American Journal of Plant Sciences
Vol.4 No.5A(2013), Article ID:32259,8 pages DOI:10.4236/ajps.2013.45A011
Irradiance and Developmental Stages of Crown Architecture Affect Shoot Production in Rhododendron reticulatum
![]()
Center for Ecological Research, Kyoto University, Otsu, Japan.
Email: y.shimuken@gmail.com
Copyright © 2013 Kenichi Yoshimura. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 12th, 2013; revised April 15th, 2013; accepted May 1st, 2013
Keywords: Branching; Crown Architecture; Daughter Shoot; Developmental Stage; Shoot Production
ABSTRACT
Plasticity in crown architecture, contributing to leaf arrangement within crown, is an important feature for whole plant carbon assimilation and survival. In this study, I examined the plasticity in crown architecture to light condition and developmental stage by the changes in shoot production. Rhododendron reticulatum expands crown with orthotropic growth in monopodial branching in young stage, but orthotropic growth is ceased in adult stage. Main stem of young crown is described with monopodial branching regardless of light environment. But multi-layer crown is observed in sun-lit environment rather than mono-layer crown in adult stage. Long shoot production for each branching system (foliage derived from sympodial branching) in young crown is associated with local light environment, but not in adult crown. Long shoot production rate is correlated with long shoot production rate of its mother shoot in young crown, but not in mono-layer crown. These results suggest that young crown expands branches to sun-lit position whereas adult crown reduces congestion of shoots with stochastic shoot production regardless of shoot production of mother shoots. I concluded that both light and developmental stage are important factors for shoot production and constructing crown architecture.
1. Introduction
Plants growing in the forest understory, where insufficient irradiance limits the assimilation rates, have minimally overlapping branches and leaves for efficient sunlight reception and better carbon benefit against carbon investment. Shrub species, growing in the forest understory throughout their lifecycle, especially have higher morphological plasticity in crown architecture to survive the shaded condition [1,2]. Crown architecture is represented as a species-specific growth strategy in response to variable light environments. Pioneer species, which grow rapidly under high light intensity, show multi-layer crown architectures to receive sun light including direct beam and sunlight transmitted through upper leaf layers [3]. Late-successional species, which grow under low light intensity, show mono-layer crown architectures that can receive at least limited sunlight and avoid self-shading because sunlight transmitted through upper leaf layers does not satisfy the sufficient assimilation [3,4]. Moreover, similar to interspecies variation, there is an intraspecies variation in plasticity of crown architecture according to light. Saplings of Abies mariesii growing in open conditions show multi-layer crown architectures and those growing in shaded conditions show monolayer crown architectures [5].
Since the changes in shoot population dynamics in response to environmental factors and internal resource allocations regulate crown architecture [6], shoot demography and dynamics are inferred to determine crown architecture [7]. Long shoots represent as crown expansion due to the large axis mass relative to leaves, while short shoots represent as foliage maintenance for adequate light reception due to large leaf mass relative to axis [8,9]. The arrangement of long shoots in areas receiving more sunlight within the crown and of short shoots in shady areas is in accordance with their functions justifies crown architecture that is adapted to light environment [10,11]. Crown dynamics was estimated using a shoot matrix model based on branch order in Betula pendula and crown development from sapling to adult tree was predicted
[12]. Variations in shoot production within different branch orders or different light environments can be assessed with matrix models of shoot population [13,14].
Not only the light environment but also crown age plays an important role for crown architecture. Branching structure and shoot morphology often change according to the developmental stages. For example, in the case of Vaccinium hirtum, most of the branches expand in an orthotropic fashion in the sapling stage, but plagiotropic branches mainly form a crown in the developed stage [15], and the shoots produced in older ramets are shorter than those in younger ramets [16].
The species examined in this study is Rhododendron reticulatum D. Don: its first order branches are usually produced from main stem with monopodial branching, while its second or higher order branches are usually produced with sympodial branching. Terminal buds produce long shoots and lateral buds produce short shoots to achieve greater vertical growth via monopodial branching, whereas terminal buds produce short shoots and lateral buds produce long shoots to achieve planar growth via sympodial branching (Figure 1). In the juvenile phase, both height growth through monopodial branching and horizontal expansion through sympodial branching lead to the development of a crown. However, after the crown height has fully developed, monopodial branching and consequently height growth ceases. Thereafter, only plagiotropic growth with sympodial branching leads to the further development of crown architecture. In addition to the development of the crown through plagiotropic growth and cessation of height growth, the crown architecture is also influenced by light environment, i.e. the crown is either multi-layered or mono-layered depending on the light condition. Thus, three types of crown architecture are observed: young crown with monopodial growth, multi-

Figure 1. Simplified drawings of branching patterns in R. reticulatum. The left is monopodial branching, terminal buds produce longer shoot. The right is sympodial branching, terminal buds produce short shoots and lateral buds produce long shoots. Arrows are growing direction characterized in each branching pattern.
layer crown with sympodial growth in areas with sunlight and mono-layer crown in shaded areas.
Dynamics of the shoot population and of crown architecture are related. Therefore, modifications in the crown architecture during developmental stages lead to modification in the shoot population. Most studies on crown architecture with regard to shoot population simulate crown dynamics with constant branching patterns, regardless of crown development [17,18]. However, it is debatable whether a single branching pattern can form the crown architecture throughout the entire life history of a plant or whole the crown because shoot elongation in the developed stage is supposed to be different from that in the initial stage. Though conceptual studies have denoted these points, experimental studies rarely denote [19,20]. I observed the changes in annual shoot production of all shoots in whole the crown of R. reticulatum in response to crown architecture and how developmental stages influence crown formation and assessed the influence of annual shoot production in crown development.
2. Materials and Methods
2.1. Study Site and Species
This study was conducted at Yamashiro Experimental Forest (34˚47'N, 135˚50'E) in central Japan. This site is a common secondary forest dominated by broadleaved deciduous species such as Quercus serrata Thunb. ex. Muuray and Lyonia ovalifolia var. elliptica Hand. Mazz. and broadleaved evergreen species such as Ilex pendunculosa Miq.
R. reticulatum is a broadleaved deciduous shrub species that dominates the understory of this forest. R. reticulatum has obviously alternative shoot types; long shoot and short shoot. On sympodial branching, terminal buds produce short shoots while lateral buds produce long shoots.
2.2. Sampling and Light Environment
Individuals with a single stem and no floral shoots growing on the ridge area of the study site under similar light environments were used for this study. The tree height (H) and height of the lowest first order branch (HB) of each individual were measured, and crown depth ratio was defined as (H-HB)/H. The age of the lowest first order branch was determined by counting the bud scars on the branches. Individuals with three types of crowns (young crowns, multi-layer crowns and mono-layer crowns) were selected on the basis of crown depth ratio and age of the lowest first order branch. 1) Three individuals in which the lowest first order branch was younger than ten years old were selected as young crowns. 2) Four individuals whose lowest first order branch was older than ten years old and whose crown depth ratio was larger than 0.3 were selected as multilayer crowns. 3) Three individuals whose lowest first order branch was older than ten years old and whose crown depth ratio was smaller than 0.3 were selected as mono-layer crowns (Table 1).
A branching system was defined as a first order branch along with the branches arising from it. The relative photosynthetic photon flux density (rPPFD) was measured for each branch system by using LI-190SA (Li-cor Inc., Lincoln, Neb, USA): the PPFD on a branch system relative to the PPFD of a completely open space was obtained. The median of seven values of rPPFD, measured from May 2005 to September 2005, was used as an index of light environment.
2.3. Branching Structure
Branch maps were drawn to represent the connection among shoots. Branch age was estimated by counting the bud scars for shoots produced from 1998 to 2005. The number of fallen shoot scars was recorded because they represent abscised shoots. Mother shoots were considered to be the shoots that produce daughter shoots. Shoots produced from same mother shoot were defined as sister shoots.
To describe yearly transition in the production of long shoots and short shoots, a shoot production matrix was determined for each year from 1999 to 2005 for crown or branch systems (Equation (1)).
 (1)
(1)
where Lt and St are the number of long shoots and short shoots in the year t, and vt[ll], vt[sl], vt[ls] and vt[ss] are
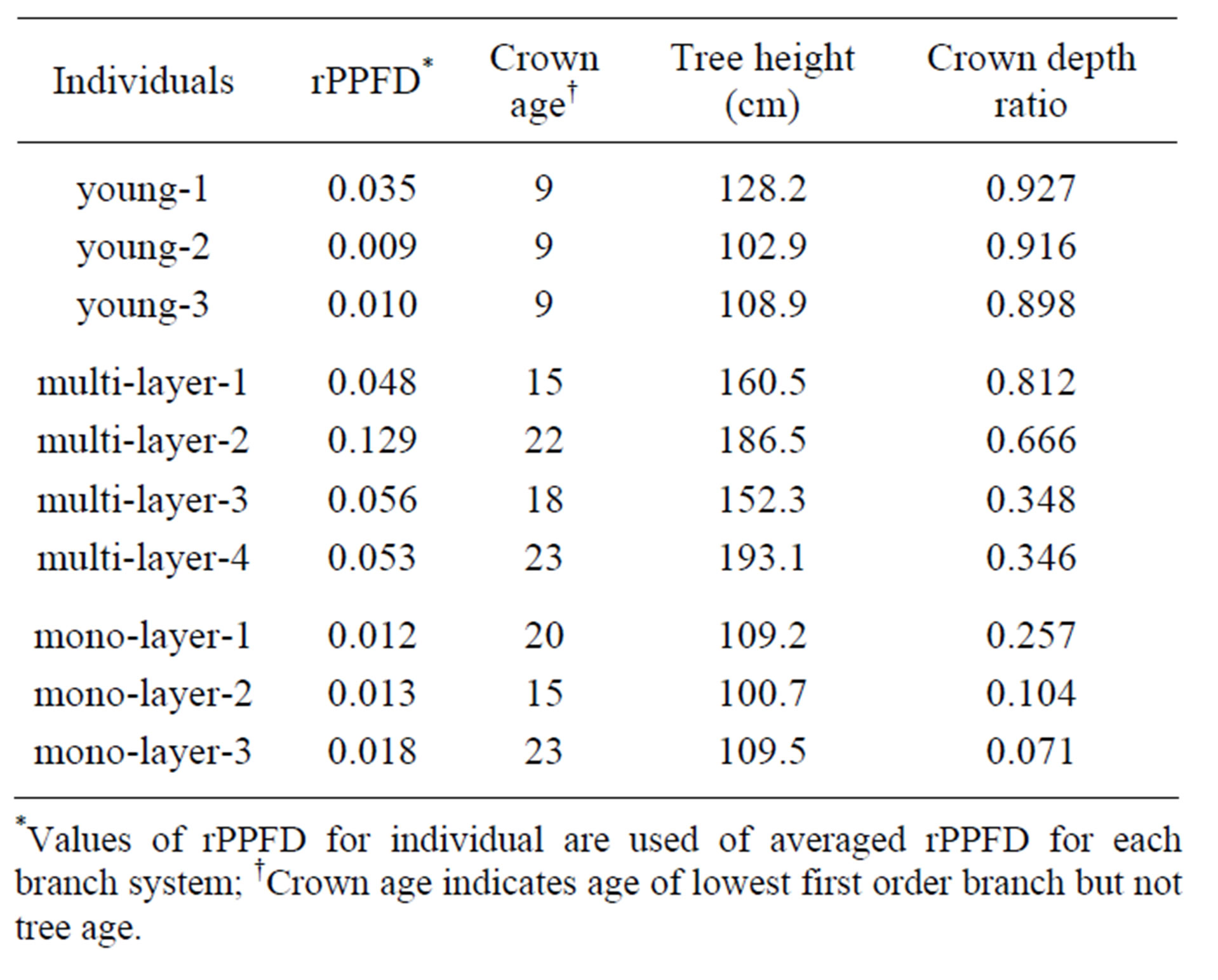
Table 1. Characteristics of sampled R. reticulatum individuals were shown.
shoot production rates in the year t from a shoot in year the t − 1, which means long shoot production rate from previous year long shoot, short shoot production rate from previous year long shoot, long shoot production rate from previous year short shoot, and short shoot production rate from previous year short shoot, respectively.
Branches of R. reticulatum grows by means of appositional sympodial branching within a branch system, so only one current year short shoot is produced at the apex of the long or short shoot grown in the previous year. This means both vt[ls] and vt[ss] values are generally one, therefore values of vt[ll] and vt[sl] determine the branching structure.
2.4. Statistical Analysis
To investigate the influence of the shoot type in the previous year shoot on the long shoot production, I compared vt[ll] and vt[sl] by using Fisher’s exact tests for each year from 1999 to 2005. Cochran-Armitage tests were used to analyze the linearity of yearly trends in the annual long shoot production rates from 1999 to 2005. To examine the relationship between shoot production and light environment, Kendall’s correlation tests were used to infer the correlation v2005[ll] and rPPFD of each branch system. Relationship between frequency in sister shoots of daughter shoots and that of mother shoots was examined using Fisher’s exact tests with Bonferroni correction.
3. Results
During the period from 1999 to 2005, vt[ll] was greater than vt[sl] for each year shoots in all individuals (Fisher’s exact test, p < 0.037), indicating that long shoot production from the previous year long shoots was more frequent than that from the previous year short shoots regardless of the crown architecture. If long shoot production rate is constant and above 1.0, the number of long shoots increases exponentially over the years. In young or multi-layer crowns, vt[ll] decreased in successive years, but mono-layer crowns did not show such an age-related trend (Figure 2 and Table 2). Decrease of long shoot production rate from the previous year long shoots over the years in young and multi-layer crowns indicates shoot production becomes lower along years in sympodial branching. Whereas in mono-layer crowns, since the long shoot production rate had already decreased to below 1.0. Some crowns showed negative trends, while others did not show such obvious trends in vt[sl] regardless of the crown type.
In young crowns, the v2005[ll] for each branch system was positively correlated with rPPFD (Table 3). However, mono-layer crowns did not show any obvious relationships between light environment and shoot produc-
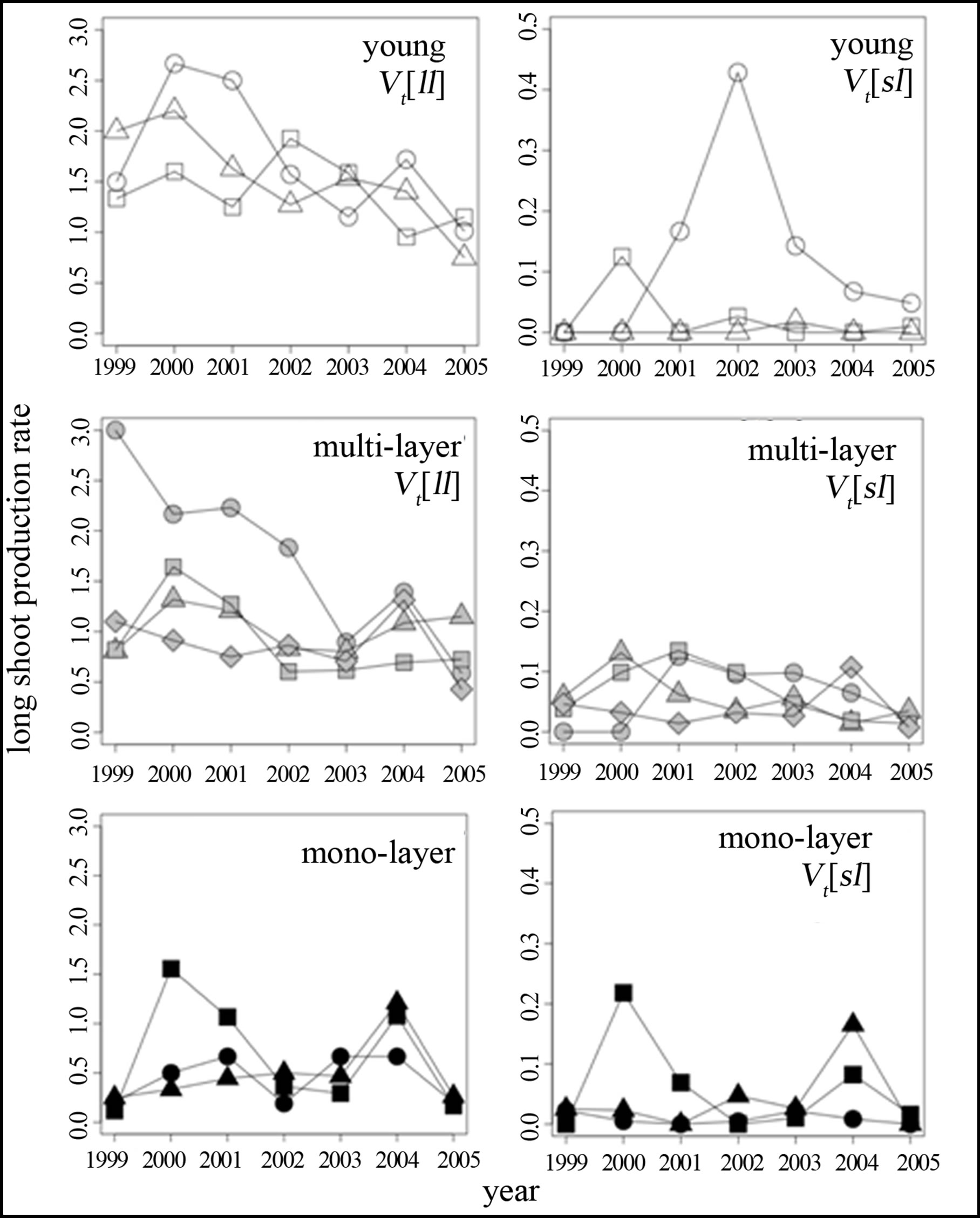
Figure 2. A-C Long shoot production rates from previous year long shoot (vt[ll]) of R. reticulatum were shown along years 1999-2005. A: Long shoot production vectors for young crowns; B: Long shoot production vectors for multi-layer crowns; C: Long shoot production vectors for mono-layer crowns. D-F: Long shoot production vectors from previous year short shoot (vt[sl]) of R. reticulatum were shown along years 1999-2005. D: Long shoot production vectors for young crowns; E: long shoot production vectors for multi-layer crowns; F: long shoot production vectors for mono-layer crowns. +: young-1, ×: young-2, ▽: young-3, ○: multi-layer-1, △: multi-layer-2, □: multi-layer-3, ◇: multilayer-4, ●: mono-layer-1, ▲: mono-layer-2, ■: monolayer-3.
tion. This indicated that more long shoots were produced in positions receiving much sunlight within a crown for young or multi-layer crowns, but not for mono-layer crowns. No obvious relationships were found between v2005[sl] and rPPFD were found regardless of the crown type.
The number of daughter shoots in young crowns varied according to the number of sister shoots. Most of the mother shoots with one or two sister shoots generally produced none or one daughter shoot, however, mother shoots with three or more sister shoots often produced two or three daughter shoots (Figure 3). Most of the shoots in mono-layer crowns produced none or one daughter shoot regardless of the number of their sister shoots. The number of daughter shoots in multi-layer crowns showed an intermediate pattern between young crowns and mono-layer crowns.

Table 2 Trends of long shoot production vectors of R. reticulatum from previous year long shoot (vt[ll]) of R. reticulatum or from previous year short shoot (vt[sl]) along years were tested with Cochran-Armitage tests. Because long shoots from previous year short shoot were rare, we could not analyze for vt[sl] of young-2.
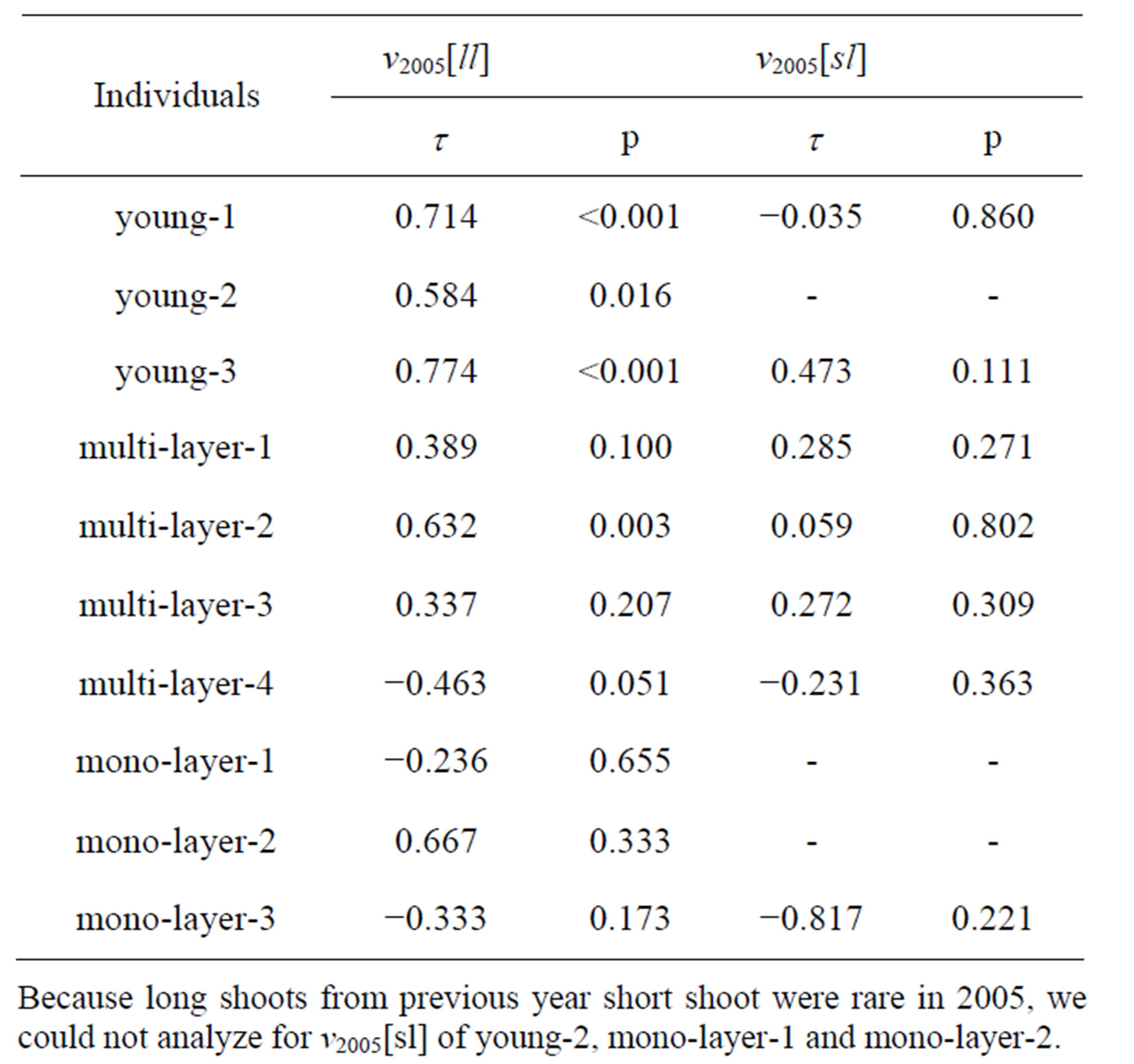
Table 3. Relationships between long shoot production vectors in 2005 from previous year long shoot (v2005[ll]) of R. reticulatum or previous year short shoot (v2005[sl]) and rPPFD for a branch system were tested with Kendall’s correlation tests.
Because long shoots from previous year short shoot were rare in 2005, we could not analyze for v2005[sl] of young-2, mono-layer-1 and mono-layer-2.
4. Discussion
The spatial arrangement of long shoots and short shoots within crown influences the crown growth patterns in the expansion and the stagnant phase [10]. Thus, the fact that long shoots are produced from the previous year long shoots rather than from the previous year short shoots in

Figure 3. Relative frequency of long shoots production for sisters of mother shoots was shown and difference of relative frequency among sisters of mother shoots was analyzed with Fisher’s exact test corrected by Bonferroni method. In the graphs, horizontal axes represent the number of long shoot and shading styles represent sisters of mother shoots. Adjusted error rates of the tests are young-1: 0.05/10, young-2: 0.05/10, young-3: 0.05/10, multi-layer-1: 0.05/10, multi-layer-2: 0.05/10, multi-layer-3: 0.05/3, multi-layer-4: 0.05/10, mono-layer-1: 0.05, mono-layer-2: 0.05/3, mono-layer-3: 0.05/6.
R. reticulatum implies that branch systems with many long shoots produce greater long shoots but those with few long shoots produce less long shoots. Short shoots, which are often found in the internal crown, are supposed to cause a decrease in long shoot production for avoiding self-shading. Moreover, the physiology of long shoots may be favorable for producing new shoots. [21] reported that the hydraulic conductivity of long shoots is greater than that of short shoots in Fagus sylvatica. The low production of long shoots from short shoots caused by inferiority in expansion of short shoots results in low values of vt[sl] regardless of the crown types.
In young crowns, the association between light environment and long shoot production rate in the branching system indicates that more long shoots are produced in sunlit environment than under shady environment within a crown. In contrast, though long shoots should be produced in sunlit position within the crown to expand to new space [22], light environment did not influence the long shoot production in multi-layer or mono-layer crowns. The long shoot production in a young crown is expected to bring about crown expansion; however, long shoots of crowns with ceased height growth have a role for crown maintenance rather than crown expansion.
Long shoots with few sister shoots were produced from the previous year shoots having few sister shoots, whereas shoots with many sister shoots were produced from the previous year shoots having many sister shoots in young crowns. The fact that branches with higher bifurcation produce new shoots with higher bifurcation and branches with lower bifurcation produce new shoots with lower bifurcation means that variation of growth among branching systems would be accelerated along crown growth. Branches with higher bifurcation have a function of crown expansion and branches with lower bifurcation have a function of foliage maintenance. Therefore, continuous variation in branching pattern for branch systems can link to functional differentiation among branching systems. In mono-layer crowns, 50% - 70% of the previous year shoots produce no daughter shoots regardless of the number of previous year sister shoots. Shoots in mono-layer crowns have less function of crown expansion to survive in forest understory. Reduction in selfshading is more important in multi-layer crowns than in young crowns because the former have more shoots according to their age and expansion for space acquisition is not so strong.
[23] observed the branching pattern in Cornus kousa and found that the branching structure of the mother shoot affects that of the daughter shoots, particularly with respect to branching intensity and functional variation. In this manner, young crowns guide the branching pattern for adequate spatial acquisition in order to expand by intense branching in sun-lit areas. The spatial arrangement of foliage that occurs in response to the gradation of light intensity is important for crowns to develop by monopodial branching. Whereas, shoots in mono-layer crowns behave foliage maintenance irrespective of light intensity. The skeleton of the crown architecture is formed in the initial stage, therefore, the arrangement of shoot tips in adult stage is considered to play a role in reducing self-shading independent of the branch order and that it is not primarily responsible for constructing the skeleton of the crown architecture. The branching pattern varies among different developmental stages due to variations in shoot production [23-24]. Foliage arrangement and shoot elongation have a trade-off relationship because leaves will be heavily congested if each shoot were to elongate randomly. The arrangement of leaves to reduce self-shading is more important than that for crown expansion in the case of crowns in the developed stage because height growth has already ceased at this stage.
Combination of orthotropic and plagiotropic branches contributes to a variety of crown architectures and the function of such branches changes during different developmental stages: orthotropic branches mainly construct the crown architecture in the young stage, while plagiotropic branches develop the crown architecture in adult stage [8,25]. [15] discovered that plagiotropic branches in Vaccinium hirtum experienced a greater role than orthotropic growth in enduring the forest understory under low light conditions after the height growth had ceased. With regard to shoot dynamics, shoot production decreases and shoot shed increases with successive developmental stages [26]. In R. reticulatum, differentiation of monopodial and sympodial branching links to orthotropic and plagiotropic growth (Figure 1). Annual shoot production in sympodial branching decreases the ratio of long shoot to short shoot because vt[ss] is usually one though vt[sl] is almost zero. In addition, uniform rate of long shoot production is not concerned with the nature of the previous year shoots in mono-layer crowns. Thus, adult crowns in R. reticulatum can be regarded as crown architecture suitable for low-light environment in terms of shoot morphology. R. reticulatum shows various patterns of crown architecture responded to crown age, that is, architecture of young crowns functions as crown expansion and that of old crowns functions as foliage mentainance. [4] observed crown architecture in nine tree species and found that pioneer species maintain their hierarchy regardless of developmental stages, but late successional species lose their hierarchy during their developmental stages. [27] compared the crown architecture of Fagus crenata, with that of Quercus crispula during their developmental stages and reported that light environment does not restrict crown architecture in F. crenata, a late successional species, but it does not affect the crown architecture in Q. crispula, a mid-successional species. Thus, the intensity of light demand determines whether light environment causes plasticity in crown architecture. Transitional intensity of architectural hierarchy observed in R. reticulatum may be an adaptive strategy of the plant to survive in the forest understory; in this strategy, first, space for foliage expansion is acquired by means of height growth in the orthotropic growth phase and then in the following phase, foliage is arranged so as to reduce overlap among leaves.
As tall trees grow, their crowns become deeper to form multi-layer crowns during the developmental stages, because the irradiance on foliage increases along height growth [28]. However, in the case of shrub species, it is known that crown depth associates to irradiance rather than developmental stages because height growth does not improve light condition [2,15]. I demonstrate that shoot production in R. reticlatum is associated with the previous year shoot production in the height growth phase, but stochastic shoot production is seen in the adult phase regardless of previous year shoot production. Since the height growth of shrubs is limited, multiple growing patterns (i.e. branching for height growth or branching to reduce self-shading) are appropriately utilized for the growth in response to the light environment. Monopodial branching is responsible for developing the crown, regardless of light environment, during the height growth phase. Light environment, however, determines the type of crown architecture, multi-layer or mono-layer during the adult phase. For taking multiple growing patterns, R. reticulatum represents both crown development and stabilizing of crown dynamics (i.e. regulating of shoot production), which are the strategies to adapt a shady forest understory.
5. Acknowledgements
I thank Mr. Shogo Hamada and Mr. Shinya Takeno for assistance of field research. Mr. Ika Heriansyah gave me important suggestions. Dr. Yoichi Kanazawa and students in Laboratory of Forest Resource, Kobe University gave me many helpful advices.
REFERENCES
- A. Nicola and S. T. Pickett, “The Adaptive Architecture of Shrub Canopies: Leaf Display and Biomass Allocation in Relation to Light Environment,” New Phytologist, Vol. 93, 1983, pp. 301-310. doi:10.1111/j.1469-8137.1983.tb03433.x
- I. P. G. Ardhana, H. Takeda, M. Sakimoto and T. Tsutsumi, “The Vertical Foliage Distributions of Six Understory Tree Species in a Chamaecyparis obtusa Endl. Forest,” Trees, Vol. 2, 1988, pp. 143-149. doi:10.1007/BF00196019
- H. S. Horn, “The Adaptive Geometry of Trees,” Princeton University Press, Princeton, 1971.
- J. Millet, A. Bouchard and C. Édelin, “Relationship between Architecture and Successional Status of Trees in the Temperate Deciduous Forest,” Écoscience, Vol. 6, No. 2, 1999, pp. 187-203.
- T. Kohyama, “Growth Pattern of Abies mariesii Saplings under Conditions of Open-Growth and Suppression,” Botanical Magazine, Tokyo, Vol. 93, No. 1, 1980, pp. 13-24.
- J. L. Harper, “Canopies as Populations,” In: G. Russel, B. Marshall and P. G. Jarvis, Eds., Plant Canopies: Their Growth, Form and Function, Cambridge University Press, Cambridge, 1989, pp. 105-128. doi:10.1017/CBO9780511752308.007
- K. Lehtilä, J. Tuomi and M. Sulkinoja, “Bud Demography of the Mountain Birch Betula pubescens ssp. tortuosa near Tree Line,” Ecology, Vol. 75, No. 4, 1994, pp. 945- 955. doi:10.2307/1939418
- F. Hallé, R. A. A. Oldeman and P. B. Tomlinson, “Tropical Trees and Forests. An Architectural Analysis,” Springer-Verlag, Berlin, 1978. doi:10.1007/978-3-642-81190-6
- P. M. Room, L. Maillette and J. S. Hanan, “Module and Metamer Dynamics and Virtual Plants,” Advances in Ecological Research, Vol. 25, 1994, pp. 105-157. doi:10.1016/S0065-2504(08)60214-7
- T. Seino, “Intermittent Shoot Growth in Saplings of Acanthopanax sciadophylloides (Araliaceae),” Annals of Botany, Vol. 81, No. 4, 1998, pp. 535-543. doi:10.1006/anbo.1998.0588
- K. Yoshimura, “Spatial Variation and Morphology of Shoots in the Variant Crown form of Rhododendron reticulatum,” Botany, Vol. 88, No. 11, 2010, pp. 995-1005. doi:10.1139/B10-071
- L. Maillette, “Structural Dynamics of Silver Birch II. A Matrix Model of the Bud Population,” Journal of Applied Ecology, Vol. 82, 1982, pp. 219-238. doi:10.2307/2403006
- J. R. Porter, “Demographic Approaches to Studies of Canopy Development in Plants,” Biomass and Bioenergy, Vol. 11, No. 6, 1996, pp. 207-214. doi:10.1016/0961-9534(96)00026-8
- F. J. Sterck, F. Bongers, H. J. During, M. Marínez-Ramos and H. de Kroon, “Module Responses in a Tropical Forest Tree Analyzed with a Matrix Model,” Ecology, Vol. 84, No. 10, 2003, pp. 2751-2761. doi:10.1890/02-0335
- K. Kawamura and H. Takeda, “Rules of Crown Development in the Clonal Shrub Vaccinium hirtum in a LowLight Understory: A Quantative Analysis of Architecture,” Canadian Journal of Botany, Vol. 82, No. 3, 2004, pp. 329-339. doi:10.1139/b04-001
- K. Kawamura and H. Takeda, “Light Environment and Crown Architecture of Two Temperate Vaccinium Species: Inherent Growth Rules versus Degree of Plastisity in Light Response,” Canadian Journal of Botany, Vol. 80, No. 10, 2002, pp. 1063-1077. doi:10.1139/b02-096
- A. Takenaka, “A Simulation Model of Tree Architecture Development Based on Growth Response to Local Light Environment,” Journal of Plant Research, Vol. 107, No. 3, 1994, pp. 321-330. doi:10.1007/BF02344260
- J. Perttunen, R. Sievänen, E. Nikinmaa, H. Salminen, H. Saarenmaa and J. Väkevä, “LIGNUM: A Tree Model Based on Simple Structural Units,” Annals of Botany, Vol. 77, No. 1, 1996, pp. 87-98. doi:10.1006/anbo.1996.0011
- R. Borchert and N. A. Slade, “Bifurcation Ratio and the Adaptive Geometry of Trees,” Botanical Gazette, Vol. 142, No. 3, 1981, pp. 394-401. doi:10.1086/337238
- D. A. Steingraeber and D. M. Waller, “Non-Stationarity of Tree Branching Patterns and Bifurcation Ratios,” Proceedings of Royal Society of London B, Vol. 228, No. 1251, 1986, pp. 187-194.
- S. Rust and R. F. Hüttl, “The Effect of Shoot Architecture on Hydraulic Conductance in Beech (Fagus sylvatica L.),” Trees, Vol. 14, No. 1, 1999, pp. 39-42. doi:10.1007/s004680050005
- A. Takenaka, “Shoot Growth Responses to Light Microenvironment and Correlative Inhibition in Tree Seedlings under a Forest Canopy,” Tree Physiology, Vol. 30, 2000, pp. 987-991. doi:10.1093/treephys/20.14.987
- H. Hatta, H. Honda and J. B. Fisher, “Branching Principles Governing the Architecture of Cornus kousa (Cornaceae),” Annals of Botany, Vol. 84, No. 2, 1999, pp. 183-193. doi:10.1006/anbo.1999.0906
- J. B. Fisher and D. E. Hibbs, “Plasticity of Tree Architecture: Specific and Ecological Variations Found in Aubréville’s Model,” American Journal of Botany, Vol. 69, 1982, pp. 690-702. doi:10.2307/2442959
- J. Millet, A. Bouchard and C. Édelin, “Plagiotropic Architectural Development of Four Tree Species of the Temperate Forest,” Canadian Journal of Botany, Vol. 76, No. 12, 1998, pp. 2100-2118.
- G. H. Buck-Sorlin and A. D. Bell, “Crown Architecture in Quercus petraea and Q. robur: The Fate of Buds and Shoots in Relation to Age, Positiopn and Environmental Perturbation,” Forestry, Vol. 73, No. 4, 2000, pp. 331- 349. doi:10.1093/forestry/73.4.331
- N. Osada, R. Tateno, F. Hyodo and H. Takeda, “Changes in Crown Architecture with Tree Height in Two Deciduous Tree Species: Developmental Constraints or Plastic Response to the Competition for Light,” Forest, Ecology and Management, Vol. 188, No. 1-3, 2004, pp. 337-347. doi:10.1016/j.foreco.2003.08.003
- Ü. Niinemets, “Changes in Foliage Distribution with Relative Irradiance and Tree Size: Differences between the Saplings of Acer platanoides and Quercus robur,” Ecological Research, Vol. 11, No. 3, 1996, 269-281. doi:10.1007/BF02347784

