (1997) Introduction to Geomagnetic Fields. Cambridge University Press, Cambridge.
- Nickolaenko, A. and Hayakawa, M. (2014) Schumann Resonance for Tyros. Springer, Tokyo. http://dx.doi.org/10.1007/978-4-431-54358-9
- Cherry, N. (2002) Schumann Resonances, a Plausible Biophysical Mechanism for the Human Health Effects of Solar. Natural Hazards, 26, 279-331. http://dx.doi.org/10.1023/A:1015637127504
- Saroka, K.S. and Persinger, M.A. (2014) Quantitative Evidence for Direct Effects between Earth-Ionospheric Schumann Resonances and Human Cerebral Cortical Activity. International Letters of Chemistry, Physics and Astronomy, 20, 166-194.
- Graf, F.E. and Cole, E.R. (1974) Precambrian ELF and Abiogenesis. In: Persinger, M.A., Ed., ELF and VLF Electromagnetic Field Effects, Praeger, New York, 243-275.
- Johnson, A.P., Cleaves, H.J., Dworkin, J.P., Glavin, D.P., Lazcano, A. and Bada, J.L. (2008) The Miller Volcanic Spark Discharge Experiment. Science, 232, 404. http://dx.doi.org/10.1126/science.1161527
- Pantev, C., Makeig, S., Hoke, M., Galambos, R., Hampson, S. and Gallen, C. (1991) Human Auditory Evoked Gamma-Band Magnetic Fields. Proceedings of the National Academy of Sciences of the United States of America, 88, 8996- 9000. http://dx.doi.org/10.1073/pnas.88.20.8996
- Persinger, M.A. (2012) Brain Electromagnetic Activity and Lightning: Potentially Congruent Scale-Invariant Quantitative Properties. Frontiers in Integrative Neuroscience, 6, 1-7. http://dx.doi.org/10.3389/fnit.2012.00019
- Hameroff, S. and Penrose, R. (2104) Consciousness in the Universe: A Review of the “Orch OR” Theory. Physics of Life Reviews, 11, 39-78. http://dx.doi.org/10.1016/j.plrev.2013.08.002
- Llinas, R.R. and Paré, D. (1991) Of Dreaming and Wakefulness. Neuroscience, 44, 521-535. http://dx.doi.org/10.1016/0306-4522(91)90075-Y
- Llinas, R.R. and Ribardy, U. (1993) Coherent 40-Hz Oscillations Characterizes Dream State in Humans. Proceedings of the National Academy of Sciences of the United States of America, 90, 2078-2081. http://dx.doi.org/10.1073/pnas.90.5.2078
- Corson, D.R. and Lorrain, P. (1962) Introduction to Electromagnetic Fields and Waves. W. H. Freeman and Company, San Francisco, 311.
- Dotta, B.T., Saroka, K.S. and Persinger, M.A. (2012) Increased Photon Emission from the Head While Imagining Light in the Dark Is Correlated with Changes in Electroencephalographic Power: Support for Bokkon’s Biophoton Hy- pothesis. Neuroscience Letters, 513, 151-154. http://dx.doi.org/10.1016/j.neulet.2012.02.021
- Trushin, M.V. (2004) Light-Mediated “Conversation” among Microorganisms. Microbiological Research, 159, 1-10. http://dx.doi.org/10.1016/j.micres.2003.11.001
- Fels, D. (2009) Cellular Communication through Light. PLoS ONE, 4, e5086. http://dx.doi.org/10.1371/journal.pone.0005086
- Dotta, B.T. and Persinger, M.A. (2012) “Doubling” of Local Photon Emissions When Two Simultaneous, Spatially-Se- parated, Chemiluminescent Reactions Share the Same Magnetic Field Configurations. Journal of Biophysical Chemistry, 3, 72-80. http://dx.doi.org/10.4236/jbpc.2012.31009
- Persinger, M.A. (2014) Schumann Resonance Frequencies Found within Quantitative Electroencephalographic Activity: Implications for Earth-Brain Interactions. International Letters of Chemistry, Physics and Astronomy, 11, 24-32.
- Pasqual-Marquis, R. (2002) Standardized Low Resolution Brain Electromagnetic Tomography (sLORETA): Technical Details, Methods and Findings. Experimental Pharmacology, 34, 5-12.
- Persinger, M.A. (2013) Billions of Human Brains Immersed within a Shared Geomagnetic Field: Quantitative Solutions and Implications for Future Adaptations. The Open Biology Journal, 6, 8-13. http://dx.doi.org/10.2174/1874196701306010008
- Koenig, T., Prichep, L., Lehmann, D., Sosa, D.V., Braker, E., Kleinlogel, H., Ishehart, R. and John, E.R. (2002) Millisecond by Millisecond, Year by Year: Normative EEG Microstates and Developmental Stages. NeuroImage, 16, 41-48. http://dx.doi.org/10.1006/nimg.2002.1070
- Hunter, M.D., Mulligan, B.P., Dotta, B.T., Saroka, K.S., Lavallee, C.F., Koren, S.A. and Persinger, M.A. (2010) Cerebral Dynamics and Discrete Energy Changes in the Personal Physical Environment during Intuitive-Like States and Perceptions. Journal of Consciousness Exploration and Research, 1, 1179-1197.
- Nunez, P.L. (1995) Towards a Physics of the Neocortex. In: Nunez, P.L., Ed., Neocortical Dynamics and the Human EEG Rhythms, Oxford, New York, 68-131.
- Isojima, Y., Isoshima, T., Nagai, K., Kickuchi, H. and Nakagawa, H. (1995) Ultraweak Biochemiluminesence Detected from Rat Hippocampal Slices. Neuroreport, 6, 658-660. http://dx.doi.org/10.1097/00001756-199503000-00018
- Bokkon, I. (2005) Dreams and Neuroholography: An Interdisciplinary Interpretation of Development of Homeotherm State in Evolutions. Sleep and Hypnosis, 7, 61-76.
- Wang, C., Bókkon, I., Dai, J.P. and Antal, I. (2011) First Experimental Demonstration of Spontaneous and Visible Light-Induced Photon Emission from Rat Eyes with Particular Emphasis on Their Roles in Discrete Dark Noise and Retinal Phosphenes. Brain Research, 1369, 1-9. http://dx.doi.org/10.1016/j.brainres.2010.10.077
- Bókkon, I., Salari, V., Tuszynski, J.A. and Antal, I. (2010) Estimation of the Numbers of Biophotons Involved with Vi- sual Perception of a Single Object-Image. Biophoton Intensity Can Be Considerably Higher Inside Cells than Outside. Journal of Photochemistry and Photobiology: B, 100, 160-166. http://dx.doi.org/10.1016/j.jphotobiol.2010.06.001
- Dotta, B.T., Buckner, C.A., Cameron, D., Lafrenie, R.M. and Persinger, M.A. (2011) Biophoton Emissions from Cell Cultures: Biochemical Evidence for the Plasma Membrane as the Primary Source. General Physiology and Biophysics, 30, 301-309.
- Scott, M.A. and Persinger, M.A. (2013) Quantitative Convergence for Cerebral Processing of Information within the Geomagnetic Environment. Journal of Signal and Information Processing, 4, 282-287. http://dx.doi.org/10.4236/jsip.2013.43036
- Persinger, M.A. and Saroka, K.S. (2014) Quantitative Support for Convergence of Intrinsic Energies from Applied Magnetic Fields and “Noise” Fluctuations of Newton’s Gravitational Value within the Human Brain. International Letters of Chemistry, Physics and Astronomy, 19, 181-190.
- Gloor, P. (1997) The Temporal Lobes and Limbic System. Oxford Press, Oxford.
- Angel, A. and Klink, R. (1993) Differential Responsiveness of Stellate and Pyramidal-Like Cells of the Medial Entorhinal Cortex Layer II. Journal of Neurophysiology, 70, 128-143.
- Mulligan, B.M. and Persinger, M.A. (2012) Experimental Simulation of the Effects of Sudden Increases in Geomagne- tic Activity upon Quantitative Measures of Brain Activity: Validation of Correlational Studies. Neuroscience Letters, 513, 151-154.
- Persinger, M.A., Saroka, K.S., Koren, S.A. and St-Pierre, L.S. (2010) The Electromagnetic Induction of Mystical and Altered States within the Laboratory. Journal of Consciousness Exploration and Research, 1, 808-830.
- Ross, M.L., Koren, S.A. and Persinger, M.A. (2006) Physiologically Patterned Weak Magnetic Fields Applied over the Left Frontal Lobe Increases Acceptance of False Statements as True. Electromagnetic Biology and Medicine, 27, 365- 371. http://dx.doi.org/10.1080/15368370802493545
- Healey, F. and Persinger, M.A. (2001) Experimental Production of Illusory (False) Memories in Reconstructions of Narratives: Effect Size and Potential Mediation by the Right Hemispheric Stimulation from Complex, Weak Magnetic Fields. International Journal of Neuroscience, 106, 195-207. http://dx.doi.org/10.3109/00207450109149749
- Booth, J.C., Koren, S.A. and Persinger, M.A. (2005) Increased Feelings of the Sensed Presence and Increased Geomagnetic Activity at the Time of the Experiences during Exposures to Transcerebral Weak Complex Magnetic Fields. International Journal of Neuroscience, 115, 1039-1065. http://dx.doi.org/10.1080/00207450590901521
- Belisheva, N.K., Popov, A.N., Petukhova, N.V., Pavlova, L.P., Osipov, K.S., Tkachenko, S.E. and Baranova, T.I. (1995) Quantitative and Qualitative Evaluations of the Effect of Geomagnetic Field Variations on the Functional State of the Human Brain. Biophysics, 40, 1007-1014.
- Persinger, M.A. (2010) 10-20 Joules as a Neuromolecular Quantum in Medicinal Chemistry: An Alternative Approach to Myriad Molecular Pathways? Current Medicinal Chemistry, 17, 3094-3098. http://dx.doi.org/10.2174/092986710791959701
- Persinger, M.A., Koren, S.A. and Lafreniere, G.F. (2008) A Neuroquantological Approach to How Human Thought Might Affect the Universe. NeuroQuantology, 6, 262-271. http://dx.doi.org/10.14704/nq.2008.6.3.182
- Palumbo, A. (1975) Lunar Tides in the Upper Atmosphere. Journal of Atmospheric and Terrestrial Physics, 38, 103- 106. http://dx.doi.org/10.1016/0021-9169(76)90201-4
- Ondraskova, A., Sevcik, S. and Kostecky, P. (2010) Decrease of Schumann Resonance Frequencies and Changes in the Effective Lightning Areas toward the Solar Cycle Minimum of 2008-2009. Journal of Atmospheric and Solar-Terres- trial Physics, 73, 534-543. http://dx.doi.org/10.1016/j.jastp.2010.11.013
- Ondraskova, A., Bor, J., Sevick, S., Kostecky, P. and Rosenberg, L. (2008) Peculiar Transient Events in Schumann Re- sonance Band and Their Possible Explanation. Journal of Atmospheric and Solar-Terrestrial Physics, 70, 937-946. http://dx.doi.org/10.1016/j.jastp.2007.04.013
- Roldugin, V.C., Malstev, P., Petrova, G.A. and Vasiljev, A.N. (2001) Decrease of the First Schuman Resonance Frequency during Solar Proton Events. Journal of Geophysical Research: Space Physics, 106, 18555-18562. http://dx.doi.org/10.1029/2000JA900118
- Streltsov, A.V., Guido, T., Tulegenov, B., Labenski, J. and Chang, C.-L. (2014) Artificial Excitation of ELF Waves wi- th Frequency of Schumann Resonance. Journal of Atmospheric and Solar-Terrestrial Physics, 119, 110-115. http://dx.doi.org/10.1016/j.jastp.2014.07.004



