Food and Nutrition Sciences
Vol. 3 No. 8 (2012) , Article ID: 21566 , 18 pages DOI:10.4236/fns.2012.38146
Jamun (Syzygium cumini (L.)): A Review of Its Food and Medicinal Uses
![]()
1Department of Agricultural Process Engineering, College of Agricultural Engineering and Technology, Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli, Ratnagiri, India; 2NAIP-Kokum, Karonda, Jamun and Jackfruit, Department of Agricultural Process Engineering, College of Agricultural Engineering and Technology, Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli, Ratnagiri, India; 3Department of Horticulture, College of Agriculture, Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli, Ratnagiri, India.
Email: *swami_shrikant1975@yahoo.co.in, nayan07@gmail.com, patil.megha2@gmail.com, parag5663@rediffmail.com
Received April 23rd, 2012; revised July 3rd, 2012; accepted July 10th, 2012
Keywords: Jamun-Syzygium cumini; Chemopreventive; Radioprotective; Antineoplastic Activities
ABSTRACT
Eugenia jambolana Lam., commonly known as black plum or “jamun” is an important medicinal plant in various traditional systems of medicine. It is effective in the treatment of diabetes mellitus, inflammation, ulcers and diarrhea and preclinical studies have also shown it to possess chemopreventive, radioprotective and antineoplastic properties. The plant is rich in compounds containing anthocyanins, glucoside, ellagic acid, isoquercetin, kaemferol and myrecetin. The seeds are claimed to contain alkaloid, jambosine, and glycoside jambolin or antimellin, which halts the diastatic conversion of starch into sugar. The present review has been primed to describe the existing data on the information on traditional and medicinal use.
1. Introduction
Syzygium cumini (Family Myrtaceae) is also known as Syzygium jambolanum and Eugenia cumini. Other common names are Jambul, Black Plum, Java Plum, Indian Blackberry, Jamblang, Jamun etc. Today these trees are found growing throughout the Asian subcontinent, Eastern Africa, South America, Madagascar and have also naturalized to Florida and Hawaii in the United States of America [1]. The tree fruits once in a year and the berries are sweetish sour to taste. The ripe fruits are used for health drinks, making preserves, squashes, jellies and wine [1]. In association to its dietary use, all parts of the tree and, importantly the seeds are used to treat a range of ailments, the most important being diabetes mellitus [2]. Different parts of the jambolan were also reported for its antioxidant, anti-inflammatory, neuropsycho-pharmacological, anti-microbial, anti-bacterial, anti-HIV, antileishmanial and antifugal, nitric oxide scavenging, free radical scavenging, anti-diarrheal, antifertility, anorexigenic, gastroprotective and anti-ulcerogenic and redioprotective activities [2]. (Figure 1) shows the Jamun fruit.
2. Composition of Fruit
Analyses of the fruit in the Philippines were reported in 1924 as follows: Waste, 25%; edible portion: water, 80.80%; ash, 0.70; protein, 0.81; sugar, 12.70 (fructose and glucose; no sucrose); acidity (as sulphuric), 0.63%; (as malic) 0.88% [3]. The following composition per 100 grams of edible portion was reported for fruits freshly picked at the Lancetilla Experimental Garden, Honduras, in 1948: Moisture, 85.8 gm; ether extract, 0.15 gm; crude fiber, 0.3 gm; nitrogen, 0.129 gm; ash, 0.32 gm; calcium, 8.3 mg; phosphorus, 16.2 mg; iron, 1.62 mg; carotene, 0.004 mg; thiamine, 0.008 mg; riboflavin, 0.009 mg; niacin, 0.290 mg; total ascorbic acid, 5.7 mg [4]. Virmani gives the following analysis: specific gravity, 1.0184; total acidity (as acetic acid), 5.33 per 100 cc; volatile acidity (as acetic acid), 5.072 per 100 cc; fixed acidity, 0.275% as citric; total solids, 4.12 per 100 cc; ash, 0.42; alkalinity of ash, 32.5 (N/10 alkali); nitrogen, 0.66131; total sugars, 0.995; reducing sugars, 0.995; non-volatile reducing sugars, 0.995; alcohol, 0.159% by weight; oxidation value (KMnO4, 186.4); iodine value, 183.7; ester value, 40.42. Other reported constituents of the seeds are: protein (6.3 to 8.5%), fat (1.18%), crude fiber (16.9%),

Figure 1. Jamun fruit in a bunch.
ash (21.72%), calcium (0.41%), phosphorus (0.17%), fatty acids (palmitic, stearic, oleic and linoleic), starch (41%), dextrin (6.1%), a trace of phytosterol, and 6 to 19% tannin [5]. The fruits are avidly eaten by birds and four footed animals (jackals and civets in India). In Australia, they are a favorite food of the large bat called “flying fox”. Analyses of the leaves show: crude protein (9.1%), fat (4.3%), crude fiber (17.0%), ash (6.0%), calcium (1.3%), phosphorus (0.19%) [6]. It consists mainly of monoor sesqui-terpene hydrocarbons which are “very common in essential oils.” Constituents of Syzgium cumini seeds are fatty oils (30 g/kg), including lauric (2.8%), myristic (31.7%), palmitic (4.7%), stearic (6.5%), oleic (32.2%), linoleic (16.1%), malvalic (1.2%), sterculic (1.8%) and vernolic acid (3%) and phytosterols such as β-sitosterol. Further constituents are tannins (6%), predominantly corilagin, ellagitannins, ellagic acid, galloyl-galactoside and gallic acid [7]. The leaf oil consists of 16.91% octadecane, 9.98% nonacosane, 9.38% triacontane, 7.38% octacosane, 4.86% Heptacosane, 4.25% hexadecanoic acid and 4.02% eicosane. The seed oil consists of 33.2% 1-chlorooctadecane, 9.24% tetratetracontane, 8.02% decahydro-8a-ethyl-1,1, 4a,6-tetramethylnapahthalene, 5.29% 4-(2-2-dimethyl-6-6-methylenecyclohexyl) butanol, 5.15% Octadecane, 3.97% octacosane, 1.72% heptacosane and 1.71% eicosane. [8]. Java Plum consist of Energy 251 kJ (60 kcal), Carbohydrates 15.56 g, fat 0.23 g, Protein 0.72 g, water 83.13 g, Vitamin A 3IU, Thiamine (vit B1) 0.006 mg (1%), Riboflavin (vit. B2) 0.012 mg (1%), 0.260 mg (2%) Niacin (vit. B3), 0.160 mg (3%) Pantothenic acid (B5), 0.038 mg (3%) Vitamin B60.038 mg (3%), 14.3 mg (17%) Vitamin C, 19 mg (2%) Calcium, 0.19 mg (1%) Iron, 15 mg (4%) Magnesium, 17 mg (2%) Phosphorus, 79 mg (2%) Potassium, 14 mg (1%) Sodium [9]. The Fruit Contain 83.70 - 85.80 g moisture, 0.70 - 0.13 g protein, 0.15 - 0.30 g fat, 0.30 - 0.90 g crude fibre, 14.00 g carbohydrate, 0.32 - 0.40 h ash, 8.30 - 15.00 mg calcium, 35.00 mg magnesium, 15.00 - 16.20 mg phosphorus, 1.20 - 1.62 mg iron, 26.20 mg sodium, 55.00 mg potassium, 0.23 mg copper, 13.00 mg sulfur, 8.00 mh chlorine, 8. I.U vitamin A, 0.01 - 0.03 mg thiamine, 0.009 - 0.01 mg riboflavin, 0.20 - 0.29 mg niacin, 5.70 - 18.00 mg ascorbic acid, 7.00 mg chlorine and 3.00 mcg folic acid per 100 g of edible portion [10].
3. Food Uses
Good quality jambolan juice is excellent for sherbet [11, 12], syrup and “squash”. In India the latter is a bottled drink prepared by cooking the crushed fruits for 5 to 10 minutes at 140˚F, pressing out the juice, combining it with sugar and water and adding citric acid and sodium benzoate as a preservative [13]. Jambolans of good size and quality, having a sweet or sub acid flavor and a minimum of astringency, are enjoyable raw and may be made into tarts [14], sauces and jam. Astringent fruits are improved in palatability by soaking them in salt water [14] or pricking them, rubbing them with a little salt, and letting them stand for an hour [15]. All but decidedly in ferior fruits can be utilized for juice which is often comparable to grape juice [16]. When extracting juice from cooked jambolans, it is recommended that it be allowed to drain out without squeezing the fruit and it will thus be less as tringent. The white-fleshed jambolan has adequate pectin and makes a very stiff jelly unless cooking is brief [17]. The more common purplefleshed yields richly colored jelly [18] but is deficient in pectin and requires the addition of a commercial jelling agent or must be combined with pectin-rich fruits such as unripe or sour guavas, or ketembillas [18]. In Goa and the Philippines [19], jambolans are an important source of wine, resembling Port [20]. Brandy and a distilled liquor called “jambava” have also been made from the fermented fruit. Jambolan vinegar, extensively made throughout India, is an attractive, clear purple, with a pleasant aroma and mild flavor.
4. Uses in Traditional Medicine
Traditionally the jambul fruits, leaves, seeds, and bark are all used in ayurvedic medicine. The bark contains tannins and carbohydrates, accounting for its long-term use as an astringent to combat ailments like dysentery [21]. A glycoside in the seed, jamboline, is considered to have antidiabetic properties [22]. Older French research shows that the seeds have a significant hypoglycemic effect in diabetic rabbits [23]. The seeds have also shown anti-inflammatory effects in rats and antioxidant properties in diabetic rat [24]. Older reports from Indian medical journals suggest jambul seed and bark can be beneficial in humans with diabetes [25]. Jamun fruit seeds and pulp have been reported to serve various purposes in diabetic patients, such as lowering blood glucose levels and delaying diabetic complications including neuropathy and cataracts [2,26]. Jamun is most often recognized as an adjuvant therapy in type-2 diabetes. This has been traced not only to its anthocyanin-rich, dark-purple fleshy pulp, but also to its seeds, which have been most studied for their antidiabetic principles. Jamun seeds are reported to be a rich source of ellagitannins (ETs), including corilagin, 3,6-hexa hydroxyl diphenoyl glucose and its isomer 4,6-hexahydroxy diphenoyl glucose, 1- galloylglucose, 3-galloylglucose, gallic acid, and ellagic acid (EA) [26]. This marker compound has anti-diabetic activity. When alloxan induced diabetic rats were fed with Jamun seed extract, the blood glucose, blood urea, serum cholesterol and serum triglyceride levels were found to decrease significantly [27]. Jamun fruit reduces the sugar in the blood and is very good in the control of diabetes. Its seeds contain Glucoside, Jamboline and Ellagic acid, which are reported to have the ability to check the conversion of starch into sugar in case of excess production of glucose [27]. All parts of the jambolan can be used medicinally and it has a long tradition in alternative medicine. The plant has been viewed as an antidiabetic plant since it became commercially available several decades ago.
From all over the word, the fruits have been used for a wide variety of ailments, including cough, diabetes, dysentery, inflammation and ringworm [28]. It is also an ancient medicinal plant with an illustrious medical history and has been the subject of classical reviews for over 100 years. It is widely distributed throughout India and Ayurvedic medicine (Indian folk medicine) mentions its use for the treatment of diabetic mellitus. Various traditional practitioners in India use the different parts of the plant in the treatment of diabetes, blisters in mouth, cancer, colic, and diarrhea, digestive complaints, dysentery, piles, pimples and stomachache [29]. During last four decades, numerous folk medicinal reports on the antidiabetic effects of this plant have been cited in the literature. In Union medicine various parts of Jambolan acts as liver tonic, enrich blood, strengthen teeth and gums and form Good lotion for removing ringworm infection of the head [30].
E. jambolana leaf extract showed hypoglycemic action in diabetic rats [30]. The seed powder of E. jambolana is reported to have hypoglycemic action in streptozotocindiabetic rats [31,32]. Its effect may be persistent, as in one study, homeostasis was maintained in the rats for two weeks after the cessation of treatment [32]. In alloxan-diabetic rabbits the water extract of E. jambolana fruit pulp was more effective than the ethanol extract at reducing fasting blood glucose and improving blood glucose levels in the glucose tolerance test. E. jambolana also increased blood insulin levels in both diabetic and severely diabetic rabbits [33,34]. The inhibition of insulinase activity from liver and kidney by extract of Eugenia jambolana also has been reported, which points out to its extra-pancreatic mechanism [34]. Another study also found that E. jambolana seed extract reduced blood glucose, glycosylated hemoglobin, and increased plasma insulin [35]. E. jambolana fruit combined with bitter melon decreased insulin levels that were raised in diabetic rats fed a fructose diet [36].
Jamun is most often recognized as an adjuvant therapy in type-2 diabetes. This has been traced not only to its anthocyanin-rich, dark-purple fleshy pulp, but also to its seeds, which have been most studied for their antidiabetic principles. Other reports from Indian medical journals suggest jambul seed and bark can be beneficial in humans with diabetes [37]. When alloxan induced diabetic rats were fed with Jamun seed extract, the blood glucose, blood urea, serum cholesterol and serum triglyceride levels were found to decrease significantly [27]. Jamun fruit reduces the sugar in the blood and is very good in the control of diabetes.
Ayurvedic texts suggest that 1 - 3 g of seed powder per day is an average dose 44 additionally, Juice of ripe fruits in the amount of 0.5 - 2 tsp (2.5 - 10 ml) at least three times daily have been recommended for the treatment of diabetes. Administration of 100 and 200 mg/kg body weight of aqueous extract of Syzygium cumini pulp significantly decreased the blood glucose level in the experimental rats suggesting that it has hypoglycemic properties. The decreased body weight in diabetic rats is due to excessive breakdown of tissue proteins. Treatment with Syzygium cumini improved body weight significantly in a dose dependent manner, indicating prevention of muscle wasting due to hyperglycemic condition.
5. Medicinal Properties
The jambolan has received far more recognition in folk medicine and in the pharmaceutical trade than in any other field. Medicinally, the fruit is stated to be astringent, stomachic, carminative, antiscorbutic and diuretic [37]. Additionally, a fruit extract showed antimicrobial and cytotoxic activities and may potentially be used on topical antimicrobial products. In comparison to other nontraditional fruits jambolao showed considerable high antioxidant activity, which can constituent such as anthocyanins, tannins and flavonols [38]. The anthocyanin composition was characterised by the presence of 3,5- diglucosides of five out of six aglycones commonly found in foods [39]. Fruits contain many different kinds of anti-oxidant compounds, including flavonoids, phenolics, carotenoids and vitamins, which are all considered beneficial to human health, for decreasing the risk of degenerative diseases by reduction of oxidative stress, and for the inhibition of macromolecular oxidation [40]. There is a very high anthocyanin content in S. cumini fruits which attributes to its antioxidant and free radical scavenging activity. These pigments can be a good source of natural food colourants for the food processing industries [41].
Fruit bark decoction for antiplasmodial activity was performed, leading to the isolation of three known ellagic acid derivatives (ellagic acid, ellagic acid 4-O-alpha-L- 2”-acetylrhamnopyranoside, 3-O-methylellagic acid 3’-Oalpha-L-rhamnopyranoside), as well as the new derivative 3-O-methylellagic acid 3’-O-beta-D-glucopyranoside [42]. 3-hydroxy androstane [16,17-C] (6’methyl, 2’-1- hydroxyl-isopropene-1-yl) 4,5,6 H pyran present in Syzygium cumini seed is one of the important marker compound [43]. Phytochemical investigation of the stem bark of Syzygium cumini (L.) Skeels (Myrtaceae) yielded four new lignan derivatives characterised as (7α,8α,2’α)-3,4, 5-trimethoxy-7,3’,1’,9’-diepoxylignan (cuminiresinol), (7α, 7’α,8α,8’α)-3,4-dioxymethylene-3’,4’-dimethoxy-7,9’,7’,9-diepoxylignan-5’-ol (5’-hydroxy-methyl-piperitol), (7α, 7’α,8α,8’α)-3’-methoxy-9-oxo-7,9’,7’,9-diepoxylignan-3,4, 4’-triol or 3-demethyl-9-oxo-pinoresinol (syzygiresinol A), (7α,7’α,8α,8’α)-9-oxo-7,9’,7’,9-diepoxylignan-3,4,3’,4’, 5’-pentaol or 3,3’-didemethyl-9-oxo-pinoresinol along with the known lignans di-demethyl-5-hydroxypinoresinol, dimethylpinoresinol, didemethoxypinoresinol, pinoresinol and 4’-methyl-5’-hydroxypinoresinol [44]. The anthocyanins occur as 3,5-, but not 3-diglucosides, of delphinidin, cyanidin, petunidin, peonidin, and malvidin. This is the report to use a combination of spectrometric and spectroscopic methods to identify unequivocally the structures of E. jambolana fruit anthocyanins [45]. For instance, flavonoids have been referred to as nature’s biological response modifiers, because of their inherent ability to modify the body’s reaction to allergies and virus and they showed their anti-allergic, anti-inflammatory, anti-microbial and anti-cancer activities. Plant steroids are known to be important for their cardiotonic activities and also possess insecticidal and antimicrobial properties. They are also used in nutrition, herbal medicine and cosmetics [46]. Seed extracts of S. cumini, the part most often used in Ayurvedic medicine, were previously shown to have high levels of total phenolics and good activity in the trolox equivalent antioxidant capacity (TEAC) and ferric reducing antioxidant power (FRAP) antioxidant assays [47].
The juice of the ripe fruit, or a decoction of the fruit, or jambolan vinegar, may be administered in India in cases of enlargement of the spleen, chronic diarrhea and urine retention [16,38]. Water-diluted juice is used as a gargle for sore throat and as a lotion for ringworm of the scalp [16,38]. Jambolan juice and mango juice, half and half, quench thirst in diabetics [38]. The seeds (marketed in-inch lengths) and the bark are official in the Dutch [16]. They are much used in tropical medicine and are shipped from India, Malaya and Polynesia, and to a small extent from the West Indies [48], to pharmaceutical supply houses in Europe and England [49].
Studies in the past one decade have also shown that Jamun possess antineoplastic [50]. Radioprotective [51- 54] and chemopreventive effects [55] all of which are useful in the prevention and treatment of cancer. The reasons for the myriad pharmacological effects are due to the presence of diverse phytochemicals like flavonoids, anthocyanins, terpenes [2] and are enlisted in Table 1.
Extracts of both, but especially the seeds, in liquid or powdered form [61], are freely given orally, two or three times a day to patients with diabetes mellitus or glycosuria [38]. In many cases, the blood sugar level reportedly is quickly reduced and there are no ill effects [38]. Fresh seeds are considered superior to dried seeds [62]. Reduction of blood sugar was obtained in alloxan diabetes in rabbits [62]. In experiments at the Central Drug Research Institute, Lucknow, the dried alcoholic extract
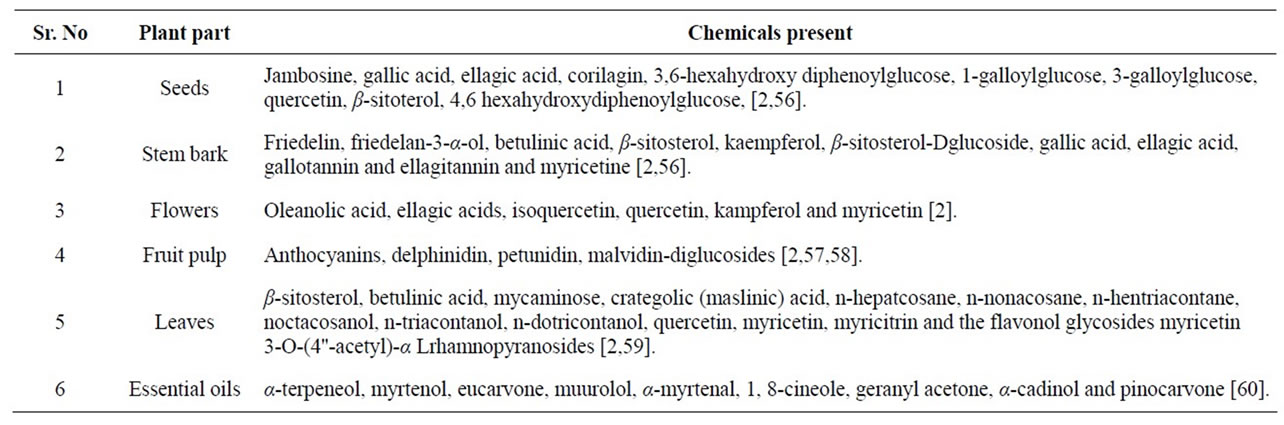
Table 1. Phytochemicals present in the jamun plant.
of jambolan seeds, given orally, reduced blood sugar and glycosuria in patients [62]. Dr. Mukerji, in 1961, called the results promising, though the action of the seed extract is milder than that of the synthetic anti-diabetics. He holds that the bark extract affects the glycogenolysis and glycogen storage in animals [38]. On the other hand, Bhatnagar and co-workers, while screening the jambolan with 174 other popular Indian medicinal plants, found no physiological activity in the bark, which they collected in the month of September [63]. Other reported constituents of the seeds are: tannin, [16] to 19%; gallic acid, 1% to 2%; chlorophyll [55]; fatty acids (palmitic, stearic, oleic, and linoleic) [16]; starch, 41% [15], dextrin, 6.1%; protein, 6.3% [15]; and a trace of phytosterol [16].The seeds are claimed by some to contain a glycoside, jambolin [16,55] or antimellin [62], which halts the diastatic conversion of starch in to sugar [16]; also a resin yielding phenolic substances named jambidol [15,16] and ellagic acid [55], and an alkaloid, jambosine [61]. The bark contains 8% to 19% tannin [15,55], gallic acid, 1.67% [16], resin [64], small amounts of ellagic acid and myricetin [65]. A decoction of the bark is taken internally for dyspepsia, dysentery and diarrhea and also serves as an enema [16]. The dried and powdered seeds and root-bark are similarly employed [16]. Powdered bark mixed with curds is given in menorrhagia. Powdered jambolan and mango seeds, with curds, are used, like the fruit juice, in treating enlarged spleen and retained urine [38]. In India, the seed powder is administered as an antidote for strychnine poisoning [15]. The leaf juice is effective in the treatment of dysentery [16], either alone or in combination with the juice of mango or emblic leaves [38]. The leaves, steeped in alcohol, are prescribed in diabetes [55]. Jambolan leaves may be helpful as poultices for skin diseases [15,16]. The leaves yield 12% to 13% tannin (by dry weight) (IS), also an essential oil containing limeonene and dipentene (20% to 30%), about 40% of sesquiterpene (cadeninic type) and a little azulenic sesquiterpene [62]. Bark decoctions are taken for asthma and bron chitis [5] and are gargled or used as mouth wash for the astringent effect on mouth ulcerations, spongy gums [16] and for stomatitis [38]. Ashes of the bark, mixed with water, are spread over local inflamations; or, blended with oil, applied to burns [38].
In the year 2008, 12.7 million new cancer cases and 7.6 million cancer deaths occurred [66]. More worryingly, predictions are that by the year 2020, the global incidence of the cancer will increase by threefold, with a disproportionate rise in cases from the developing world countries that have limited resources to tackle the problem [67]. The conventional treatment modalities used in treating cancer, the surgery, radiotherapy, hormone therapy and chemotherapy remain prohibitively expensive to the large number of population in the developing countries. With an expected rise in cancer incidence, the mortality and associated morbidity will be enormous due to the compromised financial condition of the patients [66, 67]. Since the dawn of civilization, herbal drugs have been used in the ancient civilizations and their use in the treatment of cancer is on a rise especially in the developing and underdeveloped countries primarily due to its easy affordability, non toxic nature, easy acceptability, less toxic or no toxic effects and easy availability. Plants have been the main ingredients of various medications of the traditional Indian system of medicine, the Ayurveda and one such plant of immense importance is Eugenia jambolana Lam (Syn. Syzygium cumini Skeels or Syzygium jambolana Dc or Eugenia cuminii Druce) (Figure 1), colloquially known as Java plum, Portuguese plum, Malabar plum, black plum, Indian blackberry, jaman, jambu, jambul and jambool [68].
5.1. Chemopreventive Effects
Chemoprevention, a science that has emerged during the three last decade, presents an alternative approach to reducing mortality from cancer. Chemopreventive interventions may be applied at any time during carcinogenesis, from the initial molecular defect through the accumulated molecular, cellular and histopathologic aberrations that characterize disease progression before an invasive and potentially metastatic stage [69]. It aims at blocking, reversing, or delaying carcinogenesis before the development of invasive disease by targeting key molecular derangements using pharmacological or nutritional agents [69]. Very recently [70] have also observed that administration of the jamun extract (25 mg/kg body weight/day) was effective in preventing benzo-a-pyreneinduced forestomach carcinogenesis. Recently, [71] have reported that jamun possess cancer chemopreventive properties in the DMBA-induced croton oil promoted two stage skin carcinogenesis in Swiss albino mice. Feeding of 125 mg/kg body weight/animal/day of the extract either during the perinitiation (i.e. 7 days before and 7 days after the application of DMBA) or post-initiation (i.e. from the day of start of croton oil treatment and continued till the end of the experiment) phases reduced the cumulative numbers of papillomas, the tumor incidence and increased the average latency period when compared with the control group (carcinogen alone) [71].
Jamun reduced the tumor incidence, tumor burden and cumulative number of gastric carcinomas. Reports also suggest that gallic acid, ellagic acid, flavonoids and anthocyanins (Figure 2) present in Jamun are reported to prevent experimental carcinogenesis in various organs (Table 2) and may have contributed to the anti-carcinogenesis. Additionally, recent observations also suggest that ellagitannin, a constituent of Jamun and its colonic
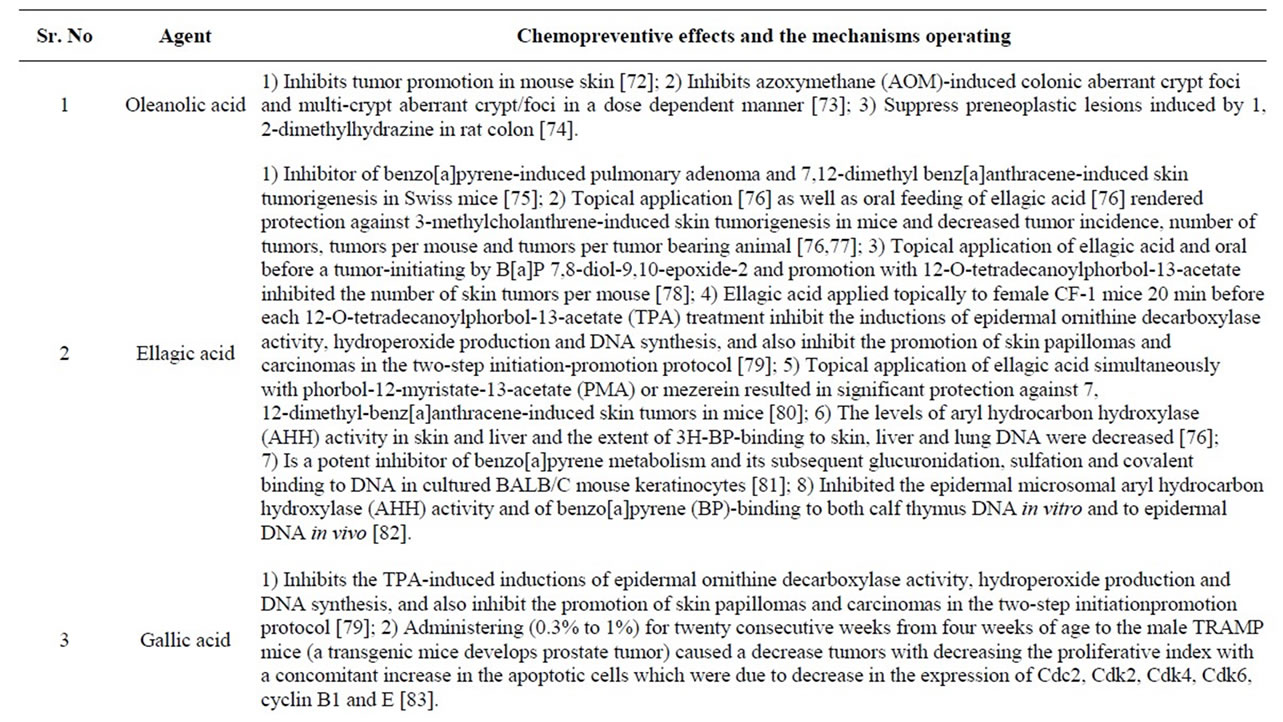

Table 2. Phytochemicals of Jamun with reported chemopreventive effects.
 (a)
(a)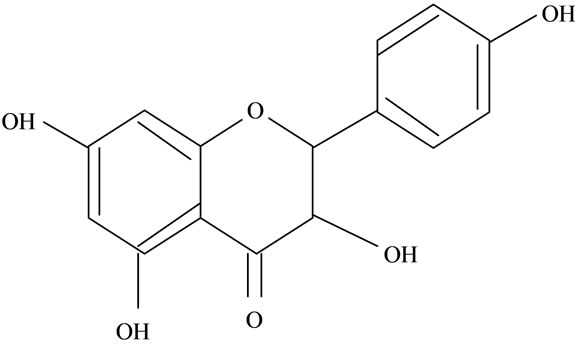 (b)
(b)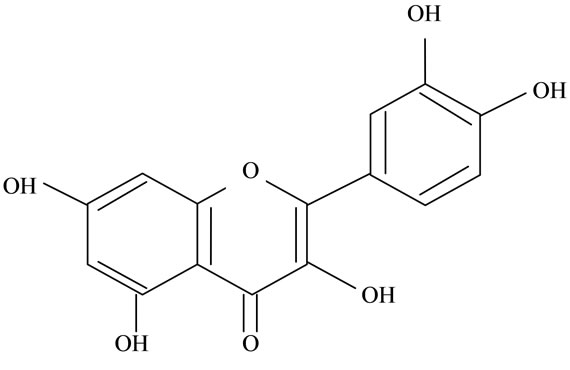 (c)
(c)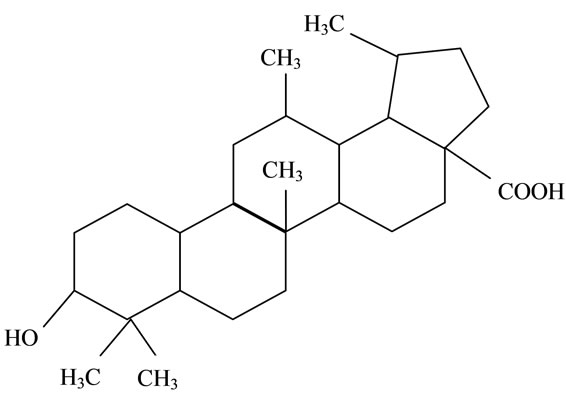 (d)
(d)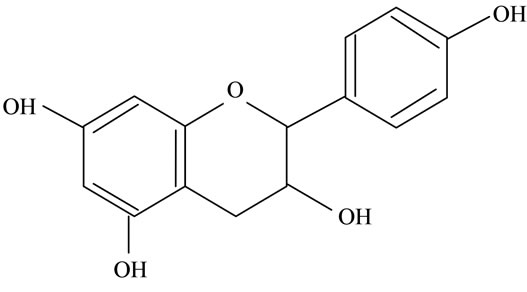 (e)
(e)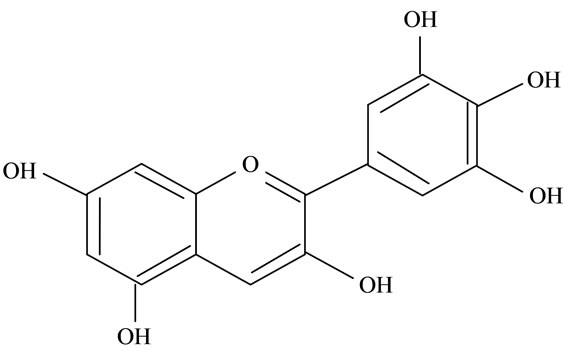 (f)
(f)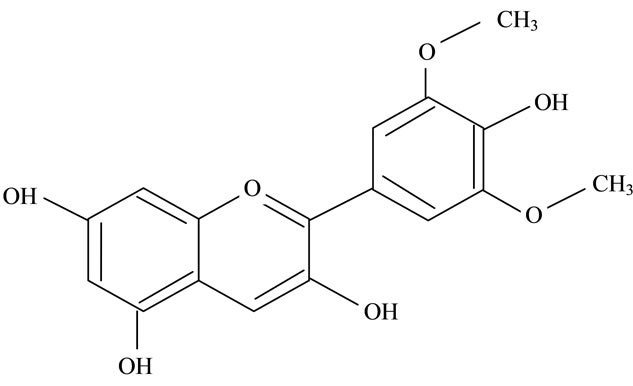 (g)
(g)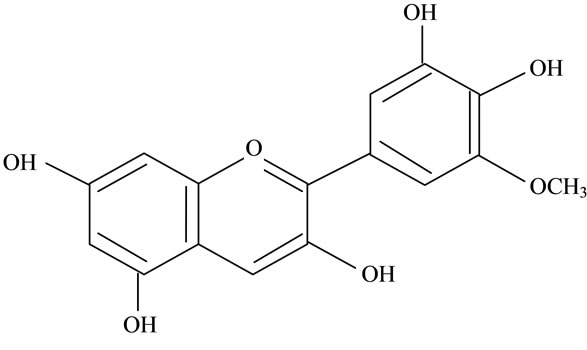 (h)
(h)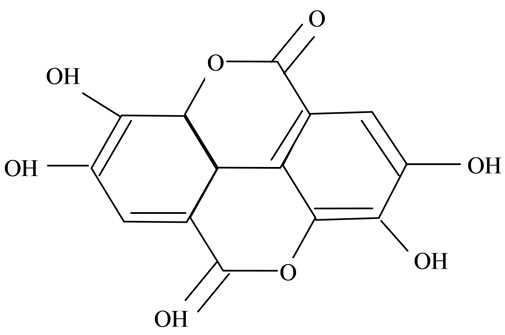 (i)
(i)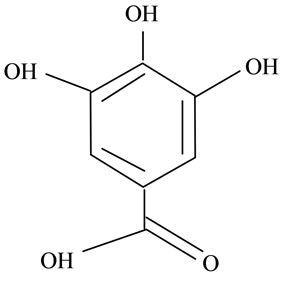 (j)
(j)
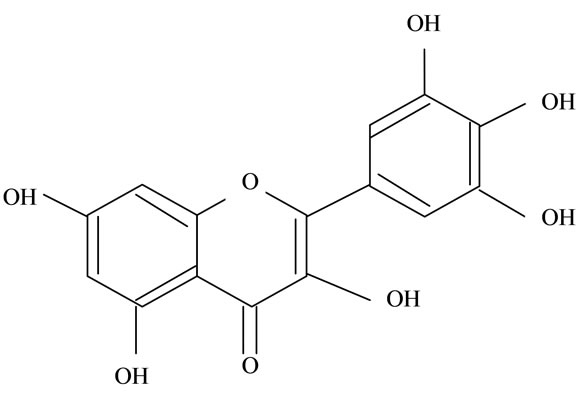
Figure 2. Structures of phytochemicals in Jamun reported to be of use in the treatment of Cancer. (a) Myricetin; (b) Kaempherol; (c) Quercetin; (d) Betulinic; (e) Anthocyanin; (f) Delphinidin; (g) Malvidian; (h) Petunidin; (i) Ellagic Acid; (j) Gallic Acid.
metabolite, urolithin A inhibit want signaling crucial in the process of colon carcinogenesis [106].
5.2. Radioprotective Effects
The affect felt by the normal cells are irreparable damage, leading to the untoward effects forcing the physicians to discontinue or reduce the treatment dose. In such situations, an agent that can render a therapeutic differential between the cancer and normal cell will be highly beneficial. Studies have shown that the intraperitoneal administration of the hydroalcoholic extract of the Jamun seed and the dichloromethane extract of Jamun leaf possess radioprotective effects [107]. Therapeutic differential may be achieved with chemical compounds that may selectively protect the normal cells from the deleterious effects of radiation termed as radio protectors. Since the observations of [108] that the natural amino acid cysteine protected mice against radiation-induced sickness and mortality, many compounds with varied pharmacological properties have been synthesized and evaluated for their radioprotective effects. Pretreatment with hydroalcoholic extract of jamun seeds (5 to 160 mg/kg body weight) for five consecutive days before exposure to supralethal dose of radiation (10 Gy) protected mice against the radiationinduced sickness and mortality. The best effect was observed at 80 mg/kg but only when administered through the intraperitoneal route as 50% of the animals survived when compare to 22% in the oral route and none in the radiation alone cohorts. The intraperitoneal administration of the organic extract (dichloromethane-methane) of leaves (5, 10, 20, 30, 40, 50, 60 and 80 mg/kg b. wt.) for five days before irradiation was also observed to be effective in preventing the radiation-induced sickness and mortality in mice. Histopathological investigations showed that Jamun leaf treatment before radiation elevated the villus height, the number of crypts and reduced the goblet and dead cells when compared with the concurrent irradiation control. The recovery and regeneration was faster in Jamun pretreated animals than the irradiation alone. Jamun extracts also provides protection to the DNA against the radiation-induced DNA damage (explained later). The phytochemicals ellagic acid, gallic acid, quercetin and oleanolic acid (Figure 2) present in Jamun also possess radioprotective effects (addressed in Table 3).
5.3. Antineoplastic Effects of Jamun
Chemotherapy has been an important modality in cancer treatment for more than five decades and is an obligatory treatment modality when metastasis has ensued. Depending on the clinical stage and the patient compliance, chemotherapy is used either alone or in combination with radiation and surgery [118]. Studies suggest that of all the antineoplastic drugs being used nearly 47% of the drugs are from natural sources [119]. With regard to Jamun many compounds exert beneficial influence (Figure 2 and Table 4).
In vitro studies by [177] have shown that whole Jamun extract possess cytotoxic effects on the cultured human cervical cancer cells, the HeLa (HPV-18 positive) and SiHa (HPV-16 positive). The extract caused a concentration dependent cell death with the effect being more pronounced in the HeLa than SiHa cells [177]. Additionally, both crude as well as the methanolic extracts of the pulp caused a time dependent increase in apoptosis when cultured with 80% concentration of the extracts. The crude extract was observed to be better than the methanolic extract in both the cell lines [177]. In a study that has wide clinical implications, recent studies by [57] have shown that the standardized Jamun fruit extract possess antiproliferative and pro-apoptotic effects in the estrogen dependent aromatase positive (MCF-7aro), and estrogen independent (MDA-MB-231) breast cancer cells. The extract was highly effective against MCF-7aro and the IC50 was observed to be 27 μg/ml to that of 40 μg/ml in MDAMB-231. Most importantly, at equivalent
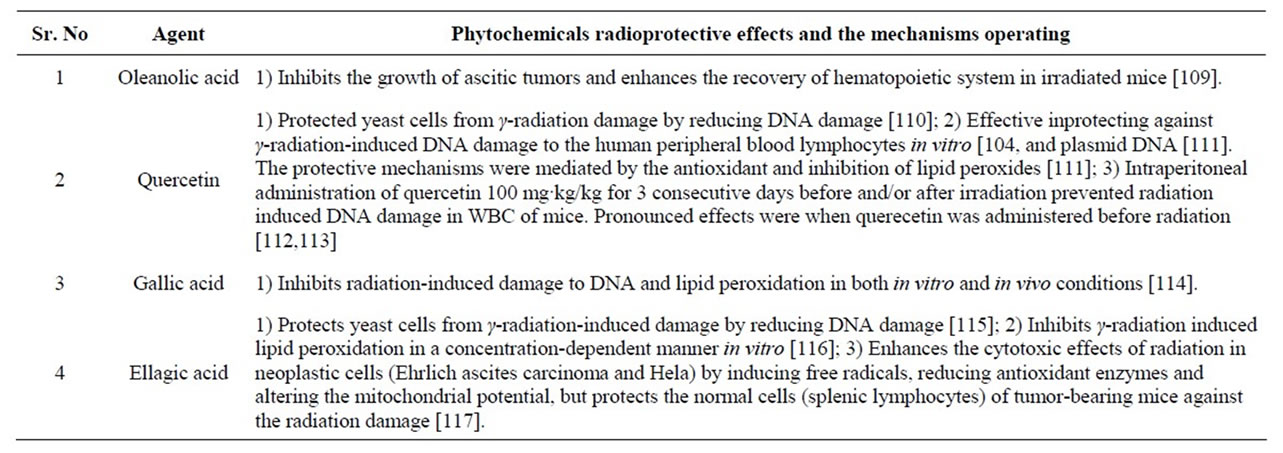
Table 3. Phytochemicals of Jamun with reported radioprotective activities.
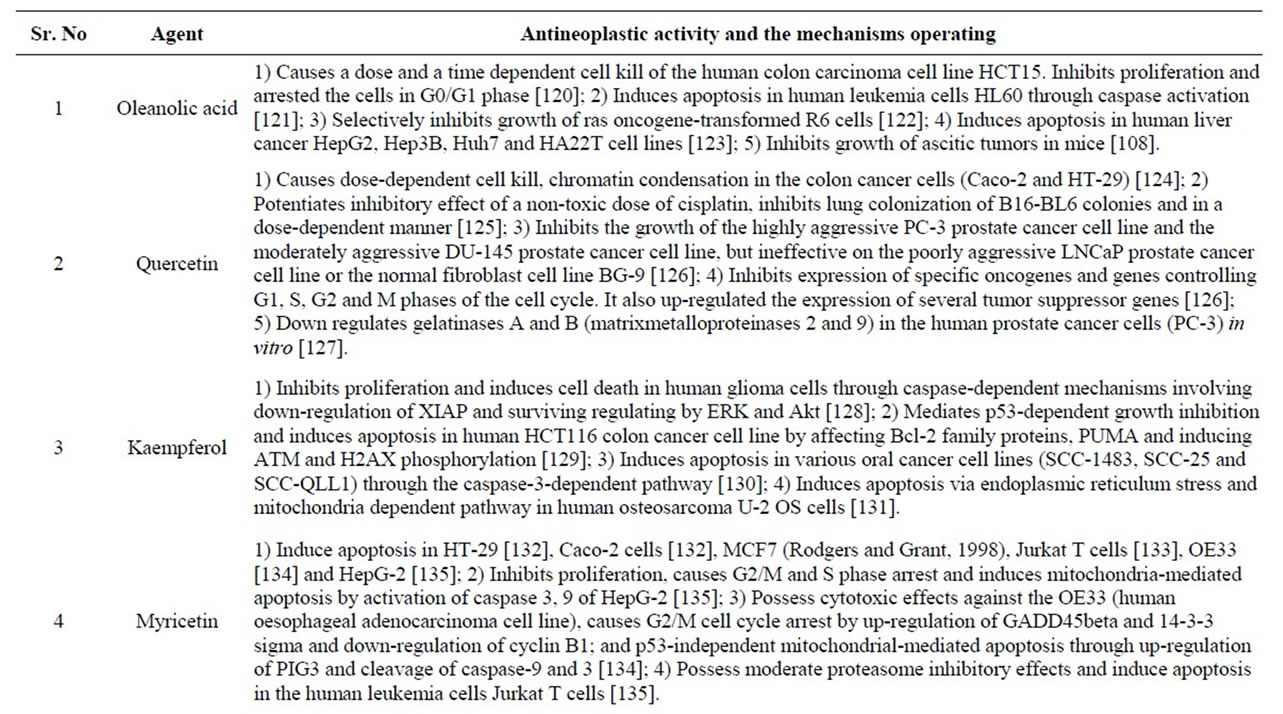
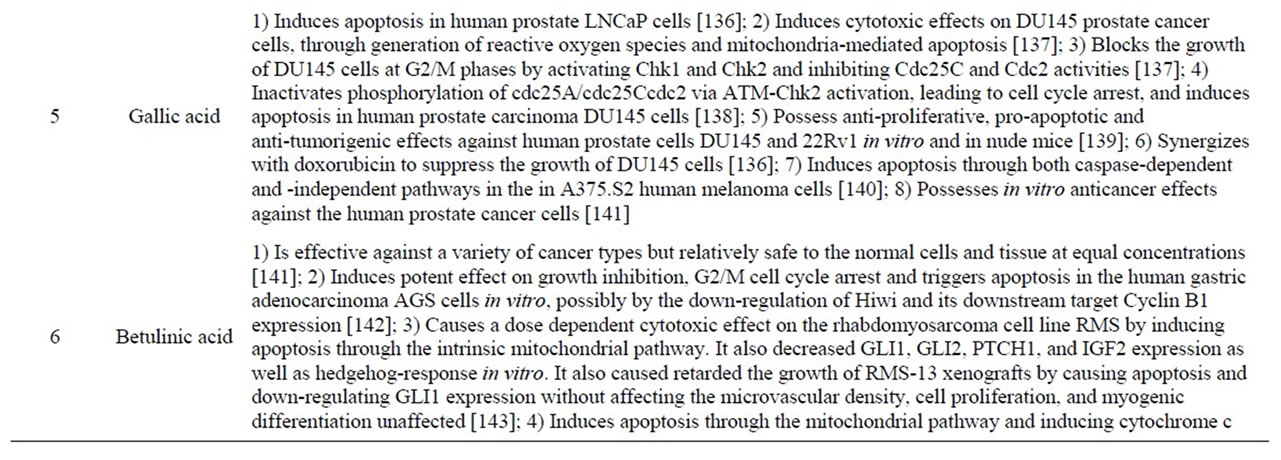


Table 4. Phytochemicals in Jamun with reported antineoplastic activities.
concentrations the extract was relatively non toxic as it did not induce cell death and apoptosis in the normal/ nontumorigenic (MCF-10A) breast cell line (IC50 > 100 μg/ml). Together these results clearly indicate that at supra dietary levels the fruit pulp extract possesses selective antineoplastic effects against breast cancer [57].
6. Conclusion
Jambolan is traditionally used for the treatment of various diseases especially diabetes and related complications. Most pharmacological works on diabetes were carried out with seeds but the pharmacological potential of the other parts of the plant is required to explore in detail. With regard to the antineoplastic activities studies suggest that Jamun is selective in its action in breast cancer cells. The effect of Jamun and its phytochemicals should also be investigated for its chemopreventive effects in other models of carcinogens, that includes chemical, radiation and viral carcinogenesis models. Mechanistic studies responsible for the chemopreventive and radioprotective effects are also lacking and need to be studied in detail. Based on these facts, this review highlights the role of jambolan in various treatments and recommend that further phytochemical and clinical research should be done on this traditional medicinal plant for the discovery of safer drugs. Studies should also be on understanding which of the phytochemicals are responsible for the observed beneficially effects and if effective, their mechanism of action.
REFERENCES
- P. Warrier, V. Nambiar and C. Ramankutty, “Indian Medical Plants,” Vol. 5, Orient Longman Ltd., Hyderabad, 1996, pp. 225-228.
- H. Sagrawat, A. Mann and M. Kharya, “Pharmacological Potential of Eugenia Jambolana: A Review,” Pharmacogenesis Magazice, Vol. 2, 2006, pp. 96-104.
- P. Wester, Journal of food Plants of the Philippines, Bulletin 39, 3rd Edition, Philippine Department of Agriculture & Natural Research, Burette of Agriculture, Manila, 1924.
- H. Munsell, L. Williams, L. Guild, C. Troescher, G. Nightingale and R. Harris, “Composition of Food Plants of Central America,” Food Research, Vol. 14, No. 2, 1949, pp. 144-164. doi:10.1111/j.1365-2621.1949.tb16218.x
- A. Ranjan, A. Jaiswal and B. Raja, “Enhancement of Syzygium cumini (Indian Jamun) Active Constituents by Ultra-Violent (UV) Irradiation Method,” Scientific Research and Essays, Vol. 6, No. 12, 2011, pp. 2457-2464.
- E. Giovannucci, E. Rimm and Y. Liu, “A Prospective Study of Tomato Products, Lycopene, and Prostate Cancer Risk,” Journal of Natural and Cancer Natural, Vol. 94, No. 5, 2002, pp. 391-398
- K. Lock, D. Stuckler, K. Charlesworth and M McKee, “Potential Uses and Health Effects of Indian Raspberry,” British Homeopathic Journal, Vol. 339, 2009, pp. 459- 452.
- A. Kumar, T. Jayachandran and P. Aravindhan, “Neutral Components in the Leaves and Seeds of Syzygium cumini,” African Journal of Pharmacy and Pharmacology, Vol. 3, No. 11, 2009, pp. 560-561.
- The US Department of Agriculture and the US Department of Health and Human, “Dietary Guidelines for Americans,” 2010. http://ndb.nal.usda.gov/2012
- M. Baliga, P. Bhat and B. Baliga, “Phytochemistry, Traditional Uses and Pharmacology of Eugenia jambolana Lam. (Black Plum): A Review,” Food Research International, Vol. 44, No. 7, 2011, pp. 1776-1789. doi:10.1016/j.foodres.2011.02.007
- A. Benthall, “Trees of Calcutta and Its Neigh Borhood. Thacker,” Spink & Co. Ltd., Calcutta, 1946.
- W. H. Brown, “Wild Food Plants of the Philippines,” Bulletin 21, Department of Agriculture and Natural Resources, Bureau of Printing, Manila, 1920.
- G. Lai, G. Siddappa and G. L. Tandon, “Preservation of Fruits and Vegetables,” Indian Council of Agriculture Research, New Delhi, 1960.
- W. Kennard and H. Winters, “Some Fruits and Nuts for the Tropics,” Misc. Pub. 801, Agricultural Research Service, US Dept. Agric, Washington, 1960.
- I. H. Burkill, “A Dictionary of the Economic Products of the Malay Peninsula,” Vol. 1, Crown Agents for the Colonies, London, 1935.
- E. Quisumbing, “Medicinal Plants of the Philip Pines,” Tech. Bui. 16, Department of Agriculture and Natural Resource, Manila, 1951.
- C. D. Miller, K. Bazore and M. Bartow, “Fruits of Hawaii,” 2nd Edition, University of Hawaii Press, Hawaii, 1955.
- O. W. Barrett, “The Tropical Crops,” The Macmillan Co., New York, 1928.
- W. Harris, “Notes on Fruits and Vegetables in Jamaica,” Government Printing Office, Kingston, 1913.
- J. Dastur, “Useful Plants of India and Pakistan,” 2nd Edition, D. B. Taraporevala Sons & Co., Mumbai, 1943.
- R. Namasivayam, B. Ramachandrani and M. Deecaraman, “Effect of Aqueous Extract of Syzygium cumini Pulp on Antioxidant Defense System in Streptozotocin Induced Diabetic Rats,” International Journal of Post Harvest Technology, Vol. 7, 2008, pp. 137-145.
- A. Ratsimamanga, A. Loiseau and S. Ratsimamanga, “Action of a Hypoglycemic Agent Found in the Young Bark of Eugenia jambolania [sic] (Myrtaceae) on Induced Hyperglycemia of the Rabbit and Continuation of Its Purification,” Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences D: Sciences Naturelles, Vol. 277, 1973, pp. 2219-2222.
- N. Chaudhuri, A. Pal and S. Gomes, “Anti-Inflammatory and Related Action of Syzygium cumini Seed Extract,” Phytotherapy Research, Vol. 4, No. 2, 1990, pp. 5-10. doi:10.1002/ptr.2650040103
- P. Prince and M. Venon, “Effect of Syzigium cumini in Plasma Antioxidants on Alloxan-Induced Diabetes in Rats,” Journal of Clinical Biochemistry and Nutrition, Vol. 25, No. 2, 1998, pp. 81-86. doi:10.3164/jcbn.25.81
- G. Sepaha and S. Bose, “Clinical Observations on the Antidiabetic Properties of Pterocarpus marsupium and Eugenia jambolana,” Journal of the Indian Medical Association, Vol. 27, 1956, pp. 388-391.
- Helmstadter, “Syzygium cumini (L.) Skeels (Myrtaceae) Against Diabetes: 125 Years of Research,” Pharmazie, Vol. 63, No. 2, 2008, pp. 91-101.
- J. Giri, T. Sathidevi and N. Dushyanth, “Effect of Jamun Seed Extract on Alloxan Induced Diabetes in Rats,” Journal of the Diabetic Association of India, Vol. 25, 1985, pp. 115-119.
- K. Reynertson. A. Basile and M. J. Kennelly, “Antioxidant Potential of Seven Mystaceous Fruits,” Ethnobotany Research and Applications, Vol. 3, 2005, pp. 25-35
- S. K. Jain, “Dictonary of Indian Folk Medicine and Ethnobotany,” Deep Publications Paschimvihar, New Delhi, 1991.
- D. C. Damasceno, G. T. Volpato and I. D. Calderon, “Study of Averrhoa carambola and Eugenia jambolana Extracts Purchased from Manipulation Drug Store on the Experimental Diabetes,” Revista Brasileira de Toxicologia, Vol. 15, 2002, pp. 9-14.
- S. B. Sridhar, U. D. Sheetal and M. R. Pai, “Preclinical Evaluation of the Antidiabetic Effect of Eugenia jambolana Seed Powder in Streptozotocin-Diabetic Rats,” Brazilian Journal of Medical and Biological Research, Vol. 38, No. 3, 2005, pp. 463-468. doi:10.1590/S0100-879X2005000300018
- K. Ravi, S. Rajasekaran and S. Subramanian, “Hypoglycemic Effect of Eugenia jambolana Seed Kernels on Streptozotocin-Induced Diabetes in Rats,” Pharmaceutical Biology, Vol. 41, No. 8, 2003, pp. 598-603. doi:10.1080/13880200390501929
- S. B. Sharma, A. Nasir and K. M. Prabhu, “Hypoglycaemic and Hypolipidemic Effect of Ethanolic Extract of Seeds of Eugenia jambolana in Alloxan-Induced Diabetic Rabbits,” Journal of Ethnopharmacology, Vol. 85, No. 2, 2003, pp. 201-206. doi:10.1016/S0378-8741(02)00366-5
- S. B. Sharma, A. Nasir and K. M. Prabhu, “Antihyperglycemic Effect of the Fruit-Pulp of Eugenia jambolana in Experimental Diabetes Mellitus,” Journal of Ethnopharmacology, Vol. 104, 2006, pp. 367-373. doi:10.1016/j.jep.2005.10.033
- K. Ravi, B. Ramachandran and S. Subramanian, “Protective Effect of Eugenia jambolana Seed Kernel on Tissue Antioxidants in Streptozotocin-Induced Diabetic Rats,” Biological & Pharmaceutical Bulletin, Vol. 27, No. 8, 2009, pp. 1212-1217. doi:10.1248/bpb.27.1212
- V. Vikrant, J. K. Grover and N. Tandon, “Treatment with Extracts of Momordica Charantia and Eugenia Jambolana Prevents Hyperglycemia and Hyperinsulinemia in Fructosefed Rats,” Journal of Ethnopharmacology, Vol. 76, No. 2, 2001, pp. 139-143. doi:10.1016/S0378-8741(01)00218-5
- Y. Srivastava, H. Venkatakrishna-Bhatt and O. P. Gupta, “Hypoglycemia Induced by Syzygium cumini Linn Seeds in Diabetes Mellitus,” Asiam Medical Journal, Vol. 26, No. 7, 1983, pp. 489-491.
- A. Gordon and E. Jungfer, “Phenolic Constituents and Antioxidant Capacity of Four Underutilized Fruits from the Amazon Region,” Journal of Agricultural and Food Chemistry, Vol. 59, 2011, pp. 7688-7699. doi:10.1021/jf201039r
- F. Adelia, C. Marcella and Z. Mercadante, “Identification of Bioactive Compounds from Jambolao (Syzygium cumini) and Antioxidant Capacity Evaluation in Different pH Conditions,” Food Chemistry, Vol. 126, No. 4, 2011, pp. 1571-1578. doi:10.1016/j.foodchem.2010.12.007
- J. Kubola, S. Siriamornpun and N. Meeso, “Phytochemicals, Vitamin C and Sugar Content of Thai Wild Fruits,” Food Chemistry, Vol. 126, No. 3, 2011, pp. 972-981. doi:10.1016/j.foodchem.2010.11.104
- B. Chaudhary and K. Mukhopadhyay, “Syzygium cumini (L.) Skeels: A Potential Source of Nutraceuticals,” International Journal of Pharmacy and Biological Sciences, Vol. 2, No. 1, 2012, pp. 46-53.
- A. C. Simoes-Pires, S. Vargas, A. Marston, et al., “Ellagic Acid Derivatives from Syzygium cumini Stem Bark: Investigation of Their Antiplasmodial Activity,” Natural Product Communications, Vol. 4, No. 10, 2009, pp. 1371- 1376.
- S. Sapana, V. Jadhav and V. Kadam, “Development and Validation of HPTLC Method for Determination of 3-Hydroxy Androstane [16, 17-C] (6’methyl, 2’-1-hydroxy-isopropene-1-yl) 4,5,6 H Pyran in Jambul Seed (Syzygium cumini),” International Journal of PharmTech Research, Vol. 1, 2009, pp. 1129-1135.
- M. Qurat and M. Ali, “Prawez, Lignan Derivatives from the Stem Bark of Syzygium cumini (L.) Skeels,” Natural Product Research, Vol. 23, 2009, pp. 422-430. doi:10.1080/14786410802038697
- L. Liya, Z. Yanjun and S. Navindra, “Structure of Anthocyanins from Eugenia jambolana Fruit,” Natural Product Communications, Vol. 4, No. 2, 2009, pp. 217-219.
- S. Gowri, S. Vasantha and K. Phytochemical, “Screening and Antibacterial Activity of Syzygium cumini (L.) (Myrtaceae) Leaves Extracts,” International Journal of PharmTech Research, Vol. 2, No. 2, 2010, pp. 1569- 1573.
- A. Reynertson, Y. Hui and B. Jiang, “Quantitative Analysis of Antiradical Phenolic Constituents from 4 Teen Edible Myrtaceae Fruits,” Food Chemistry, Vol. 109, 2008, pp. 883-890. doi:10.1016/j.foodchem.2008.01.021
- E. F. Steinmetz, “Drug Guide Published by Author,” Amsterdam, 1959.
- P. Wren, “Potter’s New Cyclopedia of Botanical Drugs and Preparations,” Sir Isaac Pitman & Sons, Ltd., London, 1956.
- D. Barh and G. Viswanathan, “Syzygium cumini Inhibits Growth and Induces Apoptosis in Cervical Cancer Cell Lines: A Primary Study,” Ecancermedicalscience, Vol. 2, 2008, p. 83.
- G. Jagetia and M. Baliga, “Syzygium cumini (Jamun) Reduces the Radiation-Induced DNA Damage in the Cultured Human Peripheral Blood Lymphocytes: A Preliminary Study,” Toxicology Letters, Vol. 132, No. 1, 2002, pp. 19-25. doi:10.1016/S0378-4274(02)00032-2
- G. Jagetia and M. Baliga, “Evaluation of the Radioprotective Effect of the Leaf Extract of Syzygium cumini (Jamun) in Mice Exposed to a Lethal Dose of Gamma-Irradiation,” Nahrung, Vol. 47, 2003, pp. 181-185. doi:10.1002/food.200390042
- G. Jagetia, M. Baliga and P. Venkatesh, “Influence of Seed Extract of Syzygium cumini (Jamun) on Mice Exposed to Different Doses of Gamma-Radiation,” Journal of Radiation Research, Vol. 46, No. 1, 2005, pp. 59-65. doi:10.1269/jrr.46.59
- G. Jagetia, P. Shetty and M. S. Vidyasagar, “Treatment of Mice with Leaf Extract of Jamun (Syzygium cumini linn. Skeels) Protects against the Radiation-Induced Damage in the Intestinal Mucosa of Mice Exposed to Different Doses of γ-Radiation,” Pharmacologyonline, Vol. 1, 2008, pp. 169-195.
- J. Parmar, P. Sharma and P. Verma, “Chemopreventive Action of Syzygium Cumini on DMBA-Induced Skin Papillomagenesis in Mice,” Asian Pacific Journal of Cancer Prevention, Vol. 11, No. 1, 2010, pp. 261-265.
- R. Rastogi and B. Mehrotra, “Compendium of Indian Medicinal Plants,” Central Drug Research Institute, Lucknow, Vol. 1, 1990, pp. 388-389.
- L. Li, L. Adams and S. Chen, “Eugenia jambolana Lam. Berry Extract Inhibits Growth and Induces Apoptosis of Human Breast Cancer but Not Non-Tumorigenic Breast Cells,” Journal of Agriculture and Food Chemistry, Vol. 57, No. 3, 2009, pp. 826-831. doi:10.1021/jf803407q
- J. Veigas, M. Narayan and P. Laxman, “Chemical Nature, Stability and Bio Efficacies of Anthocyanins from Fruit Peel of Syzygium cumini Skeels,” Food Chemistry, Vol. 10, 2007, pp. 619-627. doi:10.1016/j.foodchem.2007.04.022
- A. Ranjan, A. Jaiswal and B. Raja, “Enhancement of Syzygium cumini (Indian Jamun) Active Constituents by Ultra-Violent (UV) Irradiation Method,” Scientific Research and Essays, Vol. 6, No. 12, 2011, pp. 2457-2464.
- P. Shafi, M. Rosamma and K. Jamil, “Antibacterial Activity of Syzygium cumini and Syzygium travancoricum Leaf Essential Oils,” Fitoterapia, Vol. 73, 2002, pp. 414- 416. doi:10.1016/S0367-326X(02)00131-4
- E. F. Steinmetz, “A Botanical Drug from the Tropics Used in the Treatment of Diabetes Mellitus,” Acta Phytotherapeutica, Vol. 7, No. 2, 1960, pp. 23-25.
- B. Mukerji, “Indigenous Indian Drugs Used in the treatment of Diabetes,” Journal of Scientific & Industrial Research, Vol. 10, 1951, pp. 1-18.
- S. S. Bhatnagar, H. Santapau, F. Fernandes, V. N. Kamat, N. J. Dastoor and T. S. N. Rao, “Physiological Activity of Indian Medicinal Plants,” Journal of Scientific & Industrial Research, Vol. 8, 1961, pp. 1-24.
- E. Drafehn, “Syzygium jambolana. Apoth. Ztg,” Chemical Abstracts, Vol. 50, 1987, pp. 112-114.
- A. G. R. Nair and S. S. Subramanian, “Chemical Evaluation of the Flowers of Eugenia jambolana,” Journal of Scientific & Industrial Research, Vol. 21B, No. 9, 1962, pp. 457-458.
- J. Ferlay, H. R. Shin and F. Bray, “Estimates of Worldwidee Burden of Cancer in 2008: GLOBOCAN 2008,” International Journal of Cancer, Vol. 127, No. 12, 2010, pp. 2893-2917. doi:10.1002/ijc.25516
- C. Are, L. Colburn and S. Rajaram. “Disparities in Cancer Care between the United States of America and India and Opportunities for Surgeons to Lead,” Journal of Surgical Oncology, Vol. 102, No. 1, 2010, pp. 100-105. doi:10.1002/jso.21579
- P. K. Warrier, V. P. K. and N. C. Ramankutty, “Indian Med Plants,” Orient Longman Ltd., Hyderabad, Vol. 5, 1996, pp. 225-228.
- B. B. Aggarwal, M. E. Van Kuiken and L. H. Iyer, “Molecular Targets of Nutraceuticals Derived from Dietary Spices: Potential Role in Suppression of Inflammation and Tumorigenesis,” Experimental Biology and Medicine, Vol. 234, No. 8, 2009, pp. 825-849. doi:10.3181/0902-MR-78
- P. K. Goyal, P. Verma and P. Sharma, “Evaluation of Anti-Cancer and Anti-Oxidative Potential of Syzygium cumini against Benzo[a]pyrene (BaP) Induced Gastric Carcinogenesis in Mice,” Asian Pacific Journal of Cancer Prevention, Vol. 11, 2010, pp. 753-758.
- J. Parmar, P. Sharma and P. Verma, “Chemopreventive Action of Syzygium cumini on DMBA-Induced Skin Papillomagenesis in Mice,” Asian Pacific Journal of Cancer Prevention, Vol. 11, 2010, pp. 261-265.
- M. Sharma, L. Li and J. Celver, “Effects of Fruit Ellagitannin Extracts, Ellagic Acid, and Their Colonic Metabolite, Urolithin A, on Wnt Signaling,” Journal of Agriculture Food Chemistry, Vol. 58, No. 7, 2010, pp. 3965- 3969. doi:10.1021/jf902857v
- H. Tokuda, H. Ohigashi and K. Koshimizu, “Inhibitory Effects of Ursolic and Oleanolic Acid on Skin Tumor Promotion by 12-O-tetradecanoylphorbol-13-acetate,” Cancer Letters, Vol. 33, No. 3, 1986, pp. 279-285. doi:10.1016/0304-3835(86)90067-4
- N. Janakiram, C. Indranie and S. Malisetty, “Chemoprevention of Colon Carcinogenesis by Oleanolic Acid and Its Analog in Male F344 Rats and Modulation of COX-2 and Apoptosis in Human Colon HT-29 Cancer Cells,” Pharmaceutical Research, Vol. 25, No. 9, 2008, pp. 2151-2157. doi:10.1007/s11095-008-9582-7
- R. Furtado, E. Rodrigues and F. Araujo, “Ursolic Acid and Oleanolic Acid Suppress Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon,” Toxicology and Pathology, Vol. 36, No. 4, 2008, pp. 576-580. doi:10.1177/0192623308317423
- H. Gali, E. Perchellet and D. Klish, “Antitumorpromoting Activities of Hydrolyzable Tannins in Mouse Skin,” Carcinogenesis, Vol. 13, 1992, pp. 715-718. doi:10.1093/carcin/13.4.715
- A. Kaul and K. Khanduja, “Polyphenols Inhibit Promotional Phase of Tumorigenesis: Relevance of Superoxide Radicals,” Nutrional and Cancer, Vol. 32, No. 2, 1998, pp. 81-85. doi:10.1080/01635589809514723
- H. Mukhtar, B. Del and C. Marcelo, “Ellagic Acid: A Potent Naturally Occurring Inhibitor of Benzo[a]pyrene Metabolism and Its Subsequent Glucuronidation, Sulfation and Covalent Binding to DNA in Cultured BALB/ C Mouse Keratinocytes,” Carcinogenesis, Vol. 5, No. 12, 1984, pp. 1565-1571. doi:10.1093/carcin/5.12.1565
- B. Del, H. Mukhtar and D. Bickers, “Inhibition of Epidermal Metabolism and DNA-Binding of Benzo[a]pyrene by Ellagic Acid,” Biochemical and Biophysical Research Communication, Vol. 114, No. 1, 1983, pp. 388-394. doi:10.1016/0006-291X(83)91639-X
- H. Mukhtar, M. Das and D. Bickers, “Inhibition of 3-Methylcholanthrene-Induced Skin Tumorigenicity in BALB/c Mice by Chronic Oral Feeding of Trace Amounts of Ellagic Acid in Drinking Water,” Cancer Research, Vol. 46, 1986, pp. 2262-2265.
- R. Chang, M. Huang and A. Wood, “Effect of Ellagic Acid and Hydroxylated Flavonoids on the Tumorigenicity of Benzo[a]pyrene and (+/−)-7 Beta, 8 Alpha-Dihydroxy- 9 Alpha, 10 Alpha-Epoxy-7,8,9,10-tetrahydrobenzo[a] pyrene on Mouse Skin and in the Newborn Mouse,” Carcinogenesis, Vol. 6, 1985, pp. 1127-1133. doi:10.1093/carcin/6.8.1127
- P. Lesca, “Protective Effects of Ellagic Acid and Other Plant Phenols on Benzo [a] Pyrene-Induced Neoplasia in Mice,” Carcinogenesis, Vol. 4, No. 12, 1983, pp. 1651- 1653. doi:10.1093/carcin/4.12.1651
- H. Mukhtar, M. Das and B. Del, “Protection against 3-Methylcholanthrene-Induced Skin Tumorigenesis in Balb/C Mice by Ellagic Acid,” Biochemical and Biophysical Research Communication, Vol. 119, No. 2, 1984, pp. 751-757. doi:10.1016/S0006-291X(84)80314-9
- K. Raina, S. Rajamanickam and G. Deep, “Chemopreventive Effects of Oral Gallic Acid Feeding on Tumor Growth and Progression in TRAMP Mice,” Molecular and Cancer Therapy, Vol. 7, No. 5, 2008, pp. 1258-1267. doi:10.1158/1535-7163.MCT-07-2220
- H. Makita, T. Tanaka and H. Fujitsuka, “Chemoprevention of 4-Nitroquinoline 1-Oxide-Induced Rat Oral Carcinogenesis by the Dietary Flavonoids Chalcone, 2-Hydroxychalcone, and Quercetin,” Cancer Research, Vol. 56, No. 21, 1996, pp. 4904-4909.
- K. Khanduja, R. Gandhi and V. Pathania, “Prevention of N-Nitrosodiethylamine-Induced Lung Tumorigenesis by Ellagic Acid and Quercetin in Mice,” Food Chemistry and Toxicology, Vol. 37, No. 4, 1999, pp. 313-318. doi:10.1016/S0278-6915(99)00021-6
- S. De, J. Chakraborty and R. Chakraborty, “Chemopreventive Activity of Quercetin during Carcinogenesis in Cervix Uteri in Mice,” Phototherapy Research, Vol. 14, 2000, pp. 347-351. doi:10.1002/1099-1573(200008)14:5<347::AID-PTR613>3.0.CO;2-7
- S. De, R. Chakraborty and S. Ghosh, “Comparative Evaluation of Cancer Chemopreventive Efficacy of Alphatocopherol and Quercetin in a Murine Model,” Journal of Experience and Clinical Cancer Research, Vol. 23, 2004, pp. 251-258.
- A. Sengupta, S. Ghosh and S. Das, “Modulation of DMBA Induced Genotoxicity in Bone Marrow by Quercetin during Skin Carcinogenesis,” Journal of Experience and Clinical Cancer Research, Vol. 20, 2001, pp. 131- 134.
- T. Walle, U. Walle and D. Sedmera, “Benzo [A] Pyreneinduced Oral Carcinogenesis and Chemoprevention: Studies in Bioengineered Human Tissue,” Drug Metabolism and Disposition, Vol. 34, No. 3, 2006, pp. 346-350.
- S. Kamaraj, R. Vinodhkumar and P. Anandakumar, “The Effects of Quercetin on Antioxidant Status and Tumor Markers in the Lung and Serum of Mice Treated with Benzo (a) Pyrenean,” Biological and Pharmaceutical Bulletin, Vol. 30, No. 12, 2007, pp. 2268-2273. doi:10.1248/bpb.30.2268
- T. Kumamoto, M. Fujii and D. Hou, “Myricetin Directly Targets JAK1 to Inhibit Cell Transformation,” Cancer Letters, Vol. 275, No. 1, 2009, pp. 17-26. doi:10.1016/j.canlet.2008.09.027
- T. Kumamoto, M. Fujii and D. Hou, “Akt Is a Direct Target for Myricetin to Inhibit Cell Transformation,” Molecular and Cell Biochemistry, Vol. 332, No. 1, 2009, pp. 33-41. doi:10.1007/s11010-009-0171-9
- A. Baskar, S. Ignacimuthu and G. Paulraj, “Chemopreventive Potential of Beta-Sitosterol in Experimental Colon Cancer Model—An in Vitro and in Vivo Study,” BMC Complementary and Alternative Medicine, Vol. 10, 2010, p. 24. doi:10.1186/1472-6882-10-24
- D. Hou, K. Kai and J. Li, “Anthocyanidins Inhibit Activator Protein 1 Activity and Cell Transformation: Structure-Activity Relationship and Molecular Mechanisms,” Carcinogenesis, Vol. 25, 2004, pp. 29-36. doi:10.1093/carcin/bgg184
- D. Syed, Y. Suh and F. Afaq, “Dietary Agents for Chemoprevention of Prostate Cancer,” Cancer Letters, Vol. 26, 2008, pp. 167-176. doi:10.1016/j.canlet.2008.02.050
- K. Lee, N. Kang and J. Han, “Myricetin Down-Regulates Phorbol Ester-Induced Cyclooxygenase-2 Expression in Mouse Epidermal Cells by Blocking Activation of Nuclear Factor Kappa B,” Journal of Agriculture and Food Chemistry, Vol. 55, 2007, pp. 9678-9684. doi:10.1021/jf0717945
- K. Lee, N. Kang and E. Rogozin, “Myricetin Is a Novel Natural Inhibitor of Neoplastic Cell Transformation and MEK1,” Carcinogenesis, Vol. 28, No. 9, 2007, pp. 1918- 1927. doi:10.1093/carcin/bgm110
- S. Lee and J. Lin, “Inhibitory Effects of Phytopolyphenols on TPA-Induced Transformation, PKC Activation, and c-Jun Expression in Mouse Fibroblast Cells,” Nutrition and Cancer, Vol. 28, 1997, pp. 177-183. doi:10.1080/01635589709514572
- S. Jung, K. Lee and S. Byun, “Myricetin Suppresses UVB-Induced Skin Cancer by Targeting Fyn,” Cancer Research, Vol. 68, No. 14, 2008, pp. 6021-6029. doi:10.1158/0008-5472.CAN-08-0899
- W. Kim, H. Yang and H. Youn, “Myricetin Inhibits Akt Survival Signaling and Induces Bad-Mediated Apoptosis in a Low Dose Ultraviolet (UV)-B-Irradiated HaCaT Human Immortalized Keratinocytes,” Journal of Radiation Research, Vol. 51, No. 3, 2010, pp. 285-296. doi:10.1269/jrr.09141
- C. Ko, S. Shen and T. Lee, “Myricetin Inhibits Matrix Metalloproteinase 2 Protein Expression and Enzyme Activity in Colorectal Carcinoma Cells,” Molecular Cancer Therapeutics, Vol. 4, No. 2, 2005, pp. 281-290.
- M. Das, W. Khan and P. Asokan, “Inhibition of Polycyclic Aromatic Hydrocarbon-DNA Adduct Formation in Epidermis and Lungs of SENCAR Mice by Naturally Occurring Plant Phenols,” Cancer Research, Vol. 47, No. 3, 1987, pp. 767-773.
- K. Lee, D. Lee and S. Seo, “Phosphatidylinositol 3- Kinase, a Novel Target Molecule for the Inhibitory Effects of Kaempferol on Neoplastic Cell Transformation,” Carcinogenesis, Vol. 31, No. 8, 2010, pp. 1338-1343. doi:10.1093/carcin/bgq102
- K. Yasukawa, M. Takido and T. Matsumoto, “Sterol and Triterpene Derivatives from Plants Inhibit the Effects of a Tumor Promoter, and Sitosterol and Betulinic Acid Inhibit Tumor Formation in Mouse Skin Two-Stage Carcinogenesis,” Oncology, Vol. 48, No. 1, 1991, pp. 72-76. doi:10.1159/000226898
- J. Kwon, K. Lee, J. Kim, S. K. Jung, N. J. Kang, M. K. Hwang, Y. S. Heo, A. M. Bode, Z. Dong and H. J. Lee, “Delphinidin Suppresses Ultraviolet B-Induced Cyclooxygenases-2 Expression through Inhibition of MAPKK4 and PI-3 Kinase,” Carcinogenesis, Vol. 30, No. 11, 2009, pp. 1932-1940. doi:10.1093/carcin/bgp216
- G. Jagetia and M. Baliga, “The Evaluation of Nitric Oxide Scavenging Activity of Certain Indian Medicinal Plants in Vitro: A Preliminary Study,” Journal of Medicinal Food, Vol. 7, No. 3, 2004, pp. 343-348.
- H. Patt , E. B. Tyree, R. L.Straube and D. E. Smith, “Cysteine Protection against X Irradiation,” Science, Vol. 110, No. 2582, 1949, pp. 213-214. doi:10.1126/science.110.2852.213
- H. Hsu, J. Yang and C. Lin, “Effects of Oleanolic Acid and Ursolic Acid on Inhibiting Tumor Growth and Enhancing the Recovery of Hematopoietic System Post Irradiation in Mice,” Cancer Letters, Vol. 111, No. 1, 1997, pp. 7-13. doi:10.1016/S0304-3835(96)04481-3 P. Nemavarkar, B. Chourasia and K. Pasupathy, “Evaluation of Radioprotective Action of Compounds Using Saccharomyces cerevisiae,” Journal of Environmental Pathology, Toxicology and Oncology, Vol. 23, No. 2, 2004, pp. 145-151. doi:10.1615/JEnvPathToxOncol.v23.i2.70
- V. Benkovic, N. Kopjar and Horvat, “Evaluation of Radioprotective Effects of Propolis and Quercetin on Human White Blood Cells in Vitro,” Biological and Pharmaceutical Bulltein, Vol. 31, 2008, pp. 1778-1785. doi:10.1248/bpb.31.1778
- V. Benkovic, A. Knezevic and D. Dikic, “Radioprotective Effects of Propolis and Quercetin in Gamma-Irradiated Mice Evaluated by the Alkaline Comet Assay,” Phytomedicine, Vol. 15, 2008, pp. 851-858. doi:10.1016/j.phymed.2008.02.010
- V. Benkovic, A. Knezevic and D. Dikic “Radioprotective Effects of Quercetin And Ethanolic Extract of Propolis in Gamma-Irradiated Mice,” Archives of Industrial Hygiene and Toxicology, Vol. 60, No. 2, 2009, pp. 129-138. doi:10.2478/10004-1254-60-2009-1908
- N. Gandhi and C. Nair, “Protection of DNA and Membrane from Gamma Radiation Induced Damage by Gallic Acid,” Molecular and Cellular Biochemistry, Vol. 278, No. 1-2, 2005, pp. 111-117. doi:10.1007/s11010-005-6940-1
- P. Nemavarkar, B. Chourasia and K. Pasupathy, “Evaluation of Radioprotective Action of Compounds Using Saccharomyces cerevisiae,” Journal of Environmental Pathology Toxicology and Oncology, Vol. 23, No. 2, 2004, pp. 145-151. doi:10.1615/JEnvPathToxOncol.v23.i2.70
- K. Priyadarsini, S. Khopde and S. Kumar, “Free Radical Studies of Ellagic Acid, a Natural Phenolic Antioxidant,” Journal of Agriculture and Chemistry, Vol. 50, 2000, pp. 2200-2206. doi:10.1021/jf011275g
- S. Bhosle, N. Huilgol and K. Mishra, “Enhancement of Radiation-Induced Oxidative Stress and Cytotoxicity in Tumor Cells by Ellagic Acid,” Clinica Chimica Acta, Vol. 359, 2005, pp. 89-100. doi:10.1016/j.cccn.2005.03.037
- V. DeVita, T. Lawrence and S. Hellman, “Cancer: Principles & Practice of Oncology,” 8th Edition, Wolters Kluwer/Lippincott Williams & Wilkins a Wolters Kluwer Business, Philadelphia, 2004.
- R. Arun, M. Prakash and S. Abraham, “Role of Syzygium Cumini Seed Extract in the Chemoprevention of in Vivo Genomic Damage and Oxidative Stress,” Journal of Ethnopharmacology, Vol. 134, No. 2, 2010, pp. 329-333. doi:10.1016/j.jep.2010.12.014
- D. Barh and G. Viswanathan, “Syzygium cumini Inhibits Growth and Induces Apoptosis in Cervical Cancer Cell Lines: A Primary Study,” Ecancermedicalscience, Vol. 2, 2008, p. 83.
- J. Li, W. Guo and Q. Y. Yang, “Effects of Ursolic Acid and Oleanolic Acid on Human Colon Carcinoma Cell Line HCT15,” World Journal of Gastroenterology, Vol. 8, No. 3, 2002, pp. 493-495.
- P. Zhang, H. Li and D. Chen, “Oleanolic Acid Induces Apoptosis in Human Leukemia Cells through Caspase Activation and Poly (ADP-Ribose) Polymerase Cleavage,” Acta Biochemical and Biophysics Sinces, Vol. 39, No. 10, 2007, pp. 803-809. doi:10.1111/j.1745-7270.2007.00335.x
- P. Wu, W. Chi and Z. T. Liang, “Oleanolic Acid Isolated from Oldenlandia Diffusa Exhibits a Unique Growth Inhibitory Effect against Ras-Transformed Fibroblasts,” Life Sciences, Vol. 85, No. 3-4, 2009, pp. 113-121. doi:10.1016/j.lfs.2009.04.025
- S. Yan, C. Huang and S. Wu, “Oleanolic Acid and Ursolic Acid Induce Apoptosis in Four Human Liver Cancer Cell Lines,” Toxicology in Vitro, Vol. 24, No. 3, 2010, pp. 842-848. doi:10.1016/j.tiv.2009.12.008
- S. Kuo, “Antiproliferative Potency of Structurally Distinct Dietary Flavonoids on Human Colon Cancer Cells,” Cancer Letters, Vol. 110, No. 1-2, 1996, pp. 41-48. doi:10.1016/S0304-3835(96)04458-8
- S. Caltagirone, C. Rossi and A. Poggi, “Flavonoids Apigenin and Quercetin Inhibit Melanoma Growth and Metastatic Potential,” International Journal of Cancer, Vol. 87, 2000, pp. 595-600. doi:10.1002/1097-0215(20000815)87:4<595::AID-IJC21>3.0.CO;2-5
- H. Nair, K. Rao and R. Aalinkeel, “Inhibition of Prostate Cancer Cell Colony Formation by the Flavonoid Quercetin Correlates with Modulation of Specific Regulatory Genes,” Clinical and Diagnostic Laboratory Immunology, Vol. 11, No. 1, 2004, pp. 63-69.
- M. Vijayababu, A. Arunkumar and P. Kanagaraj, “Quercetin Downregulates Matrix Metalloproteinases 2 and 9 Proteins Expression in Prostate Cancer Cells (PC-3),” Molecular and Cellular Biochemistry, Vol. 287, No. 1-2, 2006, pp. 109-116. doi:10.1007/s11010-005-9085-3
- J. Jeong, M. Kim and T. Kim, “Kaempferol Induces Cell Death through ERK and Akt-Dependent Down-Regulation of XIAP and Survivin in Human Glioma Cells,” Neurochemical Research, Vol. 34, No. 5, 2009, pp. 991- 1001. doi:10.1007/s11064-008-9868-5
- W. Li, B. Du and T. Wang, “Kaempferol Induces Apoptosis in Human HCT116 Colon Cancer Cells via the Ataxiatelangiectasia Mutated-p53 Pathway with the Involvement of p53 Upregulated Modulator of Apoptosis,” Chemco-Biological Interactions, Vol. 177, 2009, pp. 121- 127. doi:10.1016/j.cbi.2008.10.048
- J. W. Kang, J. H. Kim, K. Song, S. H. Kim, J. H. Yoon, and K. S. Kim, “Kaempferol and Quercetin, Components of Ginkgo Biloba Extract (EGb 761), Induce Caspase- 3-Dependent Apoptosis in Oral Cavity Cancer Cells,” Phytotherapy Research (Special Issue: Flavonoids and Polyphenolics), Vol. 24, No. S1, 2010, pp. S77-S82.
- W. Huang, Y. Chiu and M. Fan, “Kaempferol Induced Apoptosis via Endoplasmic Reticulum Stress and Mitochondria-Dependent Pathway in Human Osteosarcoma U-2 OS Cells,” Molecular Nutrition and Food Research, Vol. 54, No. 11, 2010, pp. 1585-1595. doi:10.1002/mnfr.201000005
- S. Kuntz, U. Wenzel and H. Daniel, “Comparative Analysis of the Effects of Flavonoids on Proliferation, Cytotoxicity, and Apoptosis in Human Colon Cancer Cell Lines,” European Journal of Nutrition, Vol. 38, 1999, pp. 133-142. doi:10.1007/s003940050054
- D. Chen, K. Daniel and M. Chen, “Dietary Flavonoids as Proteasome Inhibitors and Apoptosis Inducers in Human Leukemia Cells,” Biochemical Pharmacology, Vol. 69, No. 10, 2005, pp. 1421-1432. doi:10.1016/j.bcp.2005.02.022
- Q. Zhang, X. Zhao and Z. Wan, “Flavones and Flavonols Exert Cytotoxic Effects on a Human Oesophageal Adenocarcinoma Cell Line (OE33) by Causing G2/M Arrest and Inducing Apoptosis,” Food and Chemical Toxicology, Vol. 46, No. 6, 2008, pp. 2042-2053. doi:10.1016/j.fct.2008.01.049
- X. Zhang, Y. Ling and H. Yu, “Studies on Mechanism of Myricetin-Induced Apoptosis in Human Hepatocellular Carcinoma HepG-2 Cells,” China Journal of Chinese Materia Medica, Vol. 35, No. 8, 2010, pp. 1046-1050.
- L. Reddivari, J. Vanamala and S. Safe, “The Bioactive Compounds Alpha-Chaconine and Gallic Acid in Potato Extracts Decrease Survival and Induce Apoptosis in LNCaP and PC3 Prostate Cancer Cells,” Nutrition and Cancer, Vol. 62, No. 5, 2010, pp. 601-610. doi:10.1080/01635580903532358
- H. Chen, Y. Wu and Y. Chia, “Gallic Acid, a Major Component of Toona Sinensis Leaf Extracts, Contains a ROS Mediated ROS Mediated Anti-Cancer Activity in Human Prostate Cancer Cells,” Cancer Letters, Vol. 286, 2009, pp. 161-171. doi:10.1016/j.canlet.2009.05.040
- C. Agarwal, A. Tyagi and R. Agarwal, “Gallic Acid Causes Inactivating Phosphorylation of cdc25A/cdc25Ccdc2 via ATM-Chk2 Activ Tion, Leading to Cell Cycle Arrest, and Induces Apoptosis in Human Prostate Carcinoma DU145 Cells,” Molecular Cancer Therapeutics, Vol. 5, No. 12, 2006, pp. 3294-3302. doi:10.1158/1535-7163.MCT-06-0483
- M. Kaur, B. Velmurugan and S. Rajamanickam, “Gallic Acid, an Active Constituent of Grape Seed Extract, Exhibits Anti-Proliferative, Pro-Apoptotic and Anti-Tumorigenic Effects against Prostate Carcinoma Xenograft Growth in Nude Mice,” Pharmaceutical Research, Vol. 26, No. 9, 2009, pp. 2133-2140.
- C. Lo, T. Lai and J. Yang, “Gallic Acid Induces Apoptosis in A375.S2 Human Melanoma Cells through Caspase-Dependent and -Independent Pathways,” International Journal of Oncology, Vol. 37, No. 2, 2010, pp. 377-385. doi:10.1007/s11095-009-9926-y
- T. Rabi, S. Shukla and S. Gupta, “Betulinic Acid Suppresses Constitutive and TNF Alpha-Induced NF-KappaB Activation and Induces Apoptosis in Human Prostate Carcinoma PC-3 Cells,” Molecular Carcinogenecis, Vol. 47, 2008, pp. 964-973. doi:10.1002/mc.20447
- L. Yang, Y. Chen and Q. Ma, “Effect of Betulinic Acid on the Regulation of Hiwi and Cyclin B1 in Human Gastric Adenocarcinoma AGS Cells,” Acta Pharmacologica Sinica, Vol. 31, No. 1, 2010, pp. 66-72. doi:10.1038/aps.2009.177
- M. Eichenmüller, B. Hemmerlein and D. Schweinitz, “Betulinic Acid Induces Apoptosis and Inhibits Hedgehog Signalling in Rhabdomyosarcoma,” British Journal of Cancer, Vol. 103, No. 1, 2010, pp. 43-51. doi:10.1038/sj.bjc.6605715
- F. Mullauer, J. Kessler and J. Medema, “Betulinic Acid Induces Cytochrome c Release and Apoptosis in a Bax, Bakindependent, Permeability Transition Pore Dependent Fashion,” Apoptosis, Vol. 14, No. 2, 2009, pp. 191-202. doi:10.1007/s10495-008-0290-x
- Z. Chen, Q. Wu and Y. Chen, “Effect of Betulinic Acid on Proliferation and Apoptosis in Jurkat Cells and Its Mechanism,” China Journal of Chinese Materia Medica, Vol. 30, 2008, pp. 588-592.
- H. Moteki, H. Hibasami and Y. Yamada, “Specific Induction of Apoptosis by 1,8-Cineole in Two Human Leukemia Cell Lines, but Not a in Human Stomach Cancer Cell Line,” Oncology Reports, Vol. 9, 2002, pp. 757-760.
- A. Awad and R. von J. Cone, “Beta-Sitosterol Inhibits Growth of HT-29 Human Colon Cancer Cells by Activating the Sphingomyelin Cycle,” Anticancer Research, Vol. 18, 1998, pp. 471-473.
- A. Awad, Y. Chen and C. Fink, “Beta-Sitosterol Inhibits HT-29 Human Colon Cancer Cell Growth and Alters Membrane Lipids,” Anticancer Research, Vol. 16, 1996, pp. 2797-2804.
- A. Awad, A. Downie and C. Fink, “Inhibition of Growth and Stimulation of Apoptosis by Beta-Sitosterol Treatment of MDA-MB-231 Human Breast Cancer Cells in Culture,” International Journal of Molecular Medicine, Vol. 5, 2000, pp. 541-545.
- A. Awad, Y. Gan and C. Fink, “Effect of Beta-Sitosterol, a Plant Sterol, on Growth, Protein Phosphatase 2A, and Phospholipase D in LNCaP Cells,” Nutrion and Cancer, Vol. 36, No. 1, 2000, pp. 74-78. doi:10.1207/S15327914NC3601_11
- A. Awad, A. Downie and C. Fink, “Dietary Phytosterol Inhibits the Growth and Metastasis of MDA-MB-231 Human Breast Cancer Cells Grown in SCID Mice,” Anticancer Research, Vol. 20, No. 2A, 2000, pp. 821- 824.
- A. Awad, B. C. Fink and H. Williams, “In Vitro and in Vivo (SCID Mice) Effects of Phytosterols on the Growth and Dissemination of Human Prostate Cancer PC-3 Cells,” European Journal of Cancer Prevention, Vol. 10, No. 6, 2001, pp. 507-513. doi:10.1097/00008469-200112000-00005
- A. Awad, R. Roy and C. Fink, “Beta-Sitosterol, a Plant Sterol, Induces Apoptosis and Activates Key Caspases in MDAMB-231 Human Breast Cancer Cells,” Oncology Reports, Vol. 10, 2003, pp. 497-500.
- A. Awad, H. Williams and C. Fink, “Phytosterols Reduce in Vitro Metastatic Ability of MDA-MB-231 Human Breast Cancer Cells,” Nutrition and Cancer, Vol. 40, No. 2, 2001, pp. 157-164. doi:10.1207/S15327914NC402_12
- Y. Choi, K. Kong and Y. Kim, “Induction of Bax and Activation of Caspases during Beta-Sitosterol-Mediated Apoptosis in Human Colon Cancer Cells,” International Journal of Oncology, Vol. 23, 2003, pp. 1657-1662.
- D. Moon , K. Lee and Y. Choi, “Beta-Sitosterolinduced Apoptosis Is Mediated by the Activation of ERK and the Downregulation of Akt in MCA-102 Murine Fibrosarcoma Cells,” International Immunopharmacology, Vol. 7, No. 8, 2007, pp. 1044-1053. doi:10.1016/j.intimp.2007.03.010
- D. Moon, M. Kim and Y. Choi, “Beta-Sitosterol Induces G2/M Arrest, Endoreduplication, and Apoptosis through the Bcl-2 and PI3K/Akt Signaling Pathways,” Cancer Letters, Vol. 264, No. 2, 2008, pp. 181-191. doi:10.1016/j.canlet.2008.01.032
- Y. Zhao, S. Chang and G. Qu, “Beta-Sitosterol Inhibits Cell Growth and Induces Apoptosis in SGC-7901 Human Stomach Cancer Cells,” Journal of Agriculture and Food Chemistry, Vol. 57, 2009, pp. 5211-5218. doi:10.1021/jf803878n
- N. Seeram, Y. Zhang and M. Nair, “Inhibition of Proliferation of Human Cancer Cells and Cyclooxygenase Enzymes by Anthocyanidins and Catechins,” Nutrition and Cancer, Vol. 46, No. 1, 2003, pp. 101-106. doi:10.1207/S15327914NC4601_13
- M. Lazzre, M. Savio and R. Pizzala, “Anthocyanins Induce Cell Cycle Perturbations and Apoptosis in Different Human Cell Lines,” Carcinogenesis, Vol. 25, No. 8, 2004, pp. 1427-1433. doi:10.1093/carcin/bgh138
- N. Katsube, K. Iwashita and T. Tsushida, “Induction of Apoptosis in Cancer Cells by Bilberry (Vaccinium Myrtillus) and the Anthocyanins,” Journal of Agriculture and Food Chemistry, Vol. 51, No. 6, 2003, pp. 68-75. doi:10.1021/jf025781x
- S. Meiers, M. Kemény and U. Weyand, “The Anthocyanidins Cyanidin and Delphinidin Are Potent Inhibitors of the Epidermal Growth-Factor Receptor,” Journal of Agriculture and Food Chemistry, Vol. 49, No. 2, 2001, pp. 958-962. doi:10.1021/jf0009100
- D. Hou and T. Ose, “Anthocyanidins Induce Apoptosis in Human Promyelocytic Leukemia Cells: Structure Activity Relationship and Mechanisms Involved,” International Journal of Oncology, Vol. 23, No. 3, 2003, pp. 705-712.
- D. Marko, N. Puppel and Z. Tjaden, “The Substitution Pattern of Anthocyanidins Affects Different Cellular Signaling Cascades Regulating Cell Proliferation,” Molecular Nutrition and Food Research, Vol. 48, No. 4, 2004, pp. 318-325. doi:10.1002/mnfr.200400034
- Y. Zhang, S. Vareed and M. Nair, “Human Tumor Cell Growth Inhibition by Nontoxic Anthocyanidins, the Pigments in Fruits and Vegetables,” Life Sciences, Vol. 76, No. 13, 2005, pp. 1465-1472. doi:10.1016/j.lfs.2004.08.025
- A. Srivastava, C. Akoh and J. Fischer, “Effect of Anthocyanin Fractions from Selected Cultivars of Georgiagrown Blueberries on Apoptosis and Phase II Enzymes,” Journal of Agriculture and Food Chemistry, Vol. 55, 2007, pp. 3180-3185. doi:10.1021/jf062915o
- D. N. Syed, F. Afaq and S. Sarfaraz, “Delphinidin Inhibits Cell Proliferation and Invasion via Modulation of Met receptor Phosphorylation,” Toxicology and Applied Pharmacology, Vol. 231, No. 1, 2008, pp. 52-60. doi:10.1016/j.taap.2008.03.023
- D. Fridrich, N. Teller and M. Esselen, “Comparison of Delphinidin, Quercetin and (-)-Epigallocatechin-3-Gallate as Inhibitors of the EGFR and the ErbB2 Receptor Phosphorylation,” Molecular Nutrional and Food Research, Vol. 52, 2008, pp. 815-822. doi:10.1002/mnfr.200800026
- F. Afaq, N. Zaman and N. Khan, “Inhibition of Epidermal Growth Factor Receptor Signaling Pathway by Delphinidin, an Anthocyanidin in Pigmented Fruits and Vegetables,” International Journal of Cancer, Vol. 123, No. 7, 2008, pp. 1508-1515. doi:10.1002/ijc.23675
- J. Yun, F. Afaq and N. Khan, “Delphinidin, an Anthocyanidin in Pigmented Fruits and Vegetables, Induces Apoptosis and Cell Cycle Arrest in Human Colon Cancer HCT116 Cells,” Molecular Carcinogenesis, Vol. 48, 2009, pp. 260-270. doi:10.1002/mc.20477
- B. Hafeez, I. Siddiqui and M. Asim, “A Dietary Anthocyanidin Delphinidin Induces Apoptosis of Human Prostate Cancer PC3 Cells in Vitro and in Vivo: Involvement of Nuclear Factor Kappab Signaling,” Cancer Research, Vol. 68, No. 20, 2008, pp. 8564-8572. doi:10.1158/0008-5472.CAN-08-2232
- N. Kang, K. Lee and J. Kwon, “Delphinidin Attenuates Neoplastic Transformation in JB6 Cl41 Mouse Epidermal Cells by Blocking Raf/Mitogen-Activated Protein Kinase Kinase/Extracellular Signal-Regulated Kinase Signaling,” Cancer Prevention Research, Vol. 1, No. 7, 2008, pp. 522-531. doi:10.1158/1940-6207.CAPR-08-0071
- D. Shin, C. Ryu and W. Lee, “Induction of Apoptosis and Inhibition of Invasion in Human Hepatoma Cells by Anthocyanins from Meoru,” Annals New Yark Academy of Sciences, Vol. 1171, No. 1, 2009, pp. 137-148. doi:10.1111/j.1749-6632.2009.04689.x
- J. Cvorovic, F. Tramer and M. Granzotto, “Oxidative Stress-Based Cytotoxicity of Delphinidin and Cyanidin in Colon Cancer Cells,” Archives of Biochemistry and Biophysics, Vol. 501, 2010, pp. 151-157. doi:10.1016/j.abb.2010.05.019
- N. Teller, W. Thiele and U. Boettler, “Delphinidin Inhibits a Broad Spectrum of Receptor Tyrosine Kinases of the ErbB and VEGFR Family,” Molecular Nutrition and Food Research, Vol. 53, No. 9, 2009, pp. 1075-1083. doi:10.1002/mnfr.200800524
- C. Yeh and G. Yen, “Induction of Apoptosis by the Anthocyanidins through Regulation of Bcl-2 Gene and Activation of c-Jun N-Terminal Kinase Cascade in Hepatoma Cells,” Journal of Agriculture and Food Chemistry, Vol. 53, No. 9, 2005, pp. 1740-1749. doi:10.1021/jf048955e
NOTES
*Corresponding author.

