Food and Nutrition Sciences
Vol. 4 No. 9A1 (2013) , Article ID: 36131 , 8 pages DOI:10.4236/fns.2013.49A1011
Specialty Lipids in Health and Disease
![]()
Department of Pharmacology and Toxicology, College of Pharmacy, Taif University, Taif, Saudi Arabia.
Email: f.hamam@tu.edu.sa, fy598884@dal.ca
Copyright © 2013 Fayez Hamam. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 14th, 2013; revised July 14th, 2013; accepted July 21st, 2013
Keywords: Structured Lipids; n-3 fatty Acids; n-6 Fatty Acids
ABSTRACT
Lipids possess a wide range of biological activities in plants, animals and humans. They also serve as important components of our daily diet and provide both energy and essential fatty acids; they also act as carriers of fat-soluble vitamins and help in their absorption. Lipids are crucial as a heating medium for food processing and affect the texture, mouth feel and flavour of foods. Structured lipids (SL) are triacylglycerols (TAG) modified to alter the fatty acid composition and/or their location in the glycerol backbone via chemical or enzymatic means. SL may offer the most efficient means of delivering target fatty acids for nutritive or therapeutic purposes as well as to alleviate specific disease and metabolic conditions. This document discusses chemistry, composition, classification, function, occurrence in food and biological activities of lipids. It also sheds light on different aspects of structured lipids, including SL applications, synthesis (chemical vs. enzymatic), SL and aquaculture and future considerations for SL.
1. Introduction
Lipids are a chemically heterogeneous group of compounds that are insoluble or sparingly soluble in water, but soluble in non-polar solvents. They serve several important biological functions including: 1) acting as structural components of all membranes; 2) serving as storage form and transport medium of metabolic fuel; 3) serving as a protective cover on the surface of several organisms; and 4) being involved as cell-surface components concerned with cell recognition, species specificity and tissue immunity. Lipids also constitute a major component of the daily diet, and provide both energy and essential fatty acids. Lipids also act as carriers of fatsoluble vitamins A, D, E and K and help in their absorption. Finally, lipids act as a heating medium for food processing and affect the texture, mouth feel and flavour of foods [1].
2. Chemistry and Composition of Lipids
2.1. Fatty Acids
Fatty acids form the basic chemical structure of fats. The triacylglycerols (TAG) constitute 80% - 95% of lipids. One molecule of TAG consists of three fatty acids and one glycerol molecule. The physical properties of TAG differ, depending on the source they are derived from. Those derived from animal fats are solid at room temperature (lard, butter, etc.); while those obtained from plant and marine oils are liquid at room temperature (cod liver oil, olive oil, etc.). Fatty acids fall into two main categories: saturated and unsaturated; the latter being further subdivided into monounsaturated (MUFA) and polyunsaturated (PUFA). PUFA is divided into two main classes, depending on the location of the first double bond from the methyl end group of the fatty acid; these are n-3 (also omega-3), n-6 (also omega-6). While saturated and monounsaturated fatty acids are also made in the human body, polyunsaturated fatty acids (PUFA) cannot be produced in the human body and must be obtained from dietary sources. Therefore PUFA are considered essential fatty acids (EFA).
2.2. Saturated Fatty Acids
Saturated fatty acids contain only single carbon-carbon bonds in the aliphatic chain and hydrogen atoms occupy all other available bonds. The most abundant saturated fatty acids in animal and plant tissues are usually straight chain compounds with 10, 12, 14, 16 and 18 carbon atoms. In general, saturated fats are solid at room temperature. They are found mainly in margarine, shortening, coconut and palm oils as well as foods of animal origin.
For a series of saturated fatty acids the melting point increases as the length of the chain increases. Typically, adding double bonds to a saturated fatty acid will lower its melting point.
2.2.1. Short-Chain Fatty Acids (SCFA)
Short-chain fatty acids (SCFA) range from C2:0 to C4:0 and include acetic (C2:0), propionic (C3:0) and butyric acids (C4:0). They are the end products of carbohydrate fermentation in the human gastrointestinal tract [2]. SCFA are quickly absorbed in the stomach because of their higher solubility in water, smaller molecular size, and shorter chain length [3] and provide fewer calories than medium-chain fatty acids (MCFA) or long-chain fatty acids (LCFA) (acetic acid, 3.5 kCal; propionic acid, 5.0 kCal; butyric acid, 6.0 kCal).
2.2.2. Medium-Chain Fatty Acids (MCFA)
Medium-chain fatty acids (MCFA) comprise 6 - 12 carbon atoms that result from hydrolysis of tropical plant oils such as those of coconut and palm kernel [4, 5]. Pure medium-chain triacylglycerols (MCT) have a caloric value of 8.3 kCal /g and do not supply essential fatty acids [6,7]. MCFA are more hydrophilic than their longchain fatty acid (LCFA) counterparts. MCFA have many distinctive features such as high oxidative stability, low viscosity and low melting point [8].
MCT exhibit unique structural and physiological characteristics; they are different from other fats and oils because they can be absorbed via the portal system without hydrolysis and reesterification because they are relatively soluble in water. MCT do not require chylomicron formation to transfer from blood stream to the cells and have a more rapid β-oxidation to form acetyl CoA end products which are further oxidized to yield CO2 in the Kreb’s cycle [9]. Absorption and metabolism of MCT is as quick as glucose and have approximately twice the caloric concentration than proteins and carbohydrates. They have little affinity to accumulate as body fat. Medium-chain triacylglycerols are not dependent on carnitine (an enzyme necessary for transport of fatty acids across the inner mitochondria membrane) to enter mitochondria. The higher solubility and smaller molecular size of MCFA make their absorption, transport and metabolism much easier than long-chain fatty acids. MCT are hydrolyzed more quickly and completely by pancreatic lipase than long-chain triacylglycerols (LCT). They may be directly absorbed by the intestinal mucosa with minimum pancreatic or biliary function [10].
Oils from tropical plant, such as those from coconut and palm kernel, contain very high amounts (approximately 50%) of lauric acid (C12:0). They also contain considerable amounts of caprylic (C8:0), capric (C10:0) and myristic (C14:0) acids.
In many medical foods, a mixture of MCT and LCT is used to provide both rapidly metabolized and slowly metabolized fuel as well as essential fatty acids. Any abnormality in the many enzymes or processes involved in the digestion of LCT can cause symptoms of fat malabsorption. Thus, patients with certain diseases (Crohn’ disease, cystic fibrosis, colitis and enteritis, etc.) have shown improvement when MCT are incorporated into their diet [11]. MCT have clinical applications in the treatment of lipid malabsorption, maldigestion, obesity and deficiency of the carnitine system.
Some reports have proposed that MCT may decrease both serum and tissue cholesterol in animals and humans, even more than traditional polyunsaturated oils [12]. However, Cater et al. [13] have shown that MCT increase plasma cholesterol and TAG levels in mildly hypercholesterolemic men fed MCT, palm oil, or high oleic acid sunflower oil diets.
2.2.3. Long-Chain Fatty Acids
Most lipids consist of long-chain fatty acids (>C12) and are referred to as long-chain triacylglycerols (LCT). Palmitic acid (16:0) is a widely occurring saturated fatty acid and is found in almost all vegetable oils, as well as in fish oils and body fat of land animals. Palmitic acid is found abundantly in palm oil, cottonseed oil, lard and tallow, among others. Stearic acid (C18:0) is another important saturated fatty acids and is also a main component of cocoa butter. Triacylglycerols containing high amounts of long-chain saturated fatty acids, especially stearic acid (C18:0), are poorly absorbed in the human body partly because they have a higher melting point than the body temperature and they also display poor emulsion properties [14]. The poor absorption of longchain saturated fatty acids makes them good candidates for the synthesis of low-calorie structured lipids (SL). SL are fats or oils modified to change the fatty acid composition and/or their location in the glycerol backbone. For example, Nabisco Food Group used this feature of C18:0 to produce a group of low-calorie SL known as Salatrium, which consist of SCFA and long-chain saturated fatty acids, mainly stearic acid [15].
2.3. Unsaturated Fatty Acids
Unsaturated fatty acids contain carbon-carbon double bonds in their aliphatic chain. In general, these fats are soft at room temperature. Monounsaturated fatty acids contain one carbon-carbon double bond. On the other hand, polyunsaturated fatty acids (PUFA) contain two or more carbon-carbon double bonds. The PUFA are liquid at room temperature due to the fact that the double bonds are rigid, thus preventing the fatty acids from packing close together. In general, they have low melting points and are susceptible to oxidation. Because most PUFA are liquid at room temperature, they are generally referred to as oils. The common sources of PUFA include grains, nuts, vegetables and seafood.
2.3.1. The n-9 Fatty Acids
The n-9 fatty acids, or monounsaturated fatty acids, contain one double bond that is located between the ninth and tenth carbon atoms from the methyl end group. They are found in vegetable oils such as olive, almond, hazelnut, canola, peanut and high-oleic sunflower as oleic acid (18:1n-9). Oleic acid is the most widely distributed and the most extensively produced of all fatty acids. Olive oil (60% - 80%), hazelnut oil (60% - 70%) and almond oil (60% - 70%) are rich sources of this fatty acid [16]. The human body can synthesize oleic acid, therefore it is not considered as an essential fatty acid. It plays a moderate role in lowering plasma cholesterol in the body [17], but increasing the uptake of oleic acid in young healthy humans is known to increase plasma high density lipoprotein (HDL) and decrease TAG [18].
2.3.2. Essential Fatty Acids (EFA)
As stated earlier PUFA with two or more double bonds in their backbone structures cannot be made in the body and hence considered EFA. There are two groups of EFA, the n-3 and the n-6 fatty acids. They are defined by the location of double bond in the molecule nearest to the methyl end of the chain. In the n-3 group of fatty acids, the first double bond occurs between the third and fourth carbon atoms and in the n-6 group of fatty acids it is situated between the sixth and seventh carbon atoms. The parent compounds of the n-6 and n-3 groups of fatty acids are linoleic acid (LA, 18:2 n-6) and α-linolenic acid (ALA, 18:3 n-3), respectively. These parent compounds are metabolized in the body via a series of alternating desaturation (in which an extra double bond is inserted by removing two hydrogen atoms) and elongation (in which two carbon atoms are added) steps.
2.3.3. The n-3 Fatty Acids
The n-3 fatty acids, such as α-linolenic acid (ALA), eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) have many health benefits related to cardiovascular disease, inflammation, allergies, cancer, immune response, diabetes, hypertension and renal disorders [19]. Epidemiological studies have linked the low incidence of coronary heart disease in Greenland Eskimos with their high dietary intake of n-3 PUFA [20, 21]. Research studies have shown that DHA is essential for appropriate function of central nervous system and visual acuity of infants [19]. The n-3 fatty acids are essential for normal growth and development throughout the life cycle of humans and therefore should be included in the diet. The n-3 fatty acids have been extensively studied for their influence on cardiovascular disease (CVD). However, the exact mechanism by which these effects are rendered remains unknown, but research results have shown that these FA in marine oils may prevent CVD by decreasing serum TAG and acting as anti-therogenetic and antithrombotic agents [1].
Marine oils are rich sources of n-3 fatty acids, especially EPA and DHA. Cod liver, menhaden and sardine oils contain approximately 30% EPA and DHA [19]. Alpha-linolenic acid (ALA; 18:3n-3), the parent of n-3 fatty acids, can be metabolically converted to DHA via desaturation and elongation reactions. However, the efficiency of conversion of ALA to DHA in human adults is very restricted (approximately 4%) and even more restricted in infants (<1%) [22]. In certain disease conditions, the rate of conversion of ALA to DHA and/or EPA is much lower, therefore long-chain polyunsaturated fatty acids (LC PUFA) such as DHA and EPA are considered conditionally essentials and must be obtained from dietary sources [22]. ALA is a main constituent of flaxseed oil (50% - 60%). When ALA is absorbed into the animal body through the diet, it forms long-chain PUFA with an n-3 terminal structure. EPA and DHA are also important n-3 fatty acids. DHA is a major constituent of the gray matter of the brain and the retina of the eye. Human milk also contains a considerable level of DHA, therefore infants fed on mother’s milk show a higher IQ and intelligence level than infants fed on formula’s that lack any DHA [23]. In addition, EPA is a precursor of a series of eicosanoids and is important in protecting against heart attacks primarily due to its antithrombotic effect [24]. EPA was also shown to raise bleeding time and to decrease serum cholesterol levels [24].
In conclusion, LC PUFA exhibit multifunctional role in promotion of health and prevention of disease in the human body. However, they are highly susceptible to oxidation when stored and are known, upon consumption, to increase the body’s load on natural antioxidants such as α-tocopherol. Therefore, it is very important to stabilize oils rich in LC PUFA during storage by incorporation of appropriate antioxidants and adequate packaging technologies.
2.3.4. The n-6 Fatty Acids
The n-6 fatty acids display a variety of physiological functions in the human body. The main functions of these fatty acids are related to their roles in the membrane structure and in the biosynthesis of short-lived derivatives (eicosanoids) which regulate several aspects of cellular activity. The n-6 fatty acids are responsible for maintaining the integrity of the water impermeability barrier of the skin. They are also involved in the regulation of cholesterol transport in the body.
Linoleic acid (LA; 18:2n-6) is the most common fatty acid of this type. LA is found in all vegetable oils and is essential for normal growth, reproduction and health. LA serves as a precursor of n-6 family of fatty acids that are formed by desaturation and chain elongation, in which the terminal (n-6) structure is retained. Of these, arachidonic acid (AA; 20:4n-6) is principally important as a fundamental constituent of the membrane phospholipids and as a precursor of eicosanoids. On the other hand, γ-linolenic acid (GLA; 18:3n-6), an important intermediate in the biosynthesis of AA from LA, is a component of certain seed oils, such as borage and evening primrose, and has been a subject of intensive studies [25,26].
It is proposed that the uptake of 1% - 2% LA in the diet is adequate to protect against chemical and clinical disorders in infants. The absence of LA in the diet is associated with manifestation of several disorders, including impaired growth and reproduction, excessive water loss via the skin, scaly dermatitis and poor wound healing [27].
3. Biological Effect of Dietary Lipids
The influence of dietary lipids on the nature and constituents of adipose tissue is well recognized [28]. This lends support to the saying that “we are what we eat” for many different species tested. In a series of studies on seals and fish, Iverson and her colleagues [29] demonstrated that dietary lipids could be easily detected in their circulatory lipids and adipose tissues.
The dietary fat composition selectively affects fatty acid and TAG deposition in the adipose tissue. In turn, the composition of the fat in the adipose tissue influences lipid mobilization and release of fatty acids into the circulatory system [28]. Lipid mobilization from adipose tissue is not a random event, but instead is affected by variables, such as chain length, degree of unsaturation, and positional isomerization of fatty acids. The most readily mobilized fatty acids are those with 16 - 20 carbon atoms and four or five double bonds, while other very long unsaturated and monounsaturated fatty acids are less easily mobilized [28]. Furthermore, the reduced fat deposition in animals fed trans fatty acids may be associated with direct influence of trans isomers on fat cell metabolism [30]. Many studies provide evidence that variations in the level and type of fat incorporated in the diet can change adipose cell size (hypertrophy) and/or number (hyperplasia) [28]. It is generally accepted that a high amount of fat in the diet may induce hypertrophy and/or hyperplasia. Launay et al. [31] suggested that the multiplication rate of adipose tissue might be increased when the degree of unsaturation of the dietary lipids is increased. On the other hand, Shillabeer and Lau [32] reported a greater degree of fat cell hyperplasia with saturated rather than unsaturated dietary fat. In contrast to the studies reported above, more consistent influences on adipose cellularity were noticed with dietary n-3 PUFA that selectively restrict fat cell size and/or number in a depot-dependent manner [33].
4. Lipid Classes
Lipids are classified based on their physical characteristics at room temperature (oils are liquid and fats are solid), their polarity (polar and neutral lipids), their essentiality for humans (essential and non-essential FA), and their structure (simple, compound and fat-derived). Simple fats are made up of a glycerol, and one (monoacylglycerol), two (diacylglycerol) or three (triacylglycerol) fatty acids. The second category (compound) is the combination of simple fats with other moieties; phospholipids are one example of compound lipids. Fat-derived compounds combine simple and not contain a fatty acid, they are considered “lipid” because they are water insoluble; sterols provide a good example for this category.
Acylglycerols
The TAG consists of a glycerol backbone esterified to three fatty acids. Partial acylglycerols, such as monoand diacylglycerols, may also be found as minor constituents in edible oils. These compounds are synthesized by enzyme systems in nature. Some 80% - 95% of lipids are generally composed of TAG. The TAG is presented in many different forms, according to the type and location of the three fatty acid components involved. Those with a single type of fatty acid in all three positions are called simple TAG and are named after their fatty acid component. However, in some cases the trivial names are more commonly used. An example of this is trioleylglycerol, which is usually referred to as triolein. The TAG with two or more different fatty acids is named by a more complex system [16].
Partial acylglycerols, such as diacylglycerols (DAG) and monoacylglycerols (MAG) are significant intermediates in the biosynthesis and catabolism of TAG and other classes of lipids. For example, 1,2-DAG is important intermediates in the biosynthesis of TAG and other lipids. On the other hand, 2-MAG is formed as intermediates or end products of the enzymatic hydrolysis of TAG.
5. Structured Lipids
5.1. Structured Lipids Applications
Structured lipids (SL) are TAG modified to change the fatty acid composition and /or their location in the glycerol backbone via chemical or enzymatic means [34]. Recently, structured lipids have attracted much attention due to their potential biological function and nutritional perspectives, including reduction in serum TAG, lowdensity lipoprotein (LDL) cholesterol and total cholesterol [35], improvement of immune function, protection against thrombosis [11], reduction of protein breakdown [36,37], improvement of absorption of other fats [38]), reduction of calories, preservation of reticuloendothelial system function [39], as well as improvement of nitrogen balance [4], and reduction of risk of cancer [40].
Strategies for lipid modification include genetic engineering of oilseed crops, production of oils containing high levels of polyunsaturated fatty acids, and lipaseor chemically-assisted interesterification reactions. Depending on the type of substrate available, chemical or enzymatic reactions can be used for the synthesis of SL, including direct esterification (reaction of fatty acids and glycerol), acidolysis (transfer of acyl group between an acid and ester), and alcoholysis (exchange of alkoxy group between an alcohol and an ester) [9]. However, the ordinary methods cited in the literature for production of SL are based on reactions between two triacylglycerol molecules (interesterification) or between a triacylglycerol and an acid (acidolysis) (Figure 1).
5.2. Synthesis of Structured Lipids
5.2.1. Chemically-Catalyzed Interesterification
Chemically-catalyzed interesterification, using alkali such as sodium methoxide, is cheap and easy to scale up. However, such reactions lack specificity and offer little or no control over the positional distribution of fatty acids in the final product [9]. In addition, the reactions carried out under harsh conditions such as high temperatures (80˚C - 90˚C) and produce side products which are difficult to eliminate.
5.2.2. Enzymatically-Catalyzed Interesterification
An alternative to the chemical synthesis of SL is enzymatic process using a variety of lipases. Lipase-assisted interesterification offers many advantages over chemical one. It produces fats or oils with a defined structure because it incorporates a specific fatty acid at a specific position of the glycerol moiety. It requires mild experimental conditions without potential for side reactions, reduction of energy consumption, reduced heat damage to reactants, and easy purification of products [5,41]. However, bioconversion of lipids with lipase is more expensive than chemical methods. Therefore, immobilization of lipids on suitable supports is desirable as it allows reuse of the enzymes. Screening of new lipases from organisms or production of a thermostable or sn-2 specific lipase that is rare in nature through bioengineering are desirable for industrial application.
Another approach is to produce structured lipids through bioengineering. Calgenes’s Inc. of Davis (California) succeeded in production of high-laurate canola oil containing 40% lauric acid (C12:0). It is now available and marketed under the name Laurical and is used in confectionary coatings, coffee whiteners, whipped top-
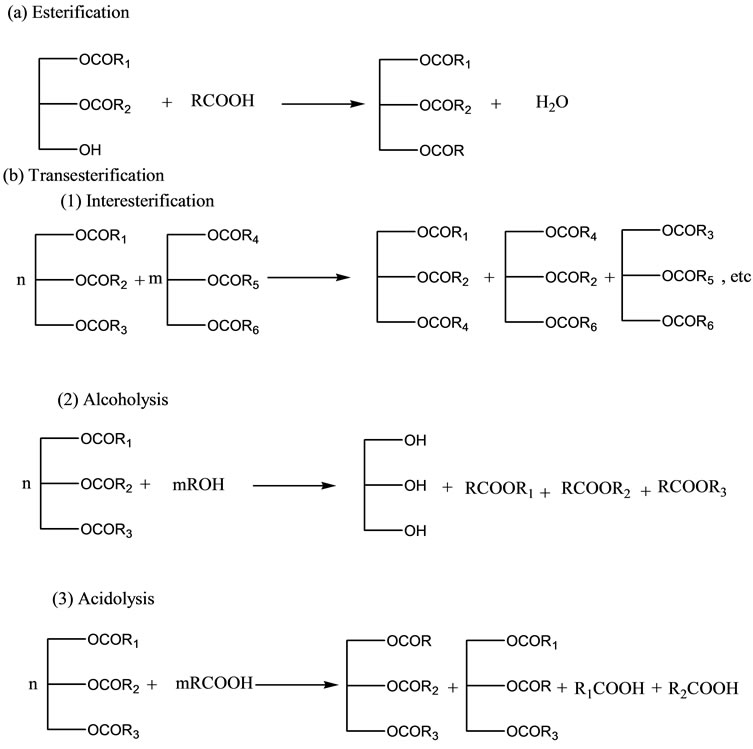
Figure 1. Schematic diagram of lipase-assisted lipid modification strategies for the synthesis of structured lipids.
pings, and filling fats. However, this genetically modified oil is deficient with essentials fatty acids. Recently Hamam and Shahidi [42-45] succeeded in enriching different kinds of high-laurate canola oil with three main kinds of n-3 fatty acids (EPA, DPA, DHA).
5.3. Structured Lipids and Aquaculture
In order to maintain an average 13 kg/person annual consumption of fish, aquaculture must continue to grow at 10 % per year. By 2010, scientists expect aquaculture to be consuming about 75% of world fish oil. The main reason for using fish oil in aqua feeds because it is a good source of n-3 fatty acids (EPA and DHA). The demand for high quality fish oil is increasing, causing the high price to remain and making vegetable oils more competitive in this market. This will result in the development of modified oils containing appropriate quality as well as providing the required amounts of n-3 fatty acids [46].
5.4. Future Considerations
Over the past two decades several research groups have successfully incorporated MCFA (caprylic or capric acids) into fish and marine oils containing PUFA [26,47-52] and into borage oil rich in γ-linolenic acid [25,26,53]. Despite their health benefits, SL containing PUFA are susceptible to rapid oxidative deterioration and thus experience stability problem. Therefore, further research is needed to stabilize these modified oils during storage by incorporation of appropriate antioxidants and adequate packaging technologies. Incorporation of SL containing n-3 PUFA into foods needs to be justified using evidence collected from animal studies and clinical trials. Further research should focus on the metabolism and medicinal importance and economic feasibility of large-scale production of SL containing a mixture of n-3 fatty acids.
Designing SL with specific fatty acids at specific locations of the TAG for use in medicine needs more studies. For example, it may be desirable to develop a SL for patients with cystic fibrosis that contains PUFA (e.g., EPA or DHA) at the sn-2 position, and MCFA at the sn-1, 3 positions.
In conclusions, fats or oils have been recognized for their nutritional, functional and sensory properties. They provide a more concentrated source of energy than do carbohydrates and proteins. There is an increasing concern about the link between a high uptake of certain types of fatty acids or an appropriate balance of the different fatty acids in the diet and certain disease conditions, such as cardiovascular disease, obesity and cancer. Thus, it is clear that there exists a need for specialty lipids that retain the physical, functional and sensory features of traditional lipids and provide specific health benefits.
REFERENCES
- I. S. Newton, “Long-Chain Fatty Acids in Health and Nutrition,” In: F. Shahidi and J. W. Finley, Eds, Omega-3 Fatty Acids: Chemistry, Nutrition, and Health Effects, American Chemical Society, Washington DC, 2001, pp. 14-27. doi:10.1021/bk-2001-0788.ch002
- M. J. Wolin, “Fermentation in the Rumen and Large Intestin,” Science, Vol. 213, No. 4515, 1981, pp. 463- 1467. doi:10.1126/science.7280665
- J. Bezard and M. Bugaut, “Absorption of Glycerides Containing Short, Medium and Long Chain Fatty Acids,” In: A. Kuksis, Ed., Fat Absorption, CRC Press, Boca Raton, 1986, pp. 119-158.
- C. C. Akoh, “Structured Lipids-Enzymatic Approach,” Inform, Vol. 6, 1995, pp. 1055-1061
- C. C. Akoh, “Making New Structured Fats by Chemical Reaction and Enzymatic Modification,” Lipid Technology, Vol. 9, 1997, pp. 61-66.
- W. C. Heird, S. M. Grundy and V. S. Hubbard, “Structured Lipids and Their Use in Clinical Nutrition,” The American Journal of Clinical Nutrition, Vol. 43, No. 2, 1986, pp. 320-324.
- T. W. Lee and C. I. Hastilow, “Quantitative Determination of Triacylglycerol Profile of Structured Lipid by Capillary Supercritical Fluid Chromatography and HighTemperature Gas Chromatogaphy,” Journal of the American Oil Chemists’ Society, Vol. 76, 1999, pp. 1405-1413.
- I.-H. Kim, H. Kim, K.-T. Lee, S.-H. Chung and S.-N. Ko, “Lipase-Catalyzed Acidolysis of Perilla Oil with Caprylic Acid to Produce Structured Lipids,” Journal of the American Oil Chemists’ Society, Vol. 79, No. 4, 2002, pp. 363-367. doi:10.1007/s11746-002-0489-3
- K.-T. Lee and C. C. Akoh, “Structured Lipids: Synthesis and Application,” Food Research International, Vol. 14, No. 1, 1998, pp. 17-34. doi:10.1080/87559129809541148
- S. J. Bell, E. A. Mascioli, B. R. Bistrian, V. K. Babayan and G. L. Blackburn, “Alternative Lipid Sources for Enteral and Parenteral Nutrition: Longand MediumChain Triglycerides, Structured Triglycerides, and Fish Oils,” Journal of the American Dietetic Association, Vol. 91, No. 1, 1991, pp. 74-78.
- J. P. Kennedy, “Structured Lipids: Fats of the Future,” Food Technology, Vol. 38, 1991, pp. 76-83.
- J. W. Stewart, K. D. Wigges, N. C. Jacobson and P. J. Berger, “Effect of Various Triglycerides on Blood and Cholesterol of Calves,” Journal of Nutrition, Vol. 108, 1978, pp. 561-565.
- N. B. Cater, J. H. Howard and M. A. Donke, “Comarison of the Effects of Medium-Chain Triacylglycerols, Palm Oil, and High Oleic Acid Sunflower Oil on Plasma Triacylglycerol Fatty Acids and Lipid and Lipoprotein Concentrations in Humans,” The American Journal of Clinical Nutrition, Vol. 65, No. 1, 1979, pp. 41-46.
- A. Hashim and V. K. Babayan, “Studies in Man of Partially Absorbed Dietary Fats,” The American Journal of Clinical Nutrition, Vol. 31, 1987, p. S273.
- J. W. Finley, L. P. Klemann, G. A. Levelle, M. S. Otterburn and C. G. Walchak, “Caloric Availability of Salatrium in Rats and Humans,” Journal of Agricultural and Food Chemistry, Vol. 42, No. 2, 1994, pp. 495-499. doi:10.1021/jf00038a046
- F. D. Gunstone, “Major Sources of Lipids,” In: F. D. Gunstone and F. B. Padley, Eds., Lipid Technologies and Application, Marcel Dekker, Inc., New York, 1997, pp. 19-50.
- J. J. Gottenbos, “Nutritional Evaluation of n-3 and n-6 Polyunsaturated Fatty Acids,” In J. Beare-Rogers, Ed., Dietary Fat Requirements in Health and Development, American Oil Chemists’ Society, Chapaign, 1988, pp. 107-119.
- R. P. Mensink and M. B. Katan, “Effect of Monosaturated Fatty Acids versus Complex Carbohydrates on High Density Lipoproteins in Health Men and Woman,” Lancet, Vol. 327, No. 8525, 1987, pp. 122-127. doi:10.1016/S0140-6736(87)91965-9
- D. J. Kyle, “The Large-Scale Production and Use of a Single-Cell Oil Highly Enriched Docosahexaenoic Acid,” In: F. Shahidi and J. W. Finley, Eds., Omega-3 Fatty Acids: Chemistry, Nutrition, and Health Effects, American Chemical Society,Washington DC, 2001, pp. 92-105.
- H. O. Bang and J. Dyerberg, “Plasma Lipids and Lipoproteins in Greenlandic West-Coast Eskimos,” ActaMedica Scandinavia, Vol. 192, No. 1-6, 1972, pp. 85-94. doi:10.1111/j.0954-6820.1972.tb04782.x
- H. O. Bang and J. Dyerberg, “Lipid Metabolism and Ischemic Heart Disease in Greenland Eskimos,” Advances in Nutritional Research, Vol. 3, 1986, pp. 1-21.
- B. J. Holub, “Docosahexaenoic Acid in Human Health, Omega-3 Fatty Acids: Chemistry, Nutrition, and Health Effects,” In: F. Shahidi and J. W. Finley, Eds., ACS Symposium Series 788, American Chemical Society, Washington DC, 2001, pp. 54-65.
- F. Shahidi and J. W. Finley, “Omega-3 Fatty Acids: Chemistry, Nutrition, and Health Effects, In: F. Shahidi and J. W. Finley, Eds., ACS Symposim Series 788, American Clinical Society, Washington DC, 2001.
- H. O. Bang, J. Dyerberg and E. Stofferson, “Eicosapentaenoic Acid and Prevention of Thrombosis and Atherosclerosis,” Lancet, Vol. 312, No. 8081, 1978, pp. 117- 122.
- S. P. Senanayake and F. Shahidi, “Enzyme-Assisted Acidolysis of Borage (Borago officinalis L.) and Evening Primrose (Oenothera biennis L.) Oils: Incorporation of Omega-3 Polyunsaturated Fatty Acids,” Journal of Agricultural and Food Chemistry, Vol. 47, No. 8, 1999, pp. 3105-3112. doi:10.1021/jf981248z
- S. P. Senanayake and F. Shahidi, “Structured Lipids via Lipase-Catalyzed Incorporation of Eicosapentaenoic Acid into Borage (Borago officinalis L.) and Evening Primrose (Oenothera biennis L.) Oils,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 3, 2002, pp. 477-483. doi:10.1021/jf010757p
- C. C. Akoh, “Structured Lipids,” In: C. C. Akoh and D. B. Min, Eds., Food Lipids, Marcel Dekker, Inc., New York, 2002, pp. 877-908. doi:10.1201/9780203908815.ch28
- D. B. Hausman, D. R. Higbee and B. M. Grossman, “Dietary Fats and Obesity,” C. C. Akoh and D. B. Min, Eds., Food Lipids, Marcel Dekker, Inc., New York, 2002, pp. 663-694.
- S. M. Budge, S. J. Iverson, W. D. Bowen and R. G. Ackman, “Amongand Within-Species Variability in Fatty Acid Signatures of Marine Fish and Invertebrates on the Scotian Shelf, George Bank, and Southern Gulf of St. Lawrence,” Canadian Journal of Fisheries and Aquatic Sciences, Vol. 59, No. 5, 2002, pp. 886-898. doi:10.1139/f02-062
- K. D. Cromer, T. C. Jenkins and E. J. Thies, “Replacing Cis Ocatdecenoic Acid with Trans Isomers in Media Containing Rat Adipocytes Stimulates Lipolysis and Inhibits Glucose Utilization,” Journal of Nutrition, Vol. 125, 1995, pp. 2394-2399.
- M. Launay, N. Vodovar and J. Raulin, “Development Du Tissue Adipeux: Nombre et Taille Des Cellules En Function De la Valent Energetique et de l’insaturation Des Lipids du Regime,” Bulletin de la Société Chimique Biologies, Vol. 50, 1986, pp. 439-445.
- G. Shillabeer and D. C. W. Lau, “Regulation of New Formation in Rats: The Role of Dietary Fats,” Journal of Lipids Reseach, Vol. 35, 1994, pp. 592-596.
- T. Raelot, R. Groscolas, D. Langin and P. Ferre, “SiteSpecific Regulation of Gene Expression by n-3 Polyunsaturated Fatty Acids in Rat White Adipose Tissue,” Journal of Lipids Reseach, Vol. 38, 1997, pp. 963-1967.
- K.-T. Lee and C. C. Akoh, “Structured Lipids: Synthesis and Application,” Food Reseach International, Vol. 14, No. 1, 1998, pp. 17-34. doi:10.1080/87559129809541148
- I. Ikeda, Y. Tomari, M. M. Sugano, S. Watanabe and J. Nagata, “Lymphatic Absorption of Structured Glycerides Containing Medium-Chain Fatty Acids and Linoleic Acid, and Their Effect on Cholestrol Absorption in Rats,” Lipids in Health and Disease, Vol. 26, 1991, pp. 369-373.
- V. K. Babayan, “Medium Chain Triglycerides and Structured Lipids,” Lipids, Vol. 22, No. 6, 1987, pp. 417- 420. doi:10.1007/BF02537271
- S. J. DeMichele, M. D. Karlstad, V. K. Babayan, N. Istfan, G. L. Blackburn and B. R. Bristrain, “Enhanced Skeletal Muscle and Liver Protein Synthesis with Structured Lipid in Enterally Fed Burned Rats,” Metabolism, Vol. 37, No. 8, 1988, pp. 788-795.
- I. Ikeda, et al., “Lymphatic Absorption of Structured Glycerolipids Containing Medium-Chain Fatty Acids and Linoleic Acid, and Their Effect on Cholesterol Absorption in Rats,” Lipids, Vol. 26, No. 5, 1991, pp. 369- 373. doi:10.1007/BF02537201 R. Sandstrom, et al., “Structured Triglycerides to Postoperative Patients: A Safety and Tolerance Study,” Journal of Parenteral and Enteral Nutrition, Vol. 17, No. 2, 1993, pp. 153-157. doi:10.1177/0148607193017002153
- L. E. Crosby, E. S. Swenson, V. K. Babayan, N. Istfan, G. L. Blackburn and B. R. Bistrain, “Effect of Structured Lipid-Enriched Total Parental Nutrition in Rats Bearing Yoshida Sarcoma,” Journal of Nutrition, Vol. 1, No. 1, 1990, pp. 41-47. C. C. Akoh, “Lipid-Based Fat Substitutes,” Critical Reviews in Food Science and Nutrition, Vol. 35, No. 5, 1995, pp. 405-430. doi:10.1080/10408399509527707
- F. Hamam and F. Shahidi, “Synthesis of Structured Lipids Containing Medium-Chain and Omega-3 Fatty Acids,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 12, 2006, pp. 4390-4396. doi:10.1021/jf052540r
- F. Shahidi and F. Hamam, “Structured Lipids Containing Medium-Chain and Omega-3 Fatty Acids,” Inform, Vol. 17, 2006, pp. 178-181.
- F. Hamam, J. Daun and F. Shahidi, “Lipase-Catalyzed Acidolysis of High-Laurate Canola Oil with Eicosapentaenoic Acid,” Journal of the American Oil Chemists’ Society, Vol. 82, 2005, pp. 875-879.
- F. Hamam and F. Shahidi, “Structured Lipids from HighLaurate Canola Oil and Long-Chain Omega-3 Fatty Acids,” Journal of the American Oil Chemists’ Society, Vol. 82, No. 10, 2005, pp. 731-736.
- A. P. Bimbo, “Fishmeal and Oil: Update Turmoil and Transition,” In: F. Shahidi, Ed., Seafood in Health and Nutrition, Sci. Tech Publishing Company, St. John’s, 2000, pp. 45-67.
- C. C. Akoh and C. O. Moussata, “Characterization and Oxidative Stability of Enzymatically Produced Fish and Canola Oil-Based Structured Lipids,” Journal of the American Oil Chemists’ Society, Vol. 78, No. 1, 2001, pp. 25-30.
- B. H. Jennings and C. C. Akoh, “Enzymatic Modification of Triacylglycerols of High Eicosapentaenoic and Docosahexaenoic Acids Content to Produce Structured Lipids,” Journal of the American Oil Chemists’ Society, Vol. 76, No. 10, 1999, pp. 1133-1137.
- A. Kawashima, Y. Shimada, M. Yamamoto, A. Sugihara, T. Nagao, S. Komemushi and Y. Tominaga, “Enzymatic Synthesis of High-Purity Structured Lipids with Caprylic Acid at 1,3-Postions and Polyunsaturated Fatty Acid at 2-Position,” Journal of the American Oil Chemists’ Society, Vol. 78, No. 6, 2001, pp. 611-616. Y. Shimada, A. Sugihara, K. Maruyama, T. Nagao, S. Nakayama, H. Nakano and Y. Tominaga, “Production of Structured Lipids Containing Docosahexaenoic and Caprylic Acids Using Immobilized Rhizopus delemar,” Journal of Fermentation and Bioengineering, Vol. 81, No. 4, 1996, pp. 299-303. doi:10.1016/0922-338X(96)80580-0
- F. Hamam and F. Shahidi, “Synthesis of Structured Lipids via Acidolysis of Docosahexaenoic Acid Single Cell Oil (DHASCO) with Capric Acid,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 10, 2004, pp. 2900-2906. doi:10.1021/jf035316f
- F. Hamam and F. Shahidi, “Enzymatic Incorporation of Capric Acid into a Single Cell Oil Rich in Docosahexaenoic Acid (DHA) and Docosapentaenoic Acid (DPA),” Food Chemistry, Vol. 91, No. 4, 2005, pp. 583- 591. doi:10.1016/j.foodchem.2004.05.024
- C. C. Akoh and C. O. Moussata, “Lipase-Catalyzed Modification of Borage Oil: Incorporation of Capric and Eicosapentaenoic Acids to Form Structured Lipids,” Journal of the American Oil Chemists’ Society, Vol. 75, No. 6, 1998, pp. 697-701.
List of Abbreviations
AA Arachidonic acid
ALA α-linolenic acid
DAG Diacylglycerol
DHA Docosahexaenoic acid
DPA Docosapentaenoic acid
EPA Eicosapentaenoic acid
FA Fatty acid
FFA Free fatty acids
EFA Essential fatty acids
LA Linoleic acid
LCFA Long-chain fatty acids
LCT Long-chain triacylglycerols
MAG Monoacylglycerols
MCFA Medium-chain fatty acids
SL Structured lipids
TAG Triacylglycerols

