Food and Nutrition Sciences
Vol.3 No.11(2012), Article ID:24493,8 pages DOI:10.4236/fns.2012.311201
Protective Effects of Nobiletin on Hypertension and Cerebral Thrombosis in Stroke-Prone Spontaneously Hypertensive Rats (SHRSP)*

1Laboratory of Physiology, Faculty of Nutrition, Kobe Gakuin University, Kobe, Japan; 2Formerly Department of Haematology, College of Medicine, Cardiff University, Cardiff, UK.
Email: #sasakiya@nutr.kobegakuin.ac.jp
Received September 18th, 2012; revised October 18th, 2012; accepted October 25th, 2012
Keywords: Nitric Oxide (NO); Nobiletin; Reactive Oxygen Species (ROS); Stroke-Prone Spontaneously Hypertensive Rat (SHRSP); Thrombosis
ABSTRACT
Some citrus flavonoids have been reported to possess antioxidant activities that moderate endothelial dysfunction and show protective effects on cardiovascular disease. We have investigated the protective effects of nobiletin (5,6,7,8,3’,4’-hexamethoxy flavone) derived from the peel of Citrus depressa Hayata (Shiikuwasha), a citrus fruit produced in Okinawa prefecture in Japan on hypertension and thrombogenicity in cerebral vessels of stroke-prone spontaneously hypertensive rats (SHRSP). Nobiletin was added to the diet of male SHRSP (7 weeks old) for 4 weeks. The age-related increase in systolic blood pressure usually observed in SHRSP was significantly suppressed in the treated animals. Thrombogenesis in pial blood vessels, determined using a He-Ne laser technique, and antioxidant activity, assessed by measuring urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG), were significantly reduced after treatment. Urinary nitric oxide (NO) metabolites and acetylcholine-induced endothelial relaxation were increased after dietary intervention. These results strongly suggested that antihypertensive and antithrombotic effects of nobiletin may be related to an increase in bioavailable NO, possibly mediated by the scavenging of reactive oxygen species (ROS).
1. Introduction
Citrus fruits are a rich source of flavanones and many polymethoxylated flavones, which are very rare in other plants [1]. Citrus flavonoids are generally categorized into two groups, flavanone glycosides (hesperidin, naringin and neohesperidin) and polymethoxylated flavones (nobiletin, tangeretin and sinensetin) [2]. They have a broad spectrum of biological activity, including antioxidant activities [2-5], anti-inflammatory [3,6,7], neuroprotective properties [8-10] and anticarcinogenic and antitumor activities [6,11-13].
Recent studies of the polymethoxylated flavones, nobiletin and tangeretin, have focused especially on antiinflammatory, anti-tumor and anti-carcinogenic activities [3,4,13-15]. For example, nobiletin has been reported to be a novel, and promising anti-inflammatory agent, inhibiting the activity of nuclear factor-kappa B (NF-kappaB) and suppressing bone resorption and the generation of reactive oxygen species (ROS) [16,17].
We have previously studied the antihypertensive and antithrombotic effects of hesperidin and naringin, and demonstrated that these citrus flavanone glycosides moderated hypertension and thrombogenesis in cerebral vessels of stroke-prone spontaneously hypertensive rats (SHRSP) [18]. Our results indicated that mechanisms underlying the effects of the flavonoids were related to strong antioxidative properties. Many studies have indicated that SHRSP are exposed to high oxidative stress and ROS contribute to the maintenance of hypertension [19-21]. Increased ROS are involved in mechanisms of vascular dysfunction and reduce the amount of bioactive nitric oxide (NO) by chemical inactivation to form toxic peroxynitrite (ONOO−) and deteriorate hypertension and endothelial dysfunction [22-24]. In addition, oxidized low-density lipoproteins (ox-LDL) appear to dysregulate the homeostasis between blood and vascular cells, alterate endothelial function, and promote inflammatory, thrombotic and atherogenetic processes [24-26]. Nobiletin has been reported to inhibit ROS production and unregulate uptake of ox-LDL via scavenger receptors in macrophages and monocytes [15,27]. Nobiletin also attenuates very-low-density lipoprotein (VLDL) overproduction, dyslipidemia, and atherosclerosis in mice [28].
The potential cardiovascular effects of polymethoxylated flavonoids were investigated by Robbins et al. [29,30] who demonstrated that nobiletin inhibited ADPinduced platelet aggregation and thrombosis. In addition, Sempiska et al. [31] showed that nobiletin and other flavonoids suppressed platelet adhesion to glasswool.
Shiikuwasha fruit (Citrus depressa Hayata) has been reported to contain higher concentrations of nobiletin and tangeretin than in other citrus fruits [1], and recent studies of extracts of Shiikuwasha have focused on various beneficial effects. Akachi et al. reported that Shiikuwasha juice suppressed liver injury by D-galactosamine [32] and obesity mediated by a high fat diet [33].
In the present communication, we have investigated the beneficial cardiovascular activities of nobiletin purified from the peel of Shiikuwasha fruits. We assessed blood pressures and thrombogenesis in cerebral vessels of SHRSP after ingestion of nobiletin added to the daily diet. We demonstrated that nobiletin moderated the usual age-related increase in blood pressure, and inhibited He-Ne laser induced thrombogenesis in cerebral vessels. Furthermore, dietary intervention significantly reduced antioxidant activity in SHRSP, and increased urinary excretion of NO metabolites and acetylcholine-induced endothelial relaxation. These results suggest that antihypertensive and antithrombogenic effects of nobiletin were possibly mediated by suppression of ROS and increased levels of bioavailable NO.
2. Materials and Methods
2.1. Animals and Diets
Male stroke prone spontaneously hypertensive rats (SHRSP), 6 weeks old, were obtained from Japan SLC (Hamamatsu, Japan). The animals were used after acclimatization in the laboratory for one week. All experiments were conducted in accordance with the ethical principles of animal care of Kobe Gakuin University and the guiding principles for the care and use of animals in the field of physiological sciences by the Physiological Society of Japan [34]. The rats were euthanized with an overdose of sodium pentobarbital following the experiments.
The powder diet was purchased from Funabashi Farm (Funabashi SP diet, Chiba, Japan). Purified nobiletin (purity 96.6%) from the peel of Okinawan Shiikuwasha was provided by ARKRAY Inc. (Karada Support Institute, Kyoto, Japan). Nobiletin was mixed with the normal powdered diet for SHRSP (Funabashi SP diet, Funabashi, Japan) at the indicated concentrations. The nobiletin treated animals were classified into three groups 1) a control group, which was given the standard Funabashi SP diet; 2) nobiletin groups which were given either 20 mg/kg/day nobiletin (c1) or 40 mg/kg/day nobiletin (c2) for 4 weeks from 7 to 11 weeks of age. All animals were allowed water ad libitum. Food and water intake and body weights were measured every day.
Blood pressures were measured once a week using a tail-cuff plethysmograph (LE5001, Pan. Lab, Barcelona, Spain).
2.2. Measurement of Thrombogenesis
Closed cranial windows were created as described by Morii et al. [35] and thrombotic tendency was measured as previously reported [36,37]. In outline, animals were anesthetized with sodium pentobarbital (60 mg/kg) and artificially ventilated with a mixture of oxygen in air. Femoral veins were exposed and blood vessels were cannulated with polyethylene tubing (PE50, Becton Dickinson and Company, Sparks, MD, USA). Evans blue was administered through the femoral vein. A craniotomy was performed using a hand drill to form a cranial window, 5 mm in diameter, in the center of the right pariet al bone. A coverslip was placed on the window and fixed with dental resin. Cerebrospinal fluid was continuously infused within the cranial window and the intracranial pressure was adjusted to 3 - 5 mm Hg to avoid brain herniation. The animals were stablized in a stereotaxic frame and were placed on the stage of an Olympus BH2 microscope equipped with a long working distance objective. The cerebral vessels were monitored using a charge-coupled device (CCD) camera (Pulnix, Takenaka System, Kyoto) and recorded on videotape. A He-Ne laser beam (15 µm in diameter) was focused on the center of the selected blood vessels through the optical path of the microscope and thrombi were formed by repeated irradiation for 10 sec at 20 sec intervals at a power 13 mW. The number of laser pulses needed to induce the formation of an occlusive thrombus was used as an index of thrombotic tendency.
2.3. Determination of Urinary 8-OHdG and NO Metabolites (NO2/NO3)
Assays were performed before and 4 weeks after each diet. The animals were kept in metabolic cages and urine samples were collected for 24 h. Samples were stored at −80˚C until assayed. 8-hydroxy-2’-deoxyguanosine (8- OHdG) was determined using a competitive enzymelinked immunosorbent assay (ELISA; 8-OHdG check, Japan Institute for the Control of Ageing, Shizuoka, Japan). Urinary NO metabolites (NO2/NO3) concentrations were determined using Griess reagent (Assay kit-C; Dojindo Laboratory, Kumamoto, Japan).
2.4. Measurement of Vasodilation with Acetylcholine
Ring preparations of thoracic aorta were excised after the experiments and dissected in ice cold Krebs-Henseleit solution (KHS; composition in mmol/L: 120 NaCl, 4.76 KCl, 25 NaHCO3, 1.18 NaH2PO4.H2O, 1.25 CaCl2, 1.18 MgSO4·H2O, 5.5 glucose) to remove connective tissue. Rings (3 mm wide) were cut and three segments from each animal were mounted on stainless steel hooks and suspended in a water-jacketed 5 ml tissue bath filled with KHS maintained at 37˚C and aerated with 95% O2 and 5% CO2 (Easy Magnus System U-5A (4-channels), Iwashiya Kishimoto Medical Instruments, Kyoto, Japan). Aortic rings were stretched to the equivalent of 1.5 g tension for 90 min before commencement of the experiment. After stretching, the aortic rings were equilibrated and contracted with phenylephrine (10−5 mol/L). Relaxation was measured in the presence or absence of endothelium, by adding acetylcholine (10−5 mol/L). Relaxation was calculated as a percentage of precontractile vascular tone. The tissue responses were recorded using isometric transducers (Easy Magnus System U-5A, Iwashiya Kishimoto Medical Instruments, Kyoto, Japan) and recorders (SEKONIC SS-250F Recorder, Tokyo, Japan).
2.5. Statistical Analyses
Statistical analyses were performed with a commercially available statistical package (Prism 5.0; GraphPad Software, Inc., San Diego, CA, USA). Results are expressed as the number of animals (N) and the mean and standard error (SE) of each experiment. When more than 2 groups were evaluated, a One-way analysis of variance (oneway ANOVA) was followed by post hoc tests of Dunnett. For comparisons of two groups, Student’s t-test was used. A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Body Weight, Systolic Blood Pressure
Nobiletin were given for 4 weeks from 7 weeks old to 11 weeks old, respectively. Body weight increased over time up to 10 weeks of age as shown in Figure 1, and was increased significantly between 7 and 9 weeks old at 40 mg/kg/day in the nobiletin treated rats. Systolic blood pressures increased with age in control animals, and nobiletin suppressed this increase significantly (Figure 2).
3.2. Effects of Nobiletin on Cerebral Thrombosis
Thrombogenesis in cerebral microvessls for each group of animals, as assessed using the He-Ne laser technique, is illustrated in Figure 3. The number of laser pulses

Figure 1. The effect of nobiletin on body weight. SHRSP were purchased from a local supplier at 6 weeks old and acclimatized for a week. Nobiletin was fed from 7 weeks old for 4 weeks. Body weights were measured weekly. Statistical analyses were performed by one-way analysis of variance (ANOVA) and by post hoc test of Dunnett. *: p < 0.05.
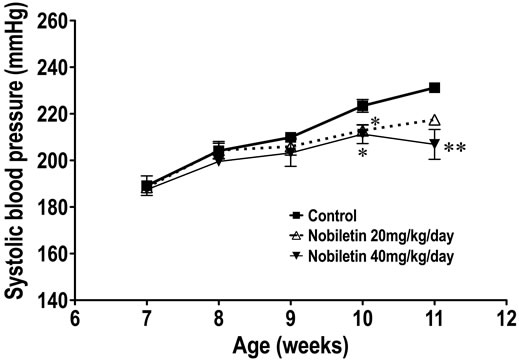
Figure 2. The effect of nobiletin on systolic blood pressure. SHRSP were purchased from a local supplier at 6 weeks old and acclimatized for a week. Nobiletin was fed from 7 weeks old for 4 weeks. Blood pressure was measured weekly. Statistical analyses were performed by one-way analysis of variance (ANOVA) and by post hoc test of Dunnett. *: p < 0.05, **: <0.01 vs Control.

Figure 3. The effect nobiletin on cerebral thombogenesis in SHRSP. Cerebral thrombogenesis was assessed using a He-Ne laser technique after ingestion of nobiletin for 4 weeks as described in Materials and Methods. Statistical analyses were performed by one-way analysis of variance (ANOVA) and by post hoc test of Dunnett. *: p < 0.05, **: < 0.01 vs Control.
required to generate occlusive thrombi in pial venules in control, nobiletin at 20, nobiletin at 40 mg/kg/day were 6.2 ± 0.4 (n = 6), 8.7 ± 0.8 (n = 6), 11.9 ± 0.5 (n = 5), respectively. The differences between the nobiletin groups at 20 mg/kg/day (p < 0.05) and 40 mg/kg/day (p < 0.01) were significantly higher than control.
3.3. Effects of Nobiletin on Oxidative Stress
The amounts of 8-OHdG in urine collected for 24 hr before and after dietary intervention, adjusted for body weight are shown in Figure 4. There were no significant differences in the amounts of urinary 8-OHdG in the control group before and after the experimental period. The amounts of urinary 8-OHdG were significantly decreased after the experimental diet in the nobiletin group (p < 0.05).
3.4. Effects of Nobiletin on NO Metabolites (NO2/NO3) and Vascular Relaxation
Changes in NO metabolites before and after ingestion of nobiletin are summarized in Figure 5. NO metabolites in the nobiletin control group were significantly decreased after 4 weeks treatment period, however. In contrast, ingestion of nobiletin at 40 mg/kg/day (p < 0.05) significantly increased NO metabolites. Ingestion of nobiletin for 4 weeks increased vascular responses. Significant increases in percentage relaxation using 10−5 mol/L acetylcholine were detected between control and nobiletin at 20 mg/kg/day and 40 mg/kg/day were 57.1% ± 3.3% (n = 5), 62.4% ± 5.9% (n = 4; n.s.) and 81.2% ± 5.1% (n = 4; p < 0.01), respectively.
4. Discussion
We have demonstrated significant cardiovascular effects

Figure 4. The antioxidant effect of nobiletin. Oxidative stress was assessed by measurements of 8-OHdG in 24 hr urine samples collected before and after ingestion of nobiletin for 4 weeks. Statistical analysis was performed by unpaired t-test. *: p < 0.05.
of nobiletin. The polymethoxylated flavone moderated the usual age-related increase in blood pressure and thrombogenicity in cerebral vessels in stroke prone spontaneously hypertensive rats (SHRSP) after ingestion for 4 weeks.
Nobiletin has been reported to exhibit anti-inflammatory, anticarcinogenic and antitumor and antioxidant activities [3,4,13,14]. Our findings indicate that the antioxidant activities of these compounds prevented conversion of nitric oxide (NO) into peroxynitrite (ONOO−) and increased the levels bioavailable NO, mediating an increase in vasorelaxation and leading to a decrease in blood pressure. In addition, we demonstrated that urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) levels were significantly decreased after ingestion of nobiletin for 4 weeks. In this context, El Haouari et al. reported that reactive oxygen species (ROS) generated in hypertensive animal models stimulated platelets [38], and others have shown that chronic exposure to oxidative stress in spontaneously hypertensive rat (SHR) and SHRSP might contribute to hypertension [19-21,22,39]. In our studies, nobiletin mixed with normal diet and given for 4 weeks to SHRSP appeared to moderate oxidative stress leading to increases in the urinary excretion of NO metabolites and suppression of enhanced blood pressures and thrombogenesis in cerebral blood vessels. Recent studies have suggested that ROS produced in receptor-mediated platelet activation participate in the regulation of platelet function [40-42]. It is conceivable, therefore, that the antioxidant activities of nobiletin inhibited platelets activities. These effects of nobiletin might have contributed to inhibition of thrombus formation in the current inves tigations. On the other hand, Guerrero et al. suggested
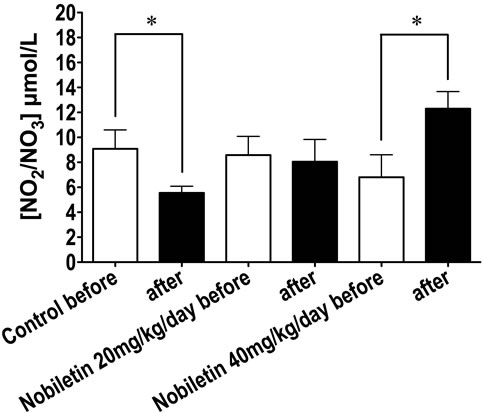
Figure 5. The effect of nobiletin on nitric oxide (NO) metabolites (NO2/NO3). NO2/NO3 was measured, using Griess reagent, in 24 hr urine samples collected before and after ingestion of nobiletin for 4 weeks. Statistical analysis was performed by unpaired t-test. NO metabolites were significantly decreased in control and increased 4 weeks after ingestion of nobiletin. *: p < 0.05.
that the mechanisms underlying inhibition of platelet function mediated by some flavonoids such as apigenin, genistein, luteolin, quercetin were related to inhibition of the thromboxane A2 signalling pathways and antagonizing thromboxane A2 receptors [43]. Further studies are required, therefore, to examine the possible role of interactions between polymethoxylated flavones and platelet thromboxane A2 receptors in the inhibition of platelet function. Our data on the antithrombotic effects of nobiletin were in keeping with the older studies of Robbins and Sempiska et al. Robbins reported that nobiletin inhibited ADP-induced pulmonary thrombosis in rats in vivo and ADP-induced platelet aggregation in vitro [29,30]. Sempiska et al., reported that nobiletin and other flavonoids suppressed adhesion of platelets to glasswool [31]. In addition, Hirata et al. suggested that nobiletin could act as an anticoagulant by inhibiting tissue factor [44]. Our findings that nobiletin inhibited thrombogenesis in cerebral blood vessels in SHRSP are consistent with these reports.
Malterud et al. reported that polymethoxylated flavones including nobiletin and tangeretin inhibited the activity of 15-lipoxygenase, which plays key role in atherogenesis [45-49]. In this context, polymethoxylated flavones are believed to possess strong anti-inflammatory activities, and many studies have indicated a relationship between inflammation and the unregulated uptake of oxidized low-density lipoproteins (ox-LDL) via macrophage scavenger receptors (SRs) [27,50]. Moreover, Eguchi et al. reported that nobiletin markedly reduced 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced lectinlike, oxidized low-density lipoprotein receptor-1 (LOX-1) mRNA expression in THP-1 human monocyte-like cells in doseand time-dependent manners, further suggesting that nobiletin may be a promising phytochemical for regulating atherosclerosis [15]. It may be that inhibition of 15-lipoxygenase and LOX-1 by nobiletin moderated age related hypertension and thrombotic tendency in SHRSP in the present study.
In conclusion, our results suggested that antioxidant activities of nobiletin contributed to antihypertensive and antithrombotic properties. Precise mechanisms underlying these effects remain to be clarified, however.
Nevertheless, the findings offer the challenging possibility that pharmacological administration of nobiletin could have beneficial effects for the prevention of cardiovascular diseases.
5. Acknowledgements
This work was supported in part by the “Academic Frontier” Project for Private Universities: matching fund subsidy from the Ministry of Education, Science, Sports, and Culture of Japan . We are also appreciated for ARKRAY Inc. (Karada Support Institute, Kyoto, Japan) for providing purified nobiletin.
REFERENCES
- Y. Nogata, K. Sakamoto, H. Shiratsuchi, T. Ishii, M. Yano and H. Ohta, “Flavonoid Composition of Fruit Tissues of Citrus Species,” Bioscience, Biotechnology, Biochemistry, Vol. 70, No. 1, 2006, pp. 178-192. doi:10.1271/bbb.70.178
- E. J. Middleton, C. Kandaswami and T. C. Theoharides, “The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer,” Pharmacological Reviews, Vol. 52, No. 4, 2000, pp. 673-751.
- A. Murakami, Y. Nakamura, Y. Ohto, M. Yano, T. Koshiba, K. Koshimizu, H. Tokuda, H. Nishino and H. Ohigashi, “Suppressive Effects of Citrus Fruits on Free Radical Generation and Nobiletin, an Anti-inflammatory Polymethoxyflavonoid,” Biofactors, Vol. 12, No. 1-4, 2000, pp. 187-192. doi:10.1002/biof.5520120130
- H. Nishino, H. Tokuda, Y. Satomi, M. Masuda, Y. Osaka, S. Yogosawa, S. Wada, X. Y. Mou, J. Takayasu, M. Murakoshi, K. Jinnno and M. Yano, “Cancer Prevention by Antioxidants,” Biofactors, Vol. 22, No. 1-4, 2004, pp. 57-61. doi:10.1002/biof.5520220110
- Y. H. Lu, M. Y. Su, H. Y. Huang, Lin-Li and C. G. Yuan, “Protective Effects of the Citrus Flavanones to PC12 Cells against Cytotoxicity Induced by Hydrogen Peroxide,” Neuroscience Letters, Vol. 484, No. 1, 2010, pp. 6-11. doi:10.1016/j.neulet.2010.07.078
- O. Benavente-Garcia and J. Castillo, “Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-Inflammatory Activity,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 15, 2008, pp. 6185-6205. doi:10.1021/jf8006568
- R. Gonzalez, I. Ballester, R. Lopez-Posadas, M. D. Suarez, A. Zarzuelo, O. Martinez-Augustin and F. Sanchez de Medina, “Effects of Flavonoids and Other Polyphenols on Inflammation,” Critical Reviews in Food Science and Nutrition, Vol. 51, No. 4, 2011, pp. 331-362. doi:10.1080/10408390903584094
- J. Nones, T. C. E Spohr and F. C. Gomes, “Hesperidin, a Flavone Glycoside, as Mediator of Neuronal Survival,” Neurochemical Research, Vol. 36, No. 10, 2011, pp. 1776-1784. doi:10.1007/s11064-011-0493-3
- K. Matsuzaki, K. Miyazaki, S. Sakai, H. Yawo, N. Nakata, S. Moriguchi, K. Fukunaga, A. Yokosuka, Y. Sashida, Y. Mimaki, T. Yamakuni and Y. Ohizumi, “Nobiletin, a Citrus Flavonoid with Neurotrophic Action, Augments Protein Kinase A-Mediated Phosphorylation of the AMPA Receptor Subunit, GluR1, and the Postsynaptic Receptor Response to Glutamate in Murine Hippocampus,” European Journal of Pharmacology, Vol. 578, No. 2-3, 2008, pp. 194-200. doi:10.1016/j.ejphar.2007.09.028
- Y. Yamamoto, N. Shioda, F. Han, S. Moriguchi, A. Nakajima, A. Yokosuka, Y. Mimaki, Y. Sashida, T. Yamakuni, Y. Ohizumi and K. Fukunaga, “Nobiletin Improves Brain Ischemia-Induced Learning and Memory Deficits through Stimulation of CaMKII and CREB Phosphorylation,” Brain Research, Vol. 1295, 2009, pp. 218-229. doi:10.1016/j.brainres.2009.07.081
- E. G. Miller, J. J. Peacock, T. C. Bourland, S. E. Taylor, J. M. Wright, B. S. Patil and E. G. Miller, “Inhibition of Oral Carcinogenesis by Citrus Flavonoids,” Nutrition and Cancer, Vol. 60, No. 1, 2008, pp. 69-74. doi:10.1080/01635580701616163
- S. Aranganathan and N. Nalini, “Efficacy of the Potential Chemopreventive Agent, Hesperetin (Citrus Flavanone), on 1,2-Dimethylhydrazine Induced Colon Carcinogenesis,” Food Chemical and Toxicology, Vol. 47, No. 10, 2009, pp. 2594-2600. doi:10.1016/j.fct.2009.07.019
- Y. Iwase, Y. Takemura, M. Ju-Ichi, M. Yano, C. Ito, H. Furukawa, T. Mukainaka, M. Kuchide, H. Tokuda and H. Nishino, “Cancer Chemopreventive Activity of 3,5,6,7,8,3’,4’-Heptamethoxyflavone from the Peel of Citrus Plants,” Cancer Letters, Vol. 163, No. 1, 2001, pp. 7-9. doi:10.1016/S0304-3835(00)00691-1
- A. Murakami and H. Ohigashi, “Cancer-Preventive AntiOxidants that Attenuate Free Radical Generation by Inflammatory Cells,” Biological Chemistry, Vol. 387, No. 4, 2006, pp. 387-392. doi:10.1515/BC.2006.052
- A. Eguchi, A. Murakami and H. Ohigashi, “Nobiletin, a Citrus Flavonoid, Suppresses Phorbol Ester-Induced Expression of Multiple Scavenger Receptor Genes in THP-1 Human Monocytic Cells,” FEBS Letters, Vol. 580, No. 13, 2006, pp. 3321-3328. doi:10.1016/j.febslet.2006.04.077
- S. Y. Choi, J. H. Hwang, H. C. Ko, J. G. Park and S. J. Kim, “Nobiletin from Citrus Fruit Peel Inhibits the DNABinding Activity of NF-Kappab and ROS Production in LPS-Activated RAW 264.7 Cells,” Journal of Ethnopharmacology, Vol. 113, No. 1, 2007, pp. 149-155. doi:10.1016/j.jep.2007.05.021
- S. Harada, T. Tominari, C. Matsumoto, M. Hirata, M. Takita, M. Inada and C. Miyaura, “Nobiletin, a Polymethoxy Flavonoid, Suppresses Bone Resorption by Inhibiting NFkappaB-dependent Prostaglandin E Synthesis in Osteoblasts and Prevents Bone Loss Due to Estrogen Deficiency,” Journal of Pharmacological Sciences, Vol. 115, No. 1, 2011, pp. 89-93. doi:10.1254/jphs.10193SC
- M. Ikemura, Y. Sasaki, J. C. Giddings and J. Yamamoto, “Preventive Effects of Hesperidin, Glucosyl Hesperidin and Naringin on Hypertension and Cerebral Thrombosis in Stroke-Prone Spontaneously Hypertensive Rats,” Phytotherapy Research, Vol. 26, No. 9, 2012, pp. 1272-1277. doi:10.1002/ptr.3724
- H. Negishi, K. Ikeda, M. Sagara, M. Sawamura and Y. Yamori, “Increased Oxidative DNA Damage in StrokeProne Spontaneously Hypertensive Rats,” Clinical and Experimental Pharmacology & Physiology, Vol. 26, No. 5-6, 1999, pp. 482-484. doi:10.1046/j.1440-1681.1999.03055.x
- K. Mizutani, K. Ikeda, T. Nishikata and Y. Yamori, “Phytoestrogens Attenuate Oxidative DNA Damage in Vascular Smooth Muscle Cells from Stroke-Prone Spontaneously Hypertensive Rats,” Journal of Hypertension, Vol. 18, No. 12, 2000, pp. 1833-1840. doi:10.1097/00004872-200018120-00018
- K. Mizutani, K. Ikeda, Y. Kawai and Y. Yamori, “Protective Effect of Resveratrol on Oxidative Damage in Male and Female Stroke-Prone Spontaneously Hypertensive Rats,” Clinical and Experimental Pharmacology & Physiology, Vol. 28, No. 1-2, 2001, pp. 55-59. doi:10.1046/j.1440-1681.2001.03415.x
- R. M. Touyz and A. M. Briones, “Reactive Oxygen Species and Vascular Biology: Implications in Human Hypertension,” Hypertension Research, Vol. 34, No. 1, 2011, pp. 5-14. doi:10.1038/hr.2010.201
- R. Rodrigo, J. Gonzalez and F. Paoletto, “The Role of Oxidative Stress in the Pathophysiology of Hypertension,” Hypertension Research, Vol. 34, No. 4, 2011, pp. 431-440. doi:10.1038/hr.2010.264
- T. Michel and P. M. Vanhoutte, “Cellular Signaling and NO Production,” Pflügers Archiv: European Journal of Physiology, Vol. 459, No. 6, 2010, pp. 807-816. doi:10.1007/s00424-009-0765-9
- C. Banfi, M. Camera, G. Giandomenico, V. Toschi, M. Arpaia, L. Mussoni, E. Tremoli and S. Colli, “Vascular Thrombogenicity Induced by Progressive LDL Oxidation: Protection by Antioxidants,” Thrombosis and Haemostasis, Vol. 89, No. 3, 2003, pp. 544-553.
- R. L. Silverstein, W. Li, Y. M. Park and S. O. Rahaman, “Mechanisms of Cell Signaling by the Scavenger Receptor CD36: Implications in Atherosclerosis and Thrombosis,” Transactions of the American Clinical Climatollgical Association, Vol. 121, 2010, pp. 206-220.
- S. C. Whitman, E. M. Kurowska, J. A. Manthey and A. Daugherty, “Nobiletin, a Citrus Flavonoid Isolated from Tangerines, Selectively Inhibits Class A Scavenger Receptor-Mediated Metabolism of Acetylated LDL by Mouse Macrophages,” Atherosclerosis, Vol. 178, No. 1, 2005, pp. 25-32. doi:10.1016/j.atherosclerosis.2004.07.034
- E. E. Mulvihill, J. M. Assini, J. K. Lee, E. M. Allister, B. G. Sutherland, J. B. Koppes, C. G. Sawyez, J. Y. Edwards, D. E. Telford, A. Charbonneau, P. St-Pierre, A. Marette and M. W. Huff, “Nobiletin Attenuates VLDL Overproduction, Dyslipidemia, and Atherosclerosis in Mice with Diet-Induced Insulin Resistance,” Diabetes, Vol. 60, No. 5, 2011, pp. 1446-1457. doi:10.2337/db10-0589
- R. C. Robbins, “Antithrombogenic Properties of a Hexamethoxylated Flavonoid. Reduction of Deaths in Rats Due to Intravascular Infusion of Adenosine Diphosphate (ADP),” Atherosclerosis, Vol. 18, No. 1, 1973, pp. 73-82. doi:10.1016/0021-9150(73)90118-4
- R. C. Robbins, “Flavones in Citrus Exhibit Antiadhesive Action on Platelets,” International Journal for Vitamin and Nutrition Research, Vol. 58, No. 4, 1988, pp. 418- 421
- E. Sempinska, B. Kostka, M. Krolikowska and E. Kalisiak, “Effect of Flavonoids on the Platelet Adhesiveness in Repeatedly Bred Rats,” Polish Journal of Pharmacology and Pharmacy, Vol. 29, No. 1, 1977, pp. 7-10.
- T. Akachi, Y. Shiina, Y. Ohishi, T. Kawaguchi, H. Kawagishi, T. Morita, M. Mori and K. Sugiyama, “Hepatoprotective Effects of Flavonoids from Shekwasha (Citrus Depressa) Against D-Galactosamine-Induced Liver Injury in Rats,” Journal of Nutritional Science and Vitaminology (Tokyo), Vol. 56, No. 1, 2010, pp. 60-67. doi:10.3177/jnsv.56.60
- Y. S. Lee, B. Y. Cha, K. Saito, S. S. Choi, X. X. Wang, B. K. Choi, T. Yonezawa, T. Teruya, K. Nagai and J. T. Woo, “Effects of a Citrus Depressa Hayata (Shiikuwasa) Extract on Obesity in High-Fat Diet-Induced Obese Mice,” Phytomedicine, Vol. 18, No. 8-9, 2011, pp. 648- 654. doi:10.1016/j.phymed.2010.11.005
- The Physiological Society of Japan, “Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences,” 2003. http://int.physiology.jp/en/ethics/
- S. Morii, A. C. Ngai and H. R. Winn, “Reactivity of Rat Pial Arterioles and Venules to Adenosine and Carbon Dioxide: With Detailed Description of the Closed Cranial Window Technique in Rats,” Journal of Cerebral Blood Flow and Metabolism, Vol. 6, No. 1, 1986, pp. 34-41. doi:10.1038/jcbfm.1986.5
- Y. Sasaki, T. Noguchi, J. Seki, J. C. Giddings and J. Yamamoto, “Protective Effects of Imidapril on He-Ne Laser-Induced Thrombosis in Cerebral Blood Vessels of Stroke-Prone Spontaneously Hypertensive Rats,” Thrombosis and Haemostasis, Vol. 83, No. 5, 2000, pp. 722- 727.
- Y. Sasaki, T. Noguchi, E. Yamamoto, J. C. Giddings, K. Ikeda, Y. Yamori and J. Yamamoto, “Effects of Ginkgo Biloba Extract (EGb 761) on Cerebral Thrombosis and Blood Pressure in Stroke-Prone Spontaneously Hypertensive Rats,” Clinical and Experimental Pharmacology & Physiology, Vol. 29, No. 11, 2002, pp. 963-967. doi:10.1046/j.1440-1681.2002.03761.x
- M. El Haouari and J. A. Rosado, “Platelet Function in Hypertension,” Blood Cells, Molecules & Diseases, Vol. 42, No. 1, 2009, pp. 38-43. doi:10.1016/j.bcmd.2008.07.003
- R. Rodrigo, J. Gonzalez and F. Paoletto, “The Role of Oxidative Stress in the Pathophysiology of Hypertension,” Hypertension Research, Vol. 34, No. 4, 2011, pp. 431-440. doi:10.1038/hr.2010.264
- T. Seno, N. Inoue, D. Gao, M. Okuda, Y. Sumi, K. Matsui, S. Yamada, K. I. Hirata, S. Kawashima, R. Tawa, S. Imajoh-Ohmi, H. Sakurai and M. Yokoyama, “Involvement of NADH/NADPH Oxidase in Human Platelet ROS Production,” Thrombosis Research, Vol. 103, No. 5, 2001, pp. 399-409. doi:10.1016/S0049-3848(01)00341-3
- B. Wachowicz, B. Olas, H. M. Zbikowska and A. Buczynski, “Generation of Reactive Oxygen Species in Blood Platelets,” Platelets, Vol. 13, No. 3, 2002, pp. 175-182. doi:10.1080/09533710022149395
- F. Krotz, H. Y. Sohn and U. Pohl, “Reactive Oxygen Species: Players in the Platelet Game,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 24, No. 11, 2004, pp. 1988-1996. doi:10.1161/01.ATV.0000145574.90840.7d
- J. A. Guerrero, L. Navarro-Nunez, M. L. Lozano, C. Martinez, V. Vicente, J. M. Gibbins and J. Rivera, “Flavonoids Inhibit the Platelet TxA(2) Signalling Pathway and Antagonize TxA(2) Receptors (TP) in Platelets and Smooth Muscle Cells,” British Journal of Clinical Pharmacology, Vol. 64, No. 2, 2007, pp. 133-144. doi:10.1111/j.1365-2125.2007.02881.x
- Y. Hirata, Y. Masuda, H. Kakutani, T. Higuchi, K. Takada, A. Ito, Y. Nakagawa and H. Ishii, “Sp1 Is an Essential Transcription Factor for LPS-induced Tissue Factor Expression in THP-1 Monocytic Cells, and Nobiletin Represses the Expression through Inhibition of NF-Kappab, AP-1, and Sp1 Activation,” Biochemical Pharmacology, Vol. 75, No. 7, 2008, pp. 1504-1514. doi:10.1016/j.bcp.2007.12.019
- D. Harats, M. A. Mulkins and E. Sigal, “A Possible Role for 15-Lipoxygenase in Atherogenesis,” Trends in Cardiovascular Medicine, Vol. 5, No. 1, 1995, pp. 29-36. doi:10.1016/1050-1738(94)00029-U
- K. E. Malterud and K. M. Rydland, “Inhibitors of 15-Lipoxygenase from Orange Peel,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 11, 2000, pp. 5576-5580. doi:10.1021/jf000613v
- D. Harats, A. Shaish, J. George, M. Mulkins, H. Kurihara, H. Levkovitz and E. Sigal, “Overexpression of 15-Lipoxygenase in Vascular Endothelium Accelerates Early Atherosclerosis in LDL Receptor-Deficient Mice,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 20, No. 9, 2000, pp. 2100-2105. doi:10.1161/01.ATV.20.9.2100
- R. Paoletti, A. M. J. Gotto and D. P. Hajjar, “Inflammation in Atherosclerosis and Implications for Therapy,” Circulation, Vol. 109, Suppl. 1, 2004, pp. 20-26. doi:10.1161/01.CIR.0000131514.71167.2e
- K. Gertow, E. Nobili, L. Folkersen, J. W. Newman, T. L. Pedersen, J. Ekstrand, J. Swedenborg, H. Kuhn, C. E. Wheelock, G. K. Hansson, U. Hedin, J. Z. Haeggstrom and A. Gabrielsen, “12- and 15-Lipoxygenases in Human Carotid Atherosclerotic Lesions: Associations with Cerebrovascular Symptoms,” Atherosclerosis, Vol. 215, No. 2, 2011, pp. 411-416. doi:10.1016/j.atherosclerosis.2011.01.015
- J. L. Mehta, J. Chen, P. L. Hermonat, F. Romeo and G. Novelli, “Lectin-Like, Oxidized Low-Density Lipoprotein Receptor-1 (LOX-1): A Critical Player in the Development of Atherosclerosis and Related Disorders,” Cardiovascular Research, Vol. 69, No. 1, 2006, pp. 36-45. doi:10.1016/j.cardiores.2005.09.006
Abbreviations
ANOVA: analysis of varianceCCD: charge-coupled deviceELISA: enzyme-linked immunosorbent assayHe-Ne: helium-neonKHS: Krebs-Henseleit solutionLDL: low-density-lipoproteinLOX-1: lectin-like ox-LDL receptor-1LPS: lipopolysaccharideNO: nitric oxideNF-kappaB: nuclear factor-kappa B8-OHdG: 8-hydroxy-2’-deoxyguanosineONOO−: peroxynitriteox-LDL: oxidized low-density lipoproteinsROS: reactive oxygen speciesSR: scavenger receptorsSHRSP: stroke prone spontaneously hypertensive ratTPA: 12-O-tetradecanoylphorbol-13-acetateVLDL: very-lowdensity lipoprotein.
NOTES
*The authors declare that they have no conflict of interest.
#Corresponding author.

