Food and Nutrition Sciences
Vol. 3 No. 1 (2012) , Article ID: 17082 , 8 pages DOI:10.4236/fns.2012.31011
Some Factors Affecting the Production of Carotenoids by Rhodotorula glutinis var. glutinis
![]()
Food Science and Technology Department, Faculty of Agriculture, University of Alexandria, Alexandria, Egypt.
Email: *amrbanna47@gmail.com
Received September 15th, 2011; revised October 19th, 2011; accepted October 26th, 2011
Keywords: Rhodotorula glutinis; Carotenoids; Yeast; Factors Affecting Carotenoids Production
ABSTRACT
A new yeast strain isolated from pin cushion flower (Scabiosa atropurpura) in our laboratory was selected from 200 yeast isolates as carotenoids producer and identified as Rhodotorula glutinis var. glutinis. The selected isolate was grown in synthetic medium to study the effect of carbon to nitrogen ratio, sources of nitrogen and carbon, mineral salts and incubation temperature on carotenoids production. The results indicated the following optimal conditions: carbon to nitrogen ratio of 5, ammonium sulphate as nitrogen source, sucrose as carbon source, presence of zinc sulphate in the medium and cultivation temperature of 25˚C. The studied factors affected the dry biomass as well as the proportion of carotenoids and consequently the colour of pellets of the yeast. The yeast strain was grown under the optimal conditions to study the changes occurring in the medium and the pellets during carotenoids production for 6 days. Carotenoids production started after the first day of incubation and most of the carotenoids content in the yeast cells was produced during stationary phase. The highest cellular (861 μg∙g–1) and volumetric (1.9 mg∙L–1) carotenoids content were obtained after 5 days of growth.
1. Introduction
Carotenoids comprise a class of natural fat-soluble pigments which are found in numerous fruits, vegetables and microorganisms. The consumption of a diet rich in carotenoids has been epidemiologically correlated with a lower risk for several diseases. The antioxidant activity of carotenoids and biochemical properties influencing signaling pathways is well documented [1,2]
Carotenoids pigment biosynthesis is a characteristic ability of the genus Rhodotorula [3]. The carotenoids yield, the type and concentration of various pigments that form carotenoids depend on species, strain, medium constituents and environmental conditions [4-6].
Synthetic media consisting of carbon source, inorganic salts, organic and/or inorganic nitrogen source and growth factors have been suggested by many authors for the production of carotenoids from Rhodotorula glutinis [5,7-11].
The effect of carbon to nitrogen (C/N) ratio, sources of nitrogen and carbon, mineral salts and incubation temperature on growth and carotenoids production by Rhodotorula glutinis have been studied by many authors [12- 19].
This work describes the effect of some factors on production of carotenoids by an isolate of Rhodotorula glutinis var. glutinis in synthetic medium. Studied factors included C/N ratio, sources of nitrogen and carbon, mineral salts and incubation temperature. Changes occurring in the medium and the pellets during carotenoids production, under the optimum growth conditions, for 6 days were also studied.
2. Materials and Methods
2.1. Materials
All chemicals were of analytical grade and obtained from different sources. Yeast extract, malt extract, peptone, casein hydrolysate and soy peptone were obtained from Oxoid (UK) and Biolife (Italy). Glucose syrup and high fructose corn syrup (HFCS) were obtained from local market.
A new strain of Rhodotorula glutinis var. glutinis, a promising carotenoids producer, was obtained from an extensive screening programme carried out in our laboratory. This strain was isolated from pin cushion flower (Scabiosa atropurpurea). It was maintained on yeast extract-malt extract agar (YMA) slant, transferred every month, and stored at 4˚C until needed.
Inoculum was prepared as follows: Ten loopful from 48 h old pure culture of Rhodotorula glutinis var. glutinis grown on YMA slant at 30˚C, were transferred to 500 mlflasks each containing 50 ml of synthetic medium (SM). The inoculated flasks were incubated by shaking in an orbital incubator (Model INR-200, Gallenkamp, UK) until absorbance at 570 nm reached its maximum (24 hours).
SM contained basal medium (BM) and glucose (25 g∙L–1). BM consists of (g∙L–1): (NH4)2∙SO4 (5), MgSO4∙7H2O (0.5), yeast extract (1), KH2PO4 (6.4) and K2HPO4 (6.4).
2.2. Factors Affecting Carotenoids Production
The following factors were studied using one variable at time method.
2.2.1. C/N Ratio of Cultivation Medium
Various concentrations of glucose (6.25, 12.5, 25.0, 37.5 and 50 g∙L–1) were added to BM to give the corresponding C/N ratios (2.5, 5, 10, 15 and 20, respectively). Yeast cultures were grown under specified conditions (SC): an initial pH of 6.2 ± 0.1, a temperature of 30˚, inoculum size of 1%, ratio of medium to flask volume of 1:5, agitation of 100 rpm and incubation time of 4 days.
2.2.2. Nitrogen Sources
Ammonium sulphate of SM was replaced by one of the following nitrogen sources: peptone, urea, ammonium nitrate, soy peptone, casein hydrolysate. The amounts of glucose and each nitrogen source were adjusted to give C/N ratio of 10. Yeast cultures were grown under SC.
2.2.3. Carbon Sources
Several carbon sources [glucose, sucrose, glucose syrup and high fructose corn syrup (HFCS)], were added to BM. The concentration of each carbon source was adjusted to give C/N ratio of 10. Yeast cultures were grown under SC for 3 days.
2.2.4. Mineral Salts
Magnesium sulphate of the SM was replaced by each of the studied salts (ZnSO4∙7H2O, MnSO4∙H2O, CaCl2 at concentration of 0.1 g∙L–1 and FeSO4∙7H2O, CuSO4∙5H2O at concentration of 0.01 g∙L–1). Also, SM with and without MgSO4∙7H2O were used for comparison. Yeast cultures were grown under SC. Media containing MnSO4 and CaCl2 had pH of 5.23 and 5.4, respectively.
2.2.5. Cultivation Temperature
Yeast cultures were grown in synthetic medium under SC except the cultivation temperature were 5, 15, 25, 35 in addition to 30˚C. Incubation time was 3 days.
2.3. Production of Carotenoids under Optimum Conditions
Yeast culture was grown under the most optimum conditions. It was grown in SM containing zinc sulphate (0.1 g∙L–1) instead of magnesium sulphate (0.5 g∙L–1) and sucrose (12.5 g∙L–1) instead of glucose (25 g∙L–1) under SC but at 25˚C, for 0 to 6 days.
2.4. Determinations of Viable Count and Yeast Dry Biomass
Viable yeast count (colony forming units) was carried out using YMA medium and both pour and spread techniques [20]. Plates were incubated at 30˚C for 48 - 72 hours before counting colony forming units (CFU∙ml–1).
Yeast dry biomass was determined by drying a known weight of the yeast pellet, which prepared by centrifugation of culture at 800× g for 5 minutes, at 85˚C to constant weight [21]. Dry biomass was expressed as g∙L–1 of culture.
2.5. Colour Measurement
The colour of yeast pellets was matched by Munsell colour charts [22].
2.6. Extraction and Quantification of Total Carotenoids
Total carotenoids were extracted from yeast pellets by solvents fortified by butylated hydroxyanisole as antioxidant to minimize the autoxidation of carotenoids [23]. The absorbance of the hexane carotenoids extract was measured at 485 nm. Total carotenoids content, as torulene, of the yeast cells was calculated and expressed as μg∙g–1 of dry biomass and as mg∙L–1 of culture. The extinction coefficient of torulene in hexane 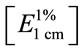 2680 was used [5].
2680 was used [5].
2.7. Quantification of Individual Carotenoids
The carotenoids extract was concentrated by evaporation of hexane solvent using a stream of nitrogen gas and kept at –8˚C under nitrogen in dark. The crude carotenoids extract was chromatographed on thin layer of a mixture of silica gel G60 and calcium hydroxide(1:1 w/w) using 5% benzene in petroleum ether (b.p. 80˚C - 100˚C) as developing system [24]. The separated carotenoids were identified by their maximum absorption [25,26] and also by their Rf values. For the quantification of individual carotenoids a simple method was used to avoid laborious separation. It is a spectrophotmetric multicomponent analysis method which is based on the assumption that the absorbencies for each component can be added in a linear manner to the absorbencies of any other component. This method was used to determine the three major carotenoids in the crude extract namely β-carotene, torulene and torularhodin directly without chromatographic separation [2]. At first the absorbance of crude carotenoids extract was spectrophotometrically measured at 500 nm, which represent the maximum absorption of torularhodin. The crude carotenoids extract (5 ml) was treated with calcium hydroxide (0.25 g) to chelate the carboxylic carotenoid torularhodin and the calcium hydroxide torularhodin chelate was removed by centrifugation at 3500 rpm (900× g) for 5 minutes. The absorbance of the torularhodin free carotenoids extract was remeasured at 500 nm and the torularhodin content (µg∙ml–1) was obtained by subtraction of absorbance at 500 nm before and after chelation and using the torularhodin extinction coefficient 2040 [25]. After that the absorbencies of the torularhodin free carotenoids extract were measured at 450 nm for β-carotene and 485 nm for torulene .At the same time known concentrations of standard β-carotene and isolated and purified torulene were measured each at the two aforementioned wave lengths. These values were used to calculate the concentrations of β-carotene and torulene in the carotenoids extract with the following interference Equations [27].
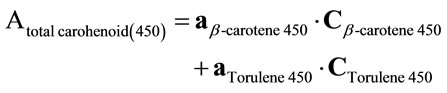
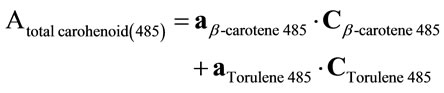
where
Atotal carotenoid (450), (485) = the absorbencies of torularhodin free carotenoids extract at 450 and 485 nm.
aβ-carotene (450), (485) = calculated constant obtained from absorbencies at 450 and 485 nm and the concentration of standard β-carotene.
aTorulene (450), (485) = calculated constant obtained from absorbencies at 450 and 485 nm and the concentration of standard torulene .
Cβ-carotene (450), (485) = The concentration of β-carotene in torularhodin free carotenoidsextract µg∙ml–1.
CTorulene (450), (485) = The concentration of torulene in torularhodin free carotenoids extract µg∙ml–1.
2.8. Chemical Determinations
Reducing sugars were determined by Somogyi and Nelson method [28]. Total reducing sugars were estimated by the same method after being hydrolyzed by HCl (Sp. Gr. 1.1029) at 60˚C for 10 min. [29] and non reducing sugars as sucrose were calculated. The distillation method of the A.O.A.C. was used to assay the ammonia content in the yeast culture supernatant [29].
3. Results and Discussion
3.1. C/N Ratio of Cultivation Medium
The dry biomass of Rhodotorula glutinis var. glutinis increased as the C/N ratio increased up to 15 (Table 1). Highest cellular carotenoids were observed in pellets of the culture cultivated in medium with C/N ratio of 5. However, pellets had highest volumetric carotenoids when cultivated with C/N ratio of 10. It was found [9] that cultivation medium with low C/N ratio, 10, resulted in a high volumetric production of carotenoids by Rhodotorula glutinis. In addition C/N ratio of cultivation medium affected the proportion of carotenoids and conesquently the colour of pellets (Table 1).
3.2. Nitrogen Sources
Varying the nitrogen sources in the culture medium affected dry biomass, carotenoid production and proportion of carotenoids (Table 2). Maximum dry biomass was occurred in medium containing casein hydrolysate. Cultivation of Rhodotorula glutinis var. glutinis in me-
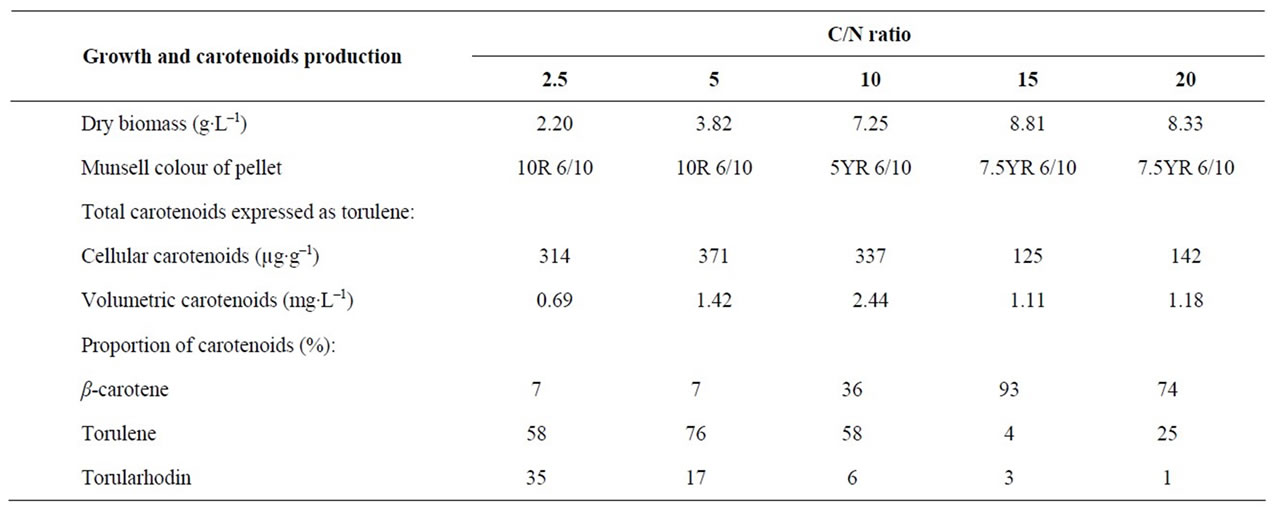
Table 1. Effect of C/N ratio of cultivation medium on biomass and carotenoids produced by Rhodotorula glutinis var. glutinis.
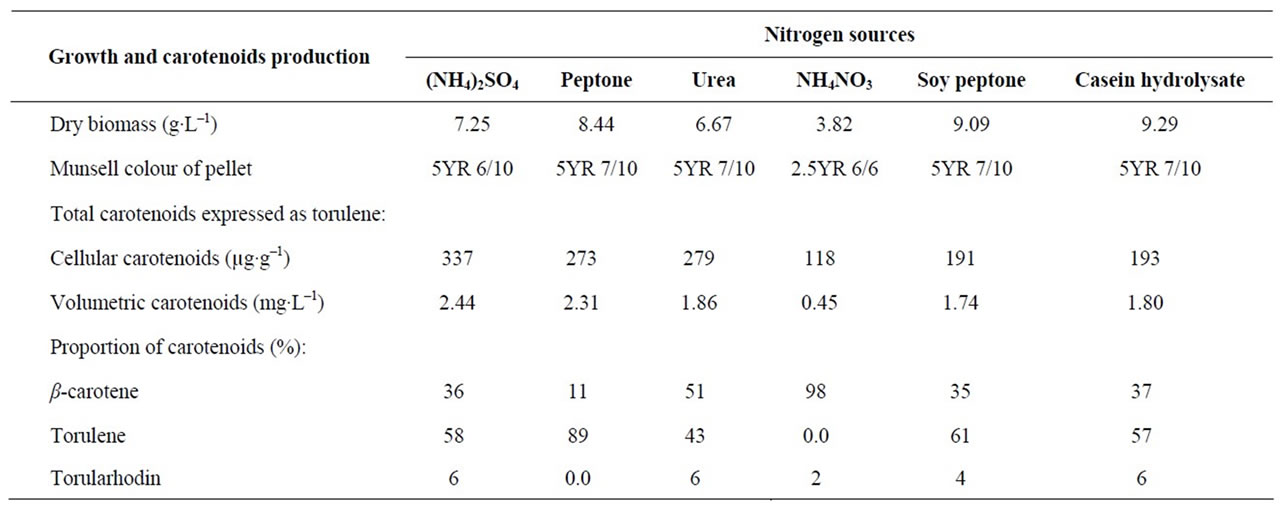
Table 2. Effect of nitrogen sources of cultivation medium on biomass and carotenoids produced by Rhodotorula glutinis var. glutinis.
dium containing ammonium sulphate resulted in production of highest cellular and volumetric carotenoids. Bhosale and Gadre [9] found that complex organic nitrogen sources (peptone, soy peptone, soybean meal and urea) resulted in higher carotenoids accumulation by mutant 32 of Rhodotorula glutinis, as compared with inorganic sources (ammonium sulphate and ammonium chloride).
The proportions of carotenoids and consequently the colour of pellets were affected by nitrogen source. Generally, ammonium nitrate resulted in highest percentage of β-carotene, while peptone gave the highest percentage of torulene (Table 2). Others [9] mentioned that when mutant 32 of Rhodotorula glutinis grown in media containing ammonium nitrate, peptone or soy peptone as nitrogen sources; higher β-carotene content was produced. While casein hydrolysate, urea or ammonium sulphate yielded higher torulene content.
3.3. Carbon Sources
Maximum dry biomass was obtained in medium containing high fructose corn syrup (Table 3). The highest total cellular and volumetric carotenoids were obtained in the medium containing sucrose. Bhosale and Gadre [9] found that cellular and volumetric carotenoids produced by mutant 33 of Rhodotorula glutinis can be arranged descendingly according to the carbon sources used as follow: Glucose then sucrose followed by fructose.
The proportion of carotenoids and consequently the colour of the pellets were changed according to the carbon source used in the medium. Generally, use of either high fructose corn syrup or glucose resulted in production of higher percentage of β-carotene. However, use of sucrose or glucose syrup gave higher percentage of torulene and torularhodin. The colour of pellets of the culture grown in media containing sucrose and glucose syrup had red hues due to the high percentages of torulene and torularhodin, respectively. While, fructose corn syrup and glucose gave pellets with yellow-red hues due to the high percentage of β-carotene. Bhosale and Gadre [9] found that the major carotenoid components produced by a mutant of Rhodotorula glutinis when glucose, fructose or sucrose were used as sole carbon source in the medium were β-carotene (69%), torulene (63%) or (60%), respectively.
3.4. Mineral Salts
Maximum dry biomass was obtained in medium containing Mg2+. The studied mineral salts resulted in higher dry biomass except Fe2+ and Cu2+ which inhibited the growth (Table 4). Other investigators [9,30] found that divalent cations act as stimulants for growth of Rhodotorula glutinis.
The highest cellular and volumetric carotenoids produced were obtained by cultivation the yeast culture in a medium containing Zn2+. All studied mineral salts resulted in an increase in cellular and volumetric carotenoids (Table 4). As illustrated [31] that certain metal ions (mg∙L–1) 0.4 Zn2+, 0.1 Cu2+ or 0.2 Fe2+ were important to produce carotenoid by Rhodotorula sp. Y1621. It was observed [32] that ferrous might be essential for the pigment production by an isolate of Rhodotorula. While addition of ZnSO4 to the growth medium of Rhodotorula completely inhibited the pigmentation. The effect of divalent cation salts on carotenoid production by mutant 32 of Rhodotorula glutinis was studied [9]. It was found that all studied divalent cation salts resulted in a higher volumetric production of carotenoids. Zinc sulphate gave the highest cellular and volumetric production of carotenoids. They assumed that the positive effect of divalent

Table 3. Effect of carbon sources of cultivation medium on biomass and carotenoids produced by Rhodotorula glutinis var. glutinis.
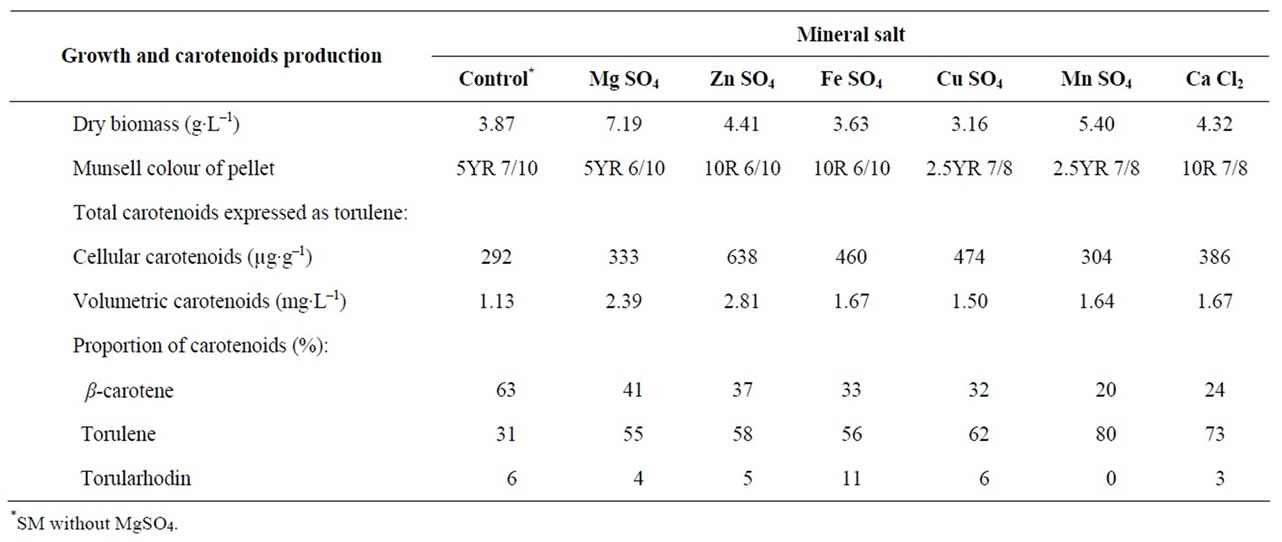
Table 4. Effect of mineral salts of cultivation medium on biomass and carotenoids produced by Rhodotorula glutinis var. glutinis.
cation salts was due to a stimulatory effect of cations on carotenoid-synthetizing enzymes.
Mineral salts affected the proportions of carotenoids and consequently the colour of pellets. Generally, all mineral salts resulted in high proportion of torulene produced (Table 4). It was mentioned [9] that most of divalent cation salts gave a higher torulene formation by mutant 32 of Rhodotorula glutinis except magnesium sulphate which increased the proportion of β-carotene (up to 77%).
3.5. Cultivation Temperature
The optimal temperature for production of cellular carotenoids was 15˚C. However, the optimal temperature for cell growth (dry biomass) and consequently production of volumetric carotenoids was 25˚C (Table 5). The most suitable temperature for growth and production of carotenoids by Rhodotorula glutinis co-cultivated with lactic acid bacteria in a whey ultrafiltrate was 30˚C [13]. Also, it was established [17] that the optimum temperature for growth of mutant 32 of Rhodotorula glutinis was 30˚C. The cellular and volumetric carotenoids produced by Rhodotorula glutinis at 30˚C were higher than those produced at 25˚C or 35˚C [15].
Cultivation temperature affected the proportions of carotenoid and consequently the colour of pellets. β-carotene content increased and the torulene and torularhodin contents decreased as cultivation temperature increased up to 30˚C (Table 5). Other investigators [13,15] concluded that lower temperatures (20˚C and 25˚C) seemed to favour synthesis of β-carotene and torulene whereas higher temperatures (30˚C and 35˚C) positively influenced torularhodin synthesis by Rhodotorula glutinis. It was established that at 30˚C, the β-carotene production by Rhodotorula glutinis mutant 32 accounted for 66% of the total carotenoids, while at 20˚C it accounted for 92% of the total carotenoids content [17].
3.6. Production of Carotenoids under Optimum Conditions
Data presented in Figure 1 illustrate the change occurring in the cultivation medium during growing Rhodotorula glutinis under optimum conditions. The count of viable yeast cells (CFU∙ml–1) increased at a rapid rate during the first day of incubation to reach its maximum, then it almost remained unchanged until third day, after that it decreased to reach its minimum at the end of cultivation time. Ammonia nitrogen of the cultivation medium dropped by 14.5% during the first two days and then remained almost unchanged. This may be due to the growth of yeast especially during the first day and its need to the simple nitrogen compounds. Sucrose in the culture supernatant disappeared by the end of the first day, due to the conversion of sucrose by yeast invertase [33,34]. The total reducing sugars decreased rapidly by 89.5% during the first two days to meet the energy requirement of yeast cells. Then it continued to decrease at slower rate to become almost zero after the third day. Others [15,16] reported that carbohydrate utilization rate of Rhodotorula glutinis was maximum within the first 24 hours.
Changes in yeast pellets are shown in Table 6 and

Table 5. Effect of cultivation temperature on biomass and carotenoids produced by Rhodotorula glutinis var. glutinis.
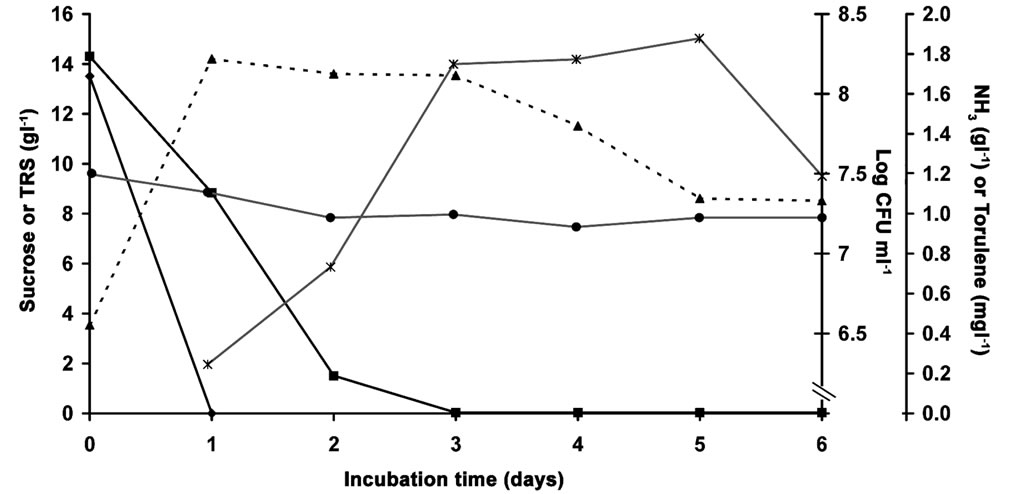
Figure 1. The effect of growth time of Rhodotorula glutinis var. glutinis on sucrose ( ), total reducing sugars (TRS) (
), total reducing sugars (TRS) ( ), HN3 (
), HN3 ( ), CFU ml–1 (
), CFU ml–1 ( )and total carotenoids (as torulene) (
)and total carotenoids (as torulene) ( ).
).
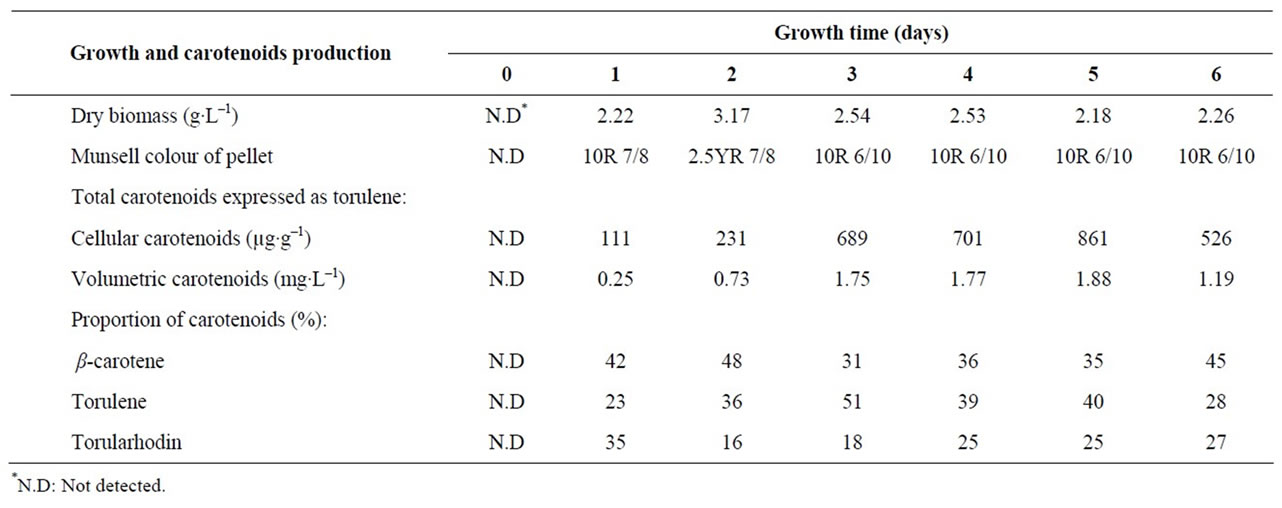
Table 6. Biomass and carotenoids production under optimum conditions by Rhodotorula glutinis var. glutinis.
Figure 1. The Munsell colour of yeast pellet became constant after three days of incubation. Dry biomass of yeast reached its maximum after the second day of cultivation and then decreased. Generally, the change in dry biomass was accorded with nitrogen and carbohydrate consumption, and count of viable yeast cells. It was reported [15] that about two thirds of total cell dry weight of Rhodotorula glutinis was produced within 48 hours. Carotenoids production started after the first day of incubation, at the end of logarithmic growth phase of the yeast, then increased rapidly until the end of the third day (during the stationary phase of the yeast) after that it continued to increase but slightly to reach its maximum at the fifth day (during the death phase of the yeast) then decreased rapidly at the sixth day (Figure 1). These results are in agreement with those obtained by many authors [10,12,14,15].
Rhodotorula glutinis var. glutinis produced moderate amount of carotenoids (861 μg∙g–1) after five days of cultivation under the selected optimum conditions (Table 6). The obtained yield is higher than those (70 - 322 μg∙g–1) obtained by some authors [6,7,9,13,35] and lower than those (915 - 2900 μg∙g–1) obtained by others [9,10,15,36].
4. Conclusion
A new yeast strain was selected from 200 carotenoids producer yeast isolates. It considered as promising carotenoids producer. Optimizing the studied factor led to an about 8-fold increase in total carotenoids production. However, further studies concerning subjecting this strain to genetic manipulation in order to increase its carotenoids production, is indeed needed.
REFERENCES
- W. Stahl and H. Sies, “Bioactivity and protective effects of natural carotenoids,” Biochimica et Biophysica Acta, Vol. 1740, No. 2, 2005, pp. 101-107
- P. Buzzini and A. Martini, “Production of Carotenoids by Strains of Rhodotorula glutinis Cultured in Raw Materials of Agro-Industrial Origin,” Bioresource Technology, Vol. 71, No. 1, 1999, pp. 41-44. doi:10.1016/S0960-8524(99)00056-5
- K. L. Simpson, C. O. Chichester and H. J. Phaff, “Carotenoid pigments of yeasts,” In: A. H. Rose and J. S. Harrison, Eds., The Yeasts Vol. 2—Physiology and Biochemistry of Yeasts, Academic Press, New York, 1971, pp. 493-515.
- P. Margalith and S. Meydav, “Some Observations on the Carotenogenesis in the Yeast Rhodotorula mucilaginosa,” Phytochemistry, Vol. 7, No. 5, 1968, pp. 765-768. doi:10.1016/S0031-9422(00)84829-3
- G. Frengova, E. Simova, K. Pavlova, D. M. Beshkova and D. Grigorova, “Formation of Carotenoids by Rhodotorula glutinis in Whey Ultrafiltrate,” Biotechnoloyg and Bioengineering, Vol. 44, No. 8, 1994, pp. 888-894. doi:10.1002/bit.260440804
- F. M. Squina, F. Yamashita, J. L. Pereira and A. Z. Mercadante, “Production of carotenoids by Rhodotorula glutinis in culture medium supplemented with sugar Cane juice,” Food Biotechnology, Vol. 16, No. 3, 2002, pp. 227-235. doi:10.1081/FBT-120016776
- I. Costa, H. L. Martelli, I. M. De Silva and D. Pomeroy, “Production of β-Carotene by a Rhodotorula Strain” Biotechnology Letters, Vol. 9, No. 5, 1987, pp. 373-375. doi:10.1007/BF01025808
- D. Müncenerová and J. Augustin, “The influence of pH on growth kinetics of yeasts in the presence of benzoate as a sole carbon source,” Folia Microbiologica, Vol. 39, No. 4, 1994, pp. 265-268. doi:10.1007/BF02814310
- P. B. Bhosale and R. V. Gadre, “Production of β-carotene by a mutant of Rhodotorula glutinis,” Applied Microbiology and Biotechnology, Vol. 55, No. 4, 2001, pp. 423- 427. doi:10.1007/s002530000570
- P. B. Bhosale and R. V. Gadre, “β-carotene production in sugar cane molasses by a Rhodotorula glutinis mutant,” Journal of Industrial Microbiology and Biotecnogy, Vol. 26, No. 6, 2001, pp. 327-332. doi:10.1038/sj.jim.7000138
- I. R. Maldonade, D. B. Rodriguez-Amaya and A. R. P. Scamparini, “Carotenoids of yeasts isolated from Brazilian ecosystem,” Food Chemistery, Vol. 107, No. 1, 2008, pp. 145-150. doi:10.1016/j.foodchem.2007.07.075
- H. S. Nam, S. Y. Cho and J. S. Rhee, “High-performance liquid chromatographic analysis of major carotenoids from Rhodotorula glutinis,” Journal of Chromatography A, Vol. 448, 1988, pp. 445-447. doi:10.1016/S0021-9673(01)84610-0
- G. I. Frengova, E. D. Simova and D. M. Beshkova, “Effect of temperature changes on the production of yeast pigments co-cultivated with lacto-acid bacteria in whey ultrafiltrate,” Biotechnology Letters, Vol. 17, No. 9, 1995, pp. 1001-1006. doi:10.1007/BF00127443
- G. I. Frengova, E. D. Simova and D. M. Beskhova, “Carotenoprotein and exopolysaccharide production by cocultures of Rhodotorula glutinis and Lactobacillus helveticus,” Journal of Industrial Microbiology and Biotecnogy, Vol. 18, No. 4, 1997, pp. 272-277.
- P. Buzzini and A. Martini, “Production of Carotenoids by strains of Rhodotorula glutinis Cultured in Raw Materials of Agro-Industrial Origin,” Bioresource Technology, Vol. 71, No. 1, 1999, pp. 41-44.
- P. Buzzini, “Batch and fed-batch carotenoid production by Rhodotorula glutinis-Debaryomyces castellii co-cultures in corn syrup,” Journal of Applied Microbiology, Vol. 90, No. 5, 2001, pp. 843-847. doi:10.1046/j.1365-2672.2001.01319.x
- P. B. Bhosale and R. V. Gadre,” Manipulation of temperature and illumination conditions for enhanced β-carotene production by mutant 32 of Rhodotorula glutinis,” Letters in Applied Microbiology, Vol. 34, No. 5, 2002, pp. 349-353. doi:10.1046/j.1472-765X.2002.01095.x
- J. Tinoi, N. Rakariyatham and R. L. Deming, “Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate,” Process Biochemistry, Vol. 40, No. 7, 2005, pp. 2551-2557. doi:10.1016/j.procbio.2004.11.005
- Z. Aksu and A. T. Eren, “Production of carotenoids by isolated yeast of Rhodotorula glutinis,” Biochemical Engineering Journal, Vol. 35, No. 2, 2007, pp. 107-113. doi:10.1016/j.bej.2007.01.004
- M. J. Pelczar and E. C. Chan, “Laboratory Exercises in Microbiology,” 14th Edition, McGraw-Hill, New York, 1977, pp. 59-61.
- C. T. Shih and Y. D. Hang, “Production of carotenoids by Rhodotorula rubra from sauerkraut brine,” Lebensmittel-Wissenschaft und-Technologie, Vol. 29, No. 5-6, 1996, pp. 570-572.
- Kallmorgen Corporation, “Munsell Colour Charts for Plant Tissues,” Munsell Color Division, Maryland, 1972.
- N. Harrd, “Astaxanthin formation by the yeast Phaffia rhodozyma on molasses,” Biotechnology Letters, Vol. 10, No. 9, 1988, pp. 609-614. doi:10.1007/BF01024710
- E. Stahl, “Thin-Layer Chromatography. A Laboratory Handbook,” Academic Press, INC. Publisher, New York, 1969, pp.210-343.
- B. H. Davies, “Analysis of Carotenoid Pigments,” In: T. W. Goodwin, Ed., Chemistry and Biochemistry of Plant Pigments, Academic Press, New York, 1965, pp. 489- 532.
- V. Perrier, E. Dubreucq and P. Galzy, “Fatty acid and carotenoid composition of Rhodotorula strains,” Archives of Microbiology, Vol. 164, No. 3, 1995, pp. 173- 179. doi:10.1007/BF02529968
- Y. Pomeranz and C. E. Meloan, “Food Analysis: Theory and Practice,” AVI Publishing Company, INC., Westport, 1978, pp. 65-67.
- B. L. Oser, “Hawk’s Physiological Chemistry,” Vol. 1, Academic Press, New York, 1965, pp. 529-544.
- AOAC, “Official Methods of Analysis of AOAC,” Arlington, Section 2.05, 31.025C, 1984.
- P. B. Bhosale and R. V. Gadre, “Production of β-carotene by a Rhodotorula glutinis mutant in sea water medium,” Bioresource Technology, Vol. 76, No. 1, 2001, pp. 53-55. doi:10.1016/S0960-8524(00)00075-4
- K. Mahattanatavee and S. Kulprecha, “Production of β-Carotene by Rhodotorula sp. Y 1621,” Microbial Utilization of Renewable Resources, Vol. 7, 1991, pp. 295- 300.
- D. K. Sandhu and V. K. Joshi, “Development of apple pomace based medium, optimizing pigment production by Rhodotorula and its characterization,” Advances in Food Science, Vol. 19, No. 1-2, 1997, pp. 31-34.
- J. D. Fontana, M. F. Guimaráes, N. T. Martins, C. A. Fontana and M. Baron, “Culture of the astaxanthinogenic yeast Phaffia rhodozyma in low cost media,” Applied Biochemistry and Biotechnology, Vol. 57-58, No. 1, 1996, pp. 413-422. doi:10.1007/BF02941721
- M. C. Rubio, R. Runcoand and A. R. Navarro, “Invertase from a Strain of Rhodotorula glutins,” Phytochemistry, Vol. 61, No. 6, 2002, pp. 605-609. doi:10.1016/S0031-9422(02)00336-9
- V. Perrier, E. Dubreucq and P. Galzy, “Fatty acid and carotenoid composition of Rhodotorula strains,” Archives Microbiology, Vol. 164, No. 3, 1995, pp. 173-179. doi:10.1007/BF02529968
- H. L. Martelli, I. M. De Silva, N. O. Souza and D. Pomeroy, “Production of β-carotene by a Rhodotorula strain grown on sugar cane juice,” Biotechnology Letters, Vol. 12, No. 3, 1990, pp. 207-208.
NOTES
*Corresponding author.

