American Journal of Plant Sciences
Vol.4 No.4(2013), Article ID:30003,6 pages DOI:10.4236/ajps.2013.44105
Characteristics of Root Caps in Four Root Types of Avicennia marina (Forsk.) Vierh.
![]()
Department of Biology, Faculty of Sciences and Technology, Universitas Airlangga, Surabaya, Indonesia.
Email: herypurba@unair.ac.id, hery-p@fst.unair.ac.id
Copyright © 2013 Hery Purnobasuki. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 26th, 2013; revised March 28th, 2013; accepted April 8th, 2013
Keywords: A. marina; Columella; Root Caps; Statocyte
ABSTRACT
The anatomy of the root caps in four root types of Avicennia marina were studied using conventional histological techniques by Ligth Microscopy (LM) in order to relate their development and structure of their function as environmental adaptation in mangrove’s root and to identify cellular polarities with respect to gravity. In columella cells, nuclei are located proximally. The result reveals that root caps consisted of two regions, i.e., central columella or statenchyma and peripheral regions. The columella cells (statocyte) are in the form of oval to rectangular. We also found that all root with marked gravitropism have statoliths that settle along different walls of that statocyte. Caps vary in form and size within root system of A. marina. The most striking feature of the root is the distinct and extensive root cap with quite long files of cells. From its shape, structure, and location, it seems clear that the root caps protects the cells under it from abrasion and assists the root in penetrating the soil.
1. Introduction
Avicennia marina (the gray mangrove) is a common mangrove species on tropical and subtropical sea shores, swamps and stream banks [1,2]. It grows in estuaries where it is subject to tidal flooding and the soil is anaerobic. It develops a fairly complex root system. In mature tree, the root system of A. marina is complicated and it has four root types, i.e. cable roots, pneumatophores, feeding root, and anchor roots ([3]). Each of them has its own particular growth direction, that is, vertical (both upwards and downwards) and horizontal, but there is lack information about the structure especially on the root cap of different root types that show the specific characters of different function and growth direction.
The root cap is a universal feature of angiosperm, gymnosperm, and pteridophyte roots. The root cap is a specialized structure located distal to the root apical meristem of nearly all higher plants. Besides providing protection against abrasive damage to the root tip, the root cap is also involved in the simultaneous perception of a number of signals-pressure, moisture, gravity, and perhaps others—that modulate growth in the main body of the root [4].
Mangrove roots can be characterized on the basis of various structural properties, but by far the most study of mangrove’s root structures were not enough to explore the characteristics of their root caps. Most of the study in mangrove’s roots were focus on the architecture [5-7], aerenchyma tissue [8-10], anatomy and morphology [11- 13], gravitropism [6,14,15], the effect of root epibiont complexity [16], degree of shading [17,18], root density [17,19], and gas exchange [20-23].
The morphology of the root and root cap is determined primarily by the root apical meristem, because typically the radially symmetric meristem forms a cylindrical root. Anatomical information for the meristem is useful to understand the root morphology of A. marina, but the cellular patterns in the meristem in relation to the root proper and cap have been analyzed in detail in only a few species. [24] described that in three species of Cladopus with either narrow or broad ribbon-like roots covered by hoodshaped dorsiventral root caps, the outermost layer of the root cap is an acroscopic extension from the ventral dermal layer of the root meristem. The crustose roots of Hydrobryum japonicum and Zeylanidium olivaceum have a moderately layered structure of the meristem along the margin of the root lobe, which is framed by a root cap (protective tissue) [25,26].
The root of Indotristicha ramosissima appears to have a meristem of the closed type, which is demarcated from the root cap ([27]). Thus, the results of previous studies differ considerably in the organization of the root meristem and cap. However, very little information is available to consider the structure of the root cap in mangrove species, especially A. marina.
Studying the anatomy structures of root cap could lead to a better understanding of the morphology and functional adaptation of mangrove roots. In the present paper, consideration is given to the structure of the root caps of four root types in A. marina and, in particular, to how the meristematic initial cells of both the central cap columella and the lateral portion of the cap which surrounds the columella are organized in relation to the production of new cells.
2. Materials and Method
All the root samples were taken from 5 adult trees (10 - 15 cm in trunk diameter at base, 0.5 - 1 m tall) of A. marina which growth in Surabaya beach (7˚12'28.82''S 112˚46'33.35''T), East Java Province in Java Island, Indonesia, at March 2010 and September 2010. Around the sample trees, we excavated root system (Figure 1) of the trees during low tide and collected 1) cable root tips, 2) pneumatophore tips, 3) feeding root tips, and 4) anchor root tips. For each root tips, we collected more than five tips.
2.1. Histology
The samples of root tips that corresponded to all types of root were fixed in the field. The samples were prepared for embbeding in paraffin wax and sectioning. They were fixed in FAA (70% ethanol, 10% formalin, and 5% acetic acid in the volume ratio 90:5:5). The air in the tissue was
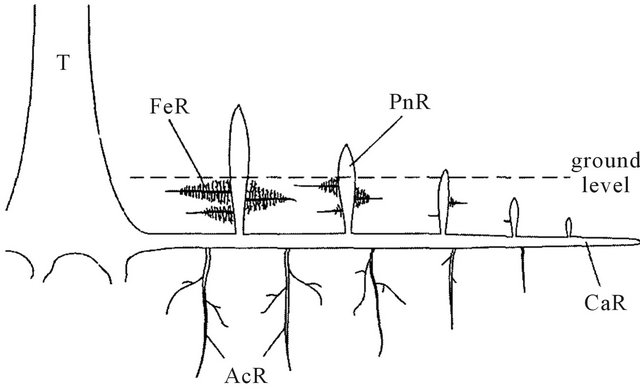
Figure 1. Simplified diagram of the mature A. marina root system showing the different types of roots observed. CaR (cable roots), primary roots with lateral horizontal growth. PnR (pneumatophores), aerial roots with upward vertical growth from CaR. FeR (feeding roots), lateral horizontal roots grown from PnR. AcR (anchor roots), vertical roots grown downward from CaR. T: trunk. Bar = 2 cm.
evacuated using an oil rotary vacuum pump. The samples were dehydrated in an ethanol series and embedded in paraplast plus (Oxford Lab, USA) at 59˚C. Longitudinal thin sections in 9 - 12 µm were cut using a rotary microtome (HM 350 Micron, Germany), stained in SafraninFast Green [28-30], and permanently mounted using entellan. Finally, all the observation was carried out using light microscope (BX 50, Olympus, Japan). Microscopic images were taken by microscopy camera (PM-C35, Olympus, Japan) and recorded on Fuji Film Neopan F ISO 32/16˚ films for black and white prints. The measurement and counts carried out on the cells of caps and the statocyst of the different root types were performed on 15 sections of each root (5 roots for each root type) using computer assisted image analysis (version 3.5 Scientific Imaging Software, IP Lab USA) by digitizing the images with a digital camera attached to a compound microscope. The results were analyzed using one-way analysis of variance (ANOVA, minitab version 13, α = 0.05).
2.2. Root Cap Dimensions and Cell Numbers
Median sections from root tips sampled of four root types of A. marina were located and the lengths and breadths of the root caps were measured, as shown in Figure 1. The total number of cells in each root cap was estimated according to a morphometric method described in [31]. The numbers of cell in each of tiers of the cap were estimated similiarly. Dimensions of cap cells in meristem, columella, lateral, and peripheral region were measured using an eyepiece graticule.
3. Results
The root cap of the four root types of Avicennia marina was a cup-shape and extended as a distinct, elongate sheath of several cell layers over more than 3 mm in all root types. Root caps consisted of two regions, i.e., central columella or statenchyma (Figure 2, co) and peripheral regions (Figure 2, pe). However in this species the differences densely staining of the horizontal layer of columella were not so clearly like in Sonneratia alba. The columella cells (statocyte) are in the form of oval to rectangular.
There were no measurable differences of columella cells between the root types in Avicennia marina (Figure 2). Of pneumatophore emerging from the mud, the apical structure rapidly changed. In the old pneumatophores, the small cap enlarged to a corky wart, and more than a dozen corky layers extended down the sides of the apex.
The cable roots had biggest of all numerical characteristics of root apex structure of A. marina compare to the other root types. Columellar initials are directly contacted with the cortex and epidermal initials. All root
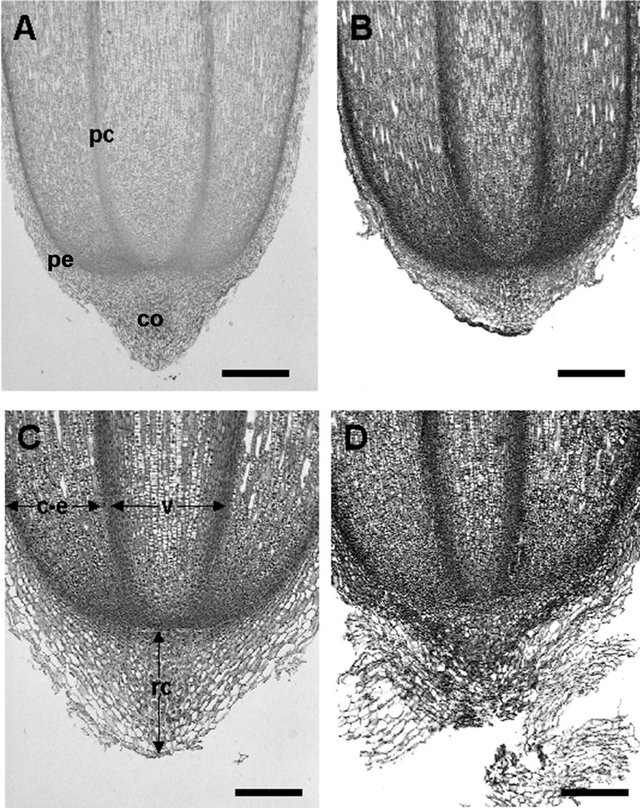
Figure 2. Median longitudinal sections of Avicennia marina root tips, showing closed type root structure with prominent root caps and three tiers initials. (A) Cable root (Bar = 400 µm); (B) Pneumatophore (Bar = 400 µm); (C) Feeding root (Bar = 100 µm), and (D) Acnhor root (Bar = 100 µm). c-e: cortex-epidermis; co: collumela; rc: root cap; v: vascular bundle; pc: procambium; pe: peripheral region.
types had such distinct columellar initials not touched by the vascular initials. The columellar initials also have cytoplasm with more lightly stained than that of adjacent cells. The number of longitudinal layers of columella was quiet different between the root types. The columella consisted of horizontal layers of cells regularly arranged. In narrower roots (anchor roots and feeding roots), the columella had 15 - 18 layers, while thicker roots (cable roots and pneumatophores) had 18 - 25 or more layers (Table 1), although exact determination of the number was fairly difficult because old root cap remained even after emergence from ground surface. Each of columella layers had horizontally arranged oval or rectangular cells (statocytes) in longitudinal section. These statocytes divided anticlinally, and cells situated on both sides of the columella began to enlarge in radial direction with development of vacuoles and became stainable with Safranin, and they were destinated to become peripheral cells. Cells at the tip of columella and outermost peripheral cells in anchor, feeding and cable roots were slashed out by soil, although collapsed residues of those cells were persistent in pneumatophores (Figure 2(B)).
Statocytes of the columella have amyloplasts (statoliths) in the cytoplasm. The distribution and amounts of the statoliths were apparently different among the different root types (Table 2). Anchor roots have the smallest statocytes (322.2 μm2) among the four root types and the number of statocytes was about 14 statoliths per cell. Smaller statoliths (about 2.5 µm in diameter) accumulated at the distal wall of the statocyte, nucleus of which was located near the proximal wall. Cable roots had the largest statocytes (about 693.6 μm2) among the four root types with the number of statocytes was about 12 statoliths per cell. The statoliths were small, 2.9 µm in diameter, and located near the proximal wall of the statocyte, nuclei of which were located near the distal wall. Feeding roots have statocytes of (about 458.7 μm2) and the numbers were about 10 statoliths per cell. The statoliths were about 2.9 µm in diameter and accumulated along one of the two longitudinal walls of the statocyte, nucleus of which was located near the proximal wall. Pneumatophores had statocytes of (about 579.5 μm2) and the numbers of statoliths were about 10 per cell. The statoliths are large (about 2.6 µm in diameter) and accumulated along one of the two longitudinal walls of the statocyte, nucleus of which was located near the distal wall.
Although the distribution and amounth of the statoliths were apparently different among the different root types, however the diameter of the statoliths in the statenchyma of the various root types was quite constant (Table 2), between 2.5 and 2.9 μm, and the means was not significant differently (ANOVA, p < 0.05). The biggest area of statoliths was found on cable roots, about 693.6 μm2. However, the area of statocyte was significantly different between root types.
Root hairs were also never observed in the feeding root of A. marina as like as in S. alba. Since root hairs often lack in marsh plants, it can not assume that persistent cap layers fundamentally responsible of hairs in mangrove roots [5].
4. Discussion
The purpose of this study was to examine the microscopic structure of the root cap of four root types of A. marina and to show their adaption in estuaries. All of four types had various sizes of root cap (RC) and the number of cells layer as well. Generally the root cap is a cup-shaped, loosely cemented mass of parenchyma cells that covers the tip of the root. As cells are lost among the soil particles, new ones are added from the meristem behind the cap. The cap is a unique feature of roots; the tip of the stem has no such structure. From its shape, structure, and location, its primary function seems clear: It protects the cells under it from abrasion and assists the root in penetrating the soil. Phenomenal numbers of cap cells are produced to replace those worn off and lost as root tips push through the soil.
Table 1. Numerical characteristics of root apex structure of Avicennia marina.
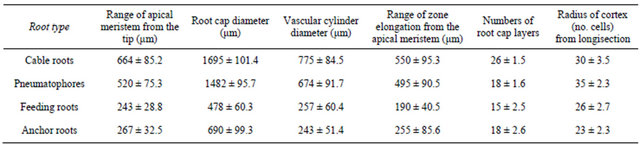
Table 2. Size of statocytes and statoliths in different root types of Avicennia marina. Different letters (superscript) show significantly different by ANOVA (p < 0.05).
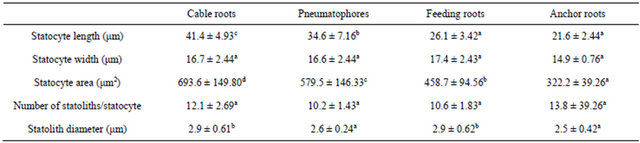
Values are means + s.e.; n = 50. Measurements were made randomly on 15 longitudinal sections of each root type.
This tissue of this RC is strictly organised into two parts: the columella (in central position), and the lateral root cap (LRC) around the columella This shape is present on roots in all major species [4,32]. Like another mangrove species such as Sonneratia alba, the most striking feature of the root is the root cap and the mature root cap extended as a distinct, elongate sheath of several cell layers over more than 3 mm in all root types.
Medial longitudinal sections through the primary root show the relationships of the various cell files to each other (Figure 2). The cells of the meristem are arranged into three tiers of cells and therefore correspond to the closed meristem organisation of [33]. The lower tier of cells (protoderm) is composed of the initials of the root cap and epidermis. The middle tier (periderm) is composed of central cells flanked by cells which are contiguous with the files of the cortex and endodermis. The upper tier of cells (plerome) is contiguous with the cells of the stele.
Histological observations show that the columella itself is composed of two different cell types: the statocytes, which are mostly located in the internal layers of columella (and occasionally in some lateral root cap cells), and the secretory cells constituting the LRC and the external columella layer. This condition appropriate to the research of [4] and the structural evidence obtained in studies of different root types indicates that the roots are effective in water and nutrient absorption. [34] describe a pattern of cell division in the cap meristem in detail. The regions considered were columella and flank meristem and the principles of the model may be regarded as general for the caps of roots, such as mangrove, with a closed meristem structure.
A striking feature of the organisation in the A. marina root is the conservation of cell file numbers in the various tissues. The conservation of file number and organisation is stringent in the primary root. There is little variation in the number of cell files in the root cap, cortex and columella, with some variability in the epidermis. Therefore the relative invariance in the resulting pattern is probably due to the fact that the primary root meristem is laid down during embryogenesis when the patterns of cell divisions are rigorously controlled and also they have a specialized patterns growth direction and function. The small variability in cell numbers observed in primary roots and the relative simplicity of the tissue pattern, facilitates the interpretation of the effects of their function on the cellular level with an accuracy uncommon in other plant systems.
The existence of various sizes of statoliths in the root cap of each root types revealed that it involved in the perception of gravity by the plant. The statoliths were denser than the cytoplasm and can sediment according to the gravity vector. The position of statoliths indicate that they are heavier than surrounding cytoplasm. A stable direction of growth (Figure 1) can be established either upwards, downwards or horizontally with the amyloplasts sedimenting on the proximal, distal or longitudinal cell walls of statocytes. This could be because amyloplast act as a ballast [35] and their sedimentation has no significantly physiological effect.
5. Conclusion
It can conclude that root caps of 4 root types consisted of two regions, i.e., central columella or statenchyma and peripheral regions. The columella cells (statocyte) are in the form of oval to rectangular. Caps vary in form and size within root system of A. marina. The most striking feature of the root is the distinct and extensive root cap with quite long files of cells. From its shape, structure, and location, it seems clear that the root caps protects the cells under it from abrasion and assists the root in penetrating the soil. We also found that all root with marked gravitropism have statoliths that settle along different walls of that statocyte. This implies that the statoliths sensing of gravity is done by gravity on mass, and that they are denser than surrounding cytoplasm and this position is related to growth direction.
REFERENCES
- V. J. Chapman, “Mangrove Vegetation,” J. Cramer, Vaduz, 1976.
- P. B. Tomlinson, “The Botany of Mangroves,” Cambridge University Press, New York, 1986, 440 p.
- H. Purnobasuki and M. Suzuki, “Functional Anatomy of Air Conducting Network on the Pneumatophores of a Mangrove Plant, Avicennia marina (Forsk.) Vierh.,” Asian Journal of Plant Sciences, Vol. 4, No. 4, 2005, pp. 334- 347. doi:10.3923/ajps.2005.334.347
- P. W. Barlow, “The Root Cap: Cell Dynamics, Cell Differentiation, and Cap Function,” Journal of Plant Growth Regulation, Vol. 21, No. 4, 2003, pp. 261-286. doi:10.1007/s00344-002-0034-z
- G. T. S. Baylis, “Root System of the New Zealand Mangrove,” Transactions of the Royal Society of New Zealand, Vol. 78, 1950, pp. 509-514.
- H. Purnobasuki and M. Suzuki, “Root System Architecture and Gravity Perception of a Mangrove Plant Sonneratia alba J. Smith,” Journal of Plant Biology, Vol. 4, No. 3, 2004, pp. 236-243. doi:10.1007/BF03030514
- I. Nagelkerken, A. M. De Schryver, M. C. Verweij, F. Dahdouh-Guebas, G. van der Velde and N. Koedam, “Differences in Root Architecture Influence Attraction of Fishes to Mangroves: A Field Experiment Mimicking Roots Different Length, Orientation, and Complexity,” Journal of Experimental Marine Biology and Ecology, Vol. 369, No. 1, 2010, pp. 27-34. doi:10.1016/j.jembe.2010.10.002
- A. E. Ashford and W. G. Allaway, “There Is a Continuum of Gas Space in Young Plants of Avicennia marina,” Hydrobiologia, Vol. 295, No. 1-3, 1995, pp. 5-11. doi:10.1007/BF00029105
- H. Purnobasuki and M. Suzuki, “Aerenchyma Tissue Development and Gas-Pathway Structure in Root of Avicennia marina (Forsk.) Vierh,” Journal of Plant Research, 118, No. 4, 2005, pp. 285-294. doi:10.1007/s10265-005-0221-7
- U. Videmsek, B. Turk and D. Vodnik, “Root Aerenchyma—Formation and Function,” Acta agriculturae Slovenica, Vol. 87, 2006, pp. 445-453.
- F. Dahdouh-Guebas, R. De Bondt, P. D. Abeysinghe, J. G. Kairo, S. Cannicci, L. Triest and N. Koedam, “Comparative Study of the Disjunct Zonation Pattern of the Grey Mangrove Avicennia marina (Forsk.) Vierh. in Gazi Bay (Kenya),” Bulletin of Marine Science, Vol. 74, No. 2, 2004, pp. 237-252.
- L. S. Evans, Y. Okawa and D. G. Searcy, “Anatomy and Morphology of Red Mangrove (Rhizopora mangle) Plants in Relation to Internal Airflow,” Journal of The Torrey Botanical Society, Vol. 132, No. 4, 2005, pp. 537-550. doi:10.3159/1095-5674(2005)132[537:AAMORM]2.0.CO;2
- L. S. Evans, M. F. de Leon and E. Sai, “Anatomy and Morphology of Rhizophora stylosa in Relation to Internal Airflow and Attim’s Plant Architecture,” Journal of The Torrey Botanical Society, Vol. 135, No. 1, 2008, pp. 114- 125. doi:10.3159/07-RA-027R.1
- W. Finney, “Comparative Growth and Propagule Viability of Louisiana-Harvested Black Mangrove, Avicennia germinans,” Thesis, Faculty of Nicholls State University, 2011.
- J. B. Fisher and P. B. Tomlinson, “Tension Wood Fibers Are Related to Gravitropic Movement of Red Mangrove (Rhizophora mangle) Seedling,” Journal of Plant Research, Vol. 115, No. 1117, 2002, pp. 39-45. doi:10.1007/s102650200006
- J. A. MacDonald, T. Glover and J. S. Weis, “The Impact of Mangrove Prop-Root Epibionts on Juvenile Reef Fishes: A Field Experiment Using Artificial Roots and Epifauna,” Estuaries and Coasts, Vol. 31, No. 5, 2008, pp. 981-993. doi:10.1007/s12237-008-9083-2
- E. Cocheret de la Morinière, I. Nagelkerken, H. van der Meij and G. van der Velde, “What Attracts Juvenile Coral Reef Fish to Mangroves: Habitat Complexity or Shade?” Marine Biology, Vol. 144, No. 1, 2004, pp. 139-145. doi:10.1007/s00227-003-1167-8
- M. C. Verweij, I. Nagelkerken, D. de Graaff, M. Peeters, E. J. Bakker and G. van der Velde, “Structure, Food and Shade Attract Juvenile Coral Reef Fish to Mangrove and Seagrass Habitats: A Field Experiment,” Marine Ecology Progress Series, Vol. 306, 2006, pp. 257-268. doi:10.3354/meps306257
- J. A. MacDonald, S. Shahrestani and J. S. Weis, “Behavior and Space Utilization of Two Common Fishes within Caribbean Mangroves: Implications for the Protective Function of Mangrove Habitats,” Estuarine, Coastal and Shelf Science, Vol. 84, No. 2, 2009, pp. 195-201. doi:10.1016/j.ecss.2009.06.010
- W. G. Allaway, M. Curran, L. M. Hollington, M. C. Ricketts and N. J. Skelton, “Gas Space and Oxygen Exchange in Roots of Avicennia marina (Forsk.) Vierh. Var. Australasica (Walp.) Moldenke ex N.C. Duke, the Grey Mangrove,” Wetlands Ecology and Management, Vol. 9, No. 3, 2008, pp. 211-218.
- K. Kitaya, K. Yabuki, M. Kiyota, A. Tani, T. Hirano and I. Aiga, “Gas Exchange and Oxygen Concentration in Pneumatophores and Prop Roots of Four Mangrove Species,” Trees, Vol. 16, No. 2-3, 2002, pp. 155-158.
- Y. Okimoto, A. Nose, Y. Katsusa, Y. Tateda, A. Agarie and K. Ikeda, “Gas Exchange Analysis for Estimating Net CO2 Fixation Capacity of Mangrove (Rhizophira stylosa) Forest in the Mouth of River Fukido, Ishigaki Island Japan,” Plant Production Science, Vol. 10, No. 3, 2007, pp. 303-313. doi:10.1626/pps.10.303
- P. F. Scholander, L. Van Dam and S. Scholander, “Gas Exchange in the Roots of Mangroves,” American Journal of Botany, Vol. 42, No. 1, 1955, pp. 92-98. doi:10.2307/2438597
- S. Koi and M. Kato, “Comparative Developmental Anatomy of the Root in Three Species of Cladopus (Podostemaceae),” Annals of Botany, Vol. 91, No. 7, 2003, pp. 927-937. doi:10.1093/aob/mcg092
- M. Ota, R. Imaichi and M. Kato, “Developmental Morphology of the Thalloid Hydrobryum japonicum (Podostemaceae),” American Journal of Botany, Vol. 88, No. 3, 2001, pp. 382-390. doi:10.2307/2657101
- Y. Hiyama, I. Tsukamoto, R. Imaichi and M. Kato, “Developmental Anatomy and Branching of Roots of Four Zeylanidium species (Podostemaceae), with Implications for Evolution of Foliose Roots,” Annals of Botany, Vol. 90, No. 6, 2002, pp. 735-744. doi:10.1093/aob/mcf259
- R. Rutishauser and K. A. Huber, “The Developmental Morphology of Indotristicha ramosissima (Podostemaceae, Tristichoideae),” Plant Systematics and Evolution, Vol. 178, No. 2-3, 1991, pp. 195-223.
- D. A. Johansen, “Plant Microtechnique,” McGraw-Hill Book Co. Inc., New York, 1990.
- T. P. O’Brian and M. E. Cully, “The Study of Plant Structures Principle and Selected Methods,” Termacarphi Pty. Ltd., Melbourne, 1981.
- J. B. Sanderson, “Biological Microtechnique,” BIOS Scientific Pub., Oxford, 1994.
- A. G. Bengough, M. Iijima and P. W. Barlow, “Image Analysis of Maize Root Caps-Estimating Cell Number from 2-D Longitudinal Sections,” Annals of Botany, Vol. 87, No. 5, 2001, pp. 693-698. doi:10.1006/anbo.2001.1392
- C. Arnaud, C. Bonnot, T. Desnos and L. Nussaume, “The Root Cap at the Forefront,” Comptes Rendus Biologies, Vol. 333, No. 4, 2010, pp. 335-343. doi:10.1016/j.crvi.2010.01.011
- H. von Guttenberg, “Die Ntwicklung der Wurzel,” Phytomorph, Vol. 14, 1964, pp. 265-287.
- P. W. Barlow, H. B. Luck and J. Luck, “Autoreproductive Cells and Plant Meristem Construction: The Case of the Tomato Cap Meristem,” Protoplasma, Vol. 215, No. 1-4, 2001, pp. 50-63. doi:10.1007/BF01280303
- R. Wayne, M. P. Staves and A. C. Leopold, “The Contribution of the Extracelluler Matrix to Gravisensing in Characean Cells,” Journal of Cell Science, Vol. 101, 1992, pp. 611-623.

