American Journal of Plant Sciences
Vol.4 No.3(2013), Article ID:28983,6 pages DOI:10.4236/ajps.2013.43071
AACA Element Negatively Regulates Expression of Protein Phosphatase 2C (PP2C) like Promoter in Arabidopsis thaliana
![]()
1Department of Biological Sciences, Birla Institute of Technology & Sciences (BITS), Pilani, India; 2National Agri-Food Biotechnology Institute (NABI), Department of Biotechnology, Government of India, Mohali, India.
Email: *rmehrotra@bits-pilani.ac.in, *rajmeh25@hotmail.com
Received January 5th, 2013; revised February 10th, 2013; accepted February 20th, 2013
Keywords: Abscisic Acid; ACGT; AACA; Abiotic Stress
ABSTRACT
Genome wide analysis of Arabidopsis thaliana reveals a unique genetic arrangement of ACGT motif in the protein phosphatase 2C (PP2C) like promoter (accession number AT5G59220). In the present study, full length, 900 base pair, 500 base pair, 400 base pair and NRM deletion variants of PP2C like promoter were constructed to investigate the activity of PP2C like promoter in the presence of inducers like abscisic acid, jasmonic acid and salicylic acid. The PP2C promoter has three ACGT elements in close vicinity. They are so positioned that they are separated by a spacer of 30 base pair and 5 base pair respectively from each other. The study has shown that ACGTN30ACGT genetic architecture is essential for the promoter to be induced in response to abscisic acid. The synergistic and antagonistic effects of cis elements were observed. AACA is a positive regulatory element in endosperm and is known to act as negative regulatory element in other tissues. In this study AACA, have been found to negatively regulate the expression of reporter gene EGFP in both induced and under uninduced conditions.
1. Introduction
Plants, being sessile in nature need to adopt different methods to combat stresses in comparison to animals, be it environmental or biological. Phytohormones like jasmonic acid (JA) and salicylic acid (SA) help the plant to face biotic stress. To cope up with the abiotic stress plants have two pathways, viz. abscisic acid (ABA) dependent and ABA independent [1]. The ABA dependent stress responsive genes have ABA responsive element (ABRE) which is present in its promoter region [2] and has ACGT as a core sequence [3]. ACGT is also the core sequence of the G box, involved in the red light signalling [4], UV response, anaerobiosis in plants and SA response elements [5].
The ABA mediated stress signaling pathway involves the receptor PYL/PYR/RCAR, a negative regulator PP2C, positive regulator SnRK2s, cis regulatory element known as ABRE and transcription factors [6]. Cis element is a consensus sequence present in the upstream region of the basal promoter, and it acts as the binding site for transcription factors. Gene expression is the result of the interaction between cis element and its complementary transcription factor, the latter being activated by a particular developmental stage or environmental cues.
A promoter consisting of TATA box is sufficient to transcribe a gene at its basal level. Diversity among promoters is an outcome of the presence of different cis regulatory elements, their variation in copy numbers, position in context to TATA box and the spacer length between two adjacent cis elements [7]. Copy number and length of the spacer is critical in determining the expression of the gene [8]. This is much evident with ACGT and GT cis elements, where both copy number and spacer length are responsible for high or low expression of gene. GT element acts as negative regulator on increasing its copy number with increase in spacer length. Reference number [7] has reported decrease in gene expression with an increasing copy number of adjacent GT motifs [9]. But unlike GT elements, an increase in copy number and spacer length to 5, 10, and 25 in case of ACGT element leads to an enhanced gene expression of the β-glucuronidase (gus A) reporter gene.
Reference number [10] showed that two ACGT elements separated by 25 base pair (bp) are induced by ABA. However, when separated by 5 nucleotides, they are induced by SA in transgenic tobacco. The genome wide analysis of Arabidopsis thaliana revealed the frequency of occurrence of two ACGT motifs separated by a spacer of 25 bp to be 62 while for 5 bp, the occurrence was 72 [11]. One such promoter located at chromosome number 5 (accession number AT5G59220) is protein phosphatase 2C (PP2C) like promoter from Arabidopsis thaliana which has ABA and SA inducible ACGT motifs. PP2C like promoter has genetic architecture which includes three ACGT elements. Two adjacent ACGT elements are separated by 30 nucleotides, followed by another set of ACGT motifs separated by 5 nucleotides (Figure 1).
The ACGT core motif serves as a binding site for the bZIP class of transcription factors. The nucleotides flanking the ACGT motif determine the binding affinity and specificity of bZIP transcription factors, which in turn leads to distinct physiological functions as in stress responses, light regulation etc. [12,13]. It has been reported that the flanking sequence is also responsible for differential distribution of stress responsive regulatory elements in stress responsive promoters [14]. In this study, PP2C like promoter was cloned and several deletion constructs were made and bombarded on to the tobacco leaves to see their effect on the expression of EGFP reporter gene cloned downstream of it. Further, considering the fact that PP2C like promoter has ABA and SA response elements positioned in close proximity, the study could also give an insight in to the relation between abiotic and biotic stress mediated responses.
Earlier, constitutive promoters were widely used in development of transgenic plants, but they suffer from the drawback of being highly energy demanding as they are always active even in favorable conditions. The demand for high energy for promoter activity in relation to constitutive promoters leads to economic imbalance in terms of energy which may significantly affect overall expression of productive genes. The beneficial solution can be through the use of a stress inducible promoter
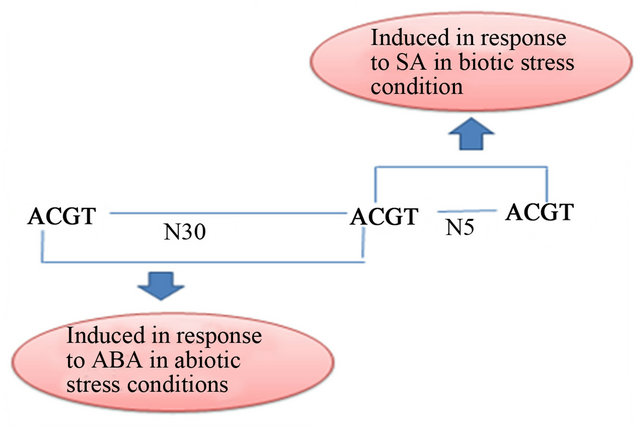
Figure 1. Genetic arrangement of NRM cassette. The bold ACGT element has been removed in NRM deletion construct of PP2C like promoter.
which is intended to stimulate under precise biotic and abiotic stresses [15].
PP2C like promoter has both biotic stress and abiotic stress response elements in close proximity and provides an added advantage over the constitutive promoters and it could be used as an inducible promoter.
In this study the expression of 400 bp construct have shown higher expression in comparison to the full length construct due to the decrease in the copy number of AACA element.
2. Material and Methods
2.1. Cloning of PP2C like Promoter from Arabidopsis thaliana
Growth conditions of Arabidopsis thaliana columbia ecotype were standardized and grown at 22˚C under 16 hr light and 8 hr dark conditions until silique formation. Total genomic DNA was isolated from the young leaves of Arabidopsis thaliana using CTAB method [16]. TAIR (The Arabidopsis Information Resource) data base was used to access the sequence of the protein phosphates 2C (PP2C) like promoter bearing the accession number AT5G59220. The primers used for amplification are mentioned in Table 1. The PCR conditions were standardized as initial denaturation at 94˚C for 2 minutes, final denaturation at 94˚C for 1 minutes, annealing temperature at 58˚C for 1.30 minutes, extension temperature at 72˚C for 1.30 minutes and final extension temperature at 72˚C for 4 minutes.
The resultant PCR amplicons were cloned into pEGFP (Clontech) vector between PstI and BamHI restriction sites (Figure 2). The E. coli DH5α competent cells were transformed with the recombinant plasmid. After transformation, plasmids were isolated and the presence of the inserted promoter was confirmed by sequencing.
Table 1. The primers used for amplification of the PP2C like promoter.
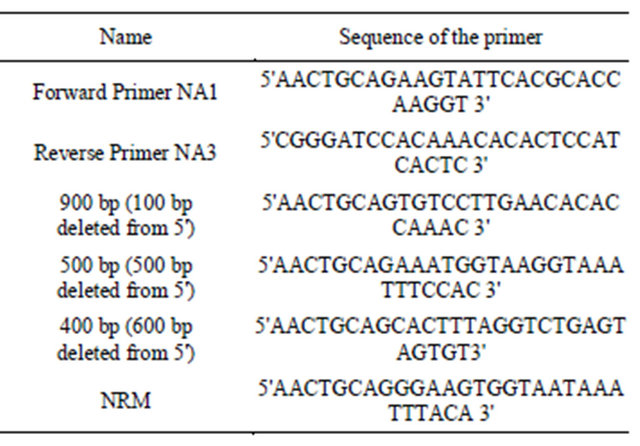
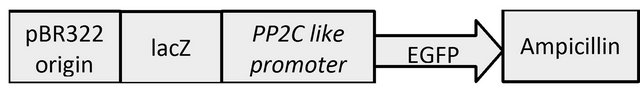
Figure 2. A pictorial representation of promoter reporter gene constructs used for plant transformation.
2.2. Transient Transformation and EGFP Assay
Transient transformation of Nicotiana tabbacum cv. Xanthi was done using Biolistic method [17]. DNA for bombardment was prepared by using Qiagen columns. The PP2C promoter reporter cassettes were coated onto gold microprojectiles and bombarded onto the tobacco leaves at 1100 psi using biolistic gene gun (BioRad PDS- 1000/He). Before bombardment the leaves were incubated on MS [18] agar plates for 12 hr. After bombardment, leaves were incubated on Hoagland media containing inducers such as 100 µM ABA, 100 µM SA, 50 µM JA at 24˚C under16 hr light and 8 hr dark photoperiod. After 48 hrs of incubation, leaves were ground in liquid nitrogen and suspended in extraction buffer (1 M Tris pH 8, 0.5 M EDTA pH 8, 0.5 M EGTA pH 8, 0.5M NaF, 1M sodium orthovandate, 1 M beta mercaptoethanol, 10% v/v glycerol) and centrifuged at 13,000 rpm for 10 minutes at 4˚C. 200 µl of the supernatant was loaded to 96 well titer plates. The excitation and emission wavelength peaks for EGFP are 484 nm and 509 nm. EGFP concentration was determined by the absorbance at 484 nm and was calculated using the reported excitation coefficients 55,000/cm/M [19,20].
3. Results and Discussion
3.1. The Functional Classification of Cis Elements Present in Full Length PP2C like Promoter
To characterize the PP2C like promoter, a series of 5’ deletion constructs were made like 900 bp, 500 bp, 400 bp and NRM. For analyzing the presence of cis elements in full length of PP2C like promoter, the PLACE database was used [21,22]. This analysis shows that different motifs responsive to inducers like ABA, JA, SA, light regulation etc. are present in the PP2C like promoter (Table 2). In our study inducers like ABA, JA and SA were used.
3.2. AACA Motif Negatively Regulates the Gene Expression of PP2C like Promoter
The full length PP2C like promoter and its deletion variants which include 900 bp, 500 bp, 400 bp and NRM constructs were bombarded on to the leaves of Nicotiana tabacum cv. Xanthi and were incubated in Hoagland medium supplemented with inducers ABA, JA and SA and without inducers. The activity of the promoter reporter construct was evaluated on the basis of the expression of EGFP reporter gene following the transient transformation of Nicotiana tabacum cv. Xanthi.
Non-recombinant pEGFP vector was used as control. The expression of EGFP was observed in the leaves bombarded with control construct which was low in comparison to other promoter reporter cassettes of PP2C like promoter, as expected. The expression of the reporter gene observed in case of the control was due to the lacZ promoter. However, in the presence of inducers like ABA, JA and SA, control does not show any induction (Figure 3).
Under uninduced conditions the full length as well as deletion variants of PP2C like promoter showed more activity than the control, i.e. pEGFP vector (Figure 3). It was observed that, the expression of EGFP is higher in 400 bp and NRM cassettes of PP2C like promoter whereas in full length, 900 bp and 500 bp constructs of PP2C like promoter the expression of EGFP was low, the latter being at par with each other. This is attributed to the presence of AACA elements in the full length, 900 and 500 bp constructs. AACA element is a tissue specific cis element and is known to express only in the endosperm [23]. Reference number [23] observed that deletion of AACA motif leads to decrease in the seed storage Glu B-1 promoter activity. The presence of AACA in
Table 2. Cis regulatory elements present in the PP2C like promoter.
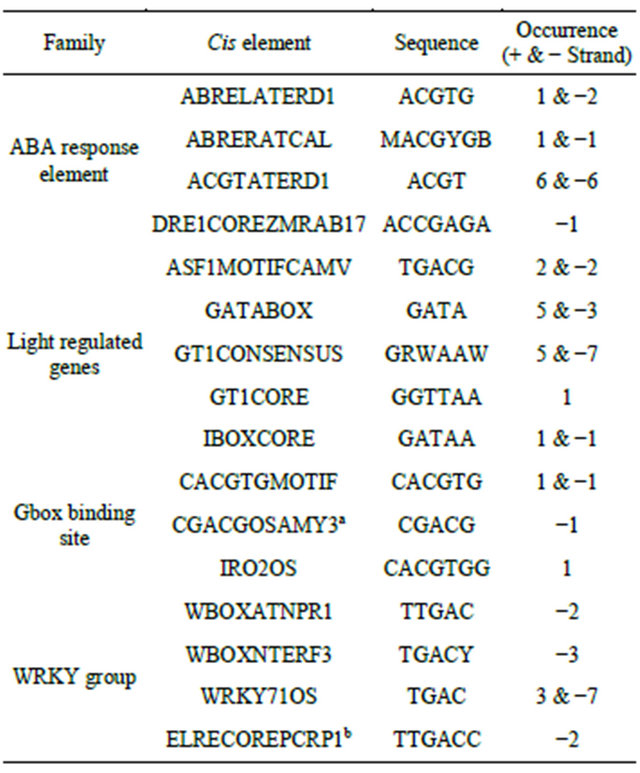
M = A/C; K = G/T; Y = T/C; N = A/G/C/T; R = A/G; W = A/T; S = G/C; H = T/C/A; D = A/T/G. aAct as coupling element for G box element; bElecitor response element.
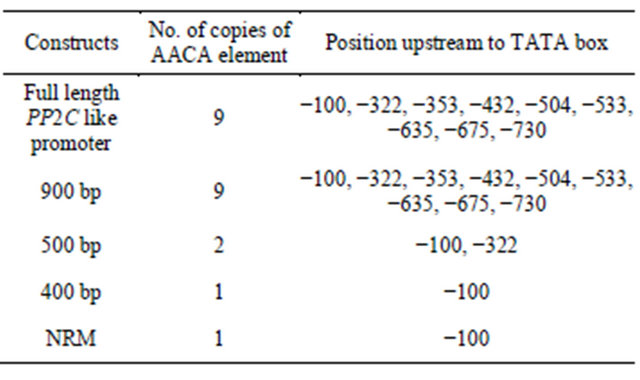
promoter of genes in any other tissue, acts as negative cis element for other elements [24]. The AACA element being present in full length, 900 bp and 500 bp could be responsible for reduced expression of reporter gene. This negative cis element AACA is present in PP2C like promoter in 9 copies. This number decreases to one in case of deletion cassettes like 400 bp and NRM construct and show high activity in terms of expression of the reporter gene (the position of the AACA motif is mentioned in Table 3).
3.3. Expression of PP2C like Promoter in the Presence of Elicitors
The induction was observed in each case of PP2C like promoter cassettes in comparison to the control incubated in Hoagland media supplemented with ABA, JA and SA, respectively.
The motifs which confer response towards ABA have ACGT as a core sequence. These motifs are like ACGTERD1 and ABRELATERD1 (as mentioned in Table 2). The successive constructs viz. full length and 900 bp of
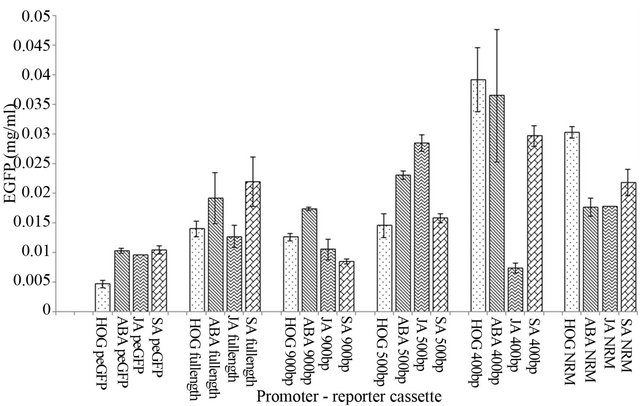
Figure 3. Expression of the PP2C like promoter (with different constructs) under un-induced and induced conditions (green: Hoagland media (H.M); blue: H.M + ABA; purple: H.M + JA; orange: H.M + SA). The vertical bar indicates standard deviation.
Table 3. The copy number and the position of the AACA motif in context to TATA box.
PP2C like promoter have six copies of ACGTATERD1 motif while 500 bp and 400 bp constructs have four copies of ACGTATERD1 motif. The ACGT motifs in close proximity lead to increase in the expression of the gene [7]. In the PP2C like promoter, other ACGT motifs are not located in close vicinity to each other. It could also be attributed to the presence of AACA, which acts as negative elements in these promoter reporter cassettes. Though the AACA motif does not have any direct relationship with the ACGT cis element, it decreases the overall expression of the reporter gene in constructs like full length, 900 bp and 500 bp deletion construct of PP2C like promoter.
The expression of NRM cassette in the presence of ABA when compared with the 500 bp and 400 bp constructs is low even though AACA copy number falls down to 1 from 9. The NRM cassette showed expression nearer to full length and 900 bp constructs. The genetic architecture of NRM construct (Figure 1) has been disturbed by removing one ACGT motif, and retains only two ACGT elements separated by 5 bp. The induction of NRM deletion construct was observed to be comparatively mild in the presence of ABA in comparison to 400 bp construct. Hence this shows that two ACGT elements separated by 30 bp are essentially responsible for induction by ABA.
In the induction studies in the presence of JA, the full length, 900 bp and NRM constructs of PP2C like promoter have not shown similar levels of EGFP expression. The highest level of expression is observed in the case of 500 bp cassette and very low expression observed in 400 bp cassette (shown in Figure 3). The deletion of 100 bp from 500 bp deletion construct led to deletion of cis regulatory elements and decrease in the copy number of the cis elements like WRKY elements which could have reduced the expression of 400 bp cassette.
When incubated in the presence of SA the full length, 500 bp, 400 bp and NRM cassette (Figure 3) showed more expression than 900 bp construct. Minimal machinery required for the induction by SA is present in full length, 900 bp and 500 bp. The 400 bp and NRM construct shows more expression, which have 1 copy of AACA element. The 400 bp and NRM has the basic structure i.e. two ACGT separated by 5 bp. This leads to increase in the expression of EGFP. In study conducted by reference number [25] similar observations were made, viz. the absence of regulatory elements in the −119/101 mutant did not lead to induction by methyl jasmonate (MJ) and SA. However, induction was observed in the case of the −112/101 mutant which had the regulatory elements, thus indicating the importance of the presence of regulatory element for MJ and SA dependent induction.
4. Conclusion
Up regulation and down regulation of expression is based on the synergistic action of cis elements present in the promoter region of the gene. Reference number [26] and [15] have reported that proper spatial arrangement of the cis elements is responsible for the transcription. The combinatorial activity of positive and negative cis elements is found to be responsible for the regulation of gene expression. The current study reveals that AACA element negatively regulates the expression of the PP2C like promoter, even in the presence of inducers viz. ABA, JA and SA. Reference number [10] had shown that two ACGT elements separated by 25 bp were induced in response to ABA and in present study; it has been shown that PP2C like promoter with an arrangement of two ACGT elements separated by 30 bp is also induced in response to ABA.
5. Acknowledgements
The authors are grateful to the Department of Science and Technology, New Delhi, India for Grant-in-Aid and financial support to carry out this work bearing the file no. SR/FT/LS-126/2008. The authors are thankful to Dr Rakesh Tuli and Dr Siddharth Tiwari for providing biolistic facilities. The authors are grateful to the administration of BITS, Pilani, Rajasthan and NABI, Mohali for providing logistic support. PB is also thankful to UGC/ BSR for providing financial support.
REFERENCES
- P. K. Busk and M. Pages, “Regulation of Abscisic AcidInduced Transcription,” Plant Molecular Biology, Vol. 37, No. 3, 1998, pp. 425-435. doi:10.1023/A:1006058700720
- Q. Shen and T. H. Ho, “Functional Dissection of an Abscisic Acid (ABA)-Inducible Gene Reveals Two Independent ABA Responsive Complexes Each Containing a G-Box and a Novel cis Acting Element,” Plant Cell, Vol. 7, No. 3, 1995, pp. 295-307.
- M. J. Guiltinan, W. R. Marcotte and R. S. Quatrano, “A Plant Leucine Zipper Protein that Recognizes an Abscisic Acid Response Element,” Science, Vol. 250, No. 4978, 1990, pp. 267-271. doi:10.1126/science.2145628
- R. G. K. Donald and A. R. Cashmore, “Mutation of Either G Box or I Box Sequences Profoundly Affects Expression from the Arabidopsis rbcS-1A Promoter,” The EMBO Journal, Vol. 9, No. 6, 1990, pp. 1717-1726.
- I. Jupin and N. H. Chua, “Activation of the CaMV as-1 cis Element by Salicylic Acid: Differential DNA-Binding of a Factor Related to TGA1a,” The EMBO Journal, Vol. 15, No. 20, 1996, pp. 5679-5689.
- T. Umezawa, K. Nakashima, T. Miyakawa, T. Kuromori, M. Tanokura, K. Shinozaki and K. Yamaguchi-Shinozaki, “Molecular Basis of the Core Regulatory Network in ABA Responses: Sensing, Signaling and Transport,” Plant and Cell Physiology, Vol. 51, No. 11, 2010, pp. 1821- 1839. doi:10.1093/pcp/pcq156
- R. Mehrotra, K. Kiran, C. P. Chaturvedi, S. A. Ansari, N. Lodhi, S. Sawant and R. Tuli, “Effect of Copy Number and Spacing of the ACGT and GT cis Elements on Transient Expression of Minimal Promoter in Plants,” Journal of Genetics, Vol. 84, No. 2, 2005, pp. 183-187. doi:10.1007/BF02715844
- T. Hobo, M. Asada, Y. Kowyama and T. Hattori, “ACGT Containing Abscisic Acid Response Element (ABRE) and Coupling Element 3 (CE3) Are Functionally Equivalent,” The Plant Journal, Vol. 19, No. 6, 1999, pp. 679-689. doi:10.1046/j.1365-313x.1999.00565.x
- R. Mehrotra and J. Panwar, “Dimerization of GT Element Interferes Negatively with Gene Activation,” Journal of Genetics, Vol. 88, No. 2, 2009, pp. 257-260. doi:10.1007/s12041-009-0037-7
- R. Mehrotra and S. Mehrotra, “Promoter Activation by ACGT in Response to Salicylic and Abscisic Acids Is Differentially Regulated by the Spacing between Two Copies of the Motif,” Journal of Plant Physiology, Vol. 167, No. 14, 2010, pp. 1214-1218. doi:10.1007/s12041-009-0037-7
- R. Mehrotra, A. Yadav, P. Bhalothia, R. Karan and S. Mehrotra, “Evidence for Directed Evolution of Larger Size Motif in Arabidopsis thaliana Genome,” The Scientific World Journal, Vol. 2012, 2012, Article ID: 983528. doi:10.1100/2012/983528
- T. Izawa, R. Foster and N. H. Chua, “Plant bZIP Protein DNA Binding Specificity,” Journal of Molecular Biology, Vol. 20, No. 4, 1993, pp. 1131-1144. doi:10.1006/jmbi.1993.1230
- R. Foster, T. Izawa and N. H. Chua, “Plant Basic Leucine Zipper Proteins Gather at ACGT Elements,” The EMBO Journal, Vol. 8, No. 2, 1994, pp. 192-200.
- M. Suzuki, M. G. Ketterling and D. R. McCarty, “Quantitative Statistical Analysis of cis-Regulatory Sequences in ABA/VP1- and CBF/DREB1-Regulated Genes of Arabidopsis,” Plant Physiology, Vol. 139, No. 1, 2005, pp. 437- 447. doi:10.1104/pp.104.058412
- R. Mehrotra, G. Gupta, R. Sethi, P. Bhalothia, N. Kumar and S. Mehrotra, “Designer Promoter: An Artwork of cis Engineering,” Plant Molecular Biology, Vol. 75, No. 6, 2011, pp. 527-536. doi:10.1007/s11103-011-9755-3
- M. G. Murray and W. F. Thompson, “Thompson Rapid Isolation of High Molecular Weight Plant DNA,” Nucleic Acids Research, Vol. 8, No. 19, 1980, pp. 4321-4326. doi:10.1093/nar/8.19.4321
- S. V. Sawant, P. K. Singh and R. Tuli, “Pretreatment of Micropojectiles to Improve the Delivery of DNA in Plant Transformation,” Biotechniques, Vol. 29, No. 2, 2000, pp. 246-248.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Plant Physiology, Vol. 15, No. 43, 1962, pp. 473- 497.
- C. Reichel, J. Mathur, P. Eckes, K. Langenkemper, C. Koncz, J. Schell, B. Reiss and C. Maas, “Enhanced Green Fluorescence by the Expression of an Aequorea victoria Green Fluorescent Protein Mutant in Monoand Dicotyledonous Plant Cells,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 93, No. 12, 1996, pp. 5888-5893. doi:10.1073/pnas.93.12.5888
- J. J. Wu, Y. W. Liu and M. X. Sun, “Improved and High throughput Quantitative Measurements of Weak GFP Expression in Transgenic Plant Materials,” Plant Cell Report, Vol. 30, No. 7, 2011, pp. 1253-1260. doi:10.1046/j.1365-313x.2000.00797.x
- D. S. Prestridge, “SIGNAL SCAN: A Computer Program That Scans DNA Sequences for Eukaryotic Transcriptional Elements,” Computer Applications in the Biosciences, Vol. 7, No. 2, 1991, pp. 203-206.
- K. Higo, Y. Ugawa, M. Iwamoto and T. Korenaga, “Plant cis-Acting Regulatory DNA Elements (PLACE) Database,” Nucleic Acids Research, Vol. 27, No. 1, 1999, pp. 297-300. doi:10.1093/nar/27.1.297
- C. Wu, H. Washida, Y. Onodera, K. Harada and F. Takaiwa, “Quantitative Nature of the Prolamin-Box, ACGT and AACA Motifs in a Rice Glutelin Gene Promoter: Minimal cis-Element Requirements for Endosperm-Specific Gene Expression,” The Plant Journal, Vol. 23, No. 3, 2000, pp. 415-421. doi:10.1046/j.1365-313x.2000.00797.x
- T. Yoshihara, H. Washida and F. Takaiwa, “A 45-bp Proximal Region Containing AACA and GCN4 Motif Is Sufficient to Confer Endosperm-Specific Expression of the Rice Storage Protein Glutelin Gene, GluA-3,” FEBS Letters, Vol. 383, No. 3, 1996, pp. 2133-2138. doi:10.1016/0014-5793(96)00233-5
- S. R. Kim, Y. Kim and G. An, “Identification of Methyl Jasmonate and Salicylic Acid Response Elements from the Nopaline Synthase (nos) Promoter,” Plant Physiology, Vol. 103, No. 1, 1993, pp. 97-103. doi:10.1104/pp.103.1.97
- S. Sawant, K. Kiran, R. Mehrotra, C. P. Chaturvedi, S. A. Ansari, P. Singh, N. Lodhi and R. Tuli, “A Variety of Synergistic and Antagonistic Interactions Mediated by cis-Acting DNA Motifs Regulate Gene Expression in Plant Cells and Modulate Stability of the Transcription Complex Formed on a Basal Promoter,” Journal of Experimental Botany, Vol. 56, No. 419, pp. 2345-2353. doi:10.1093/jxb/eri227
Abbreviations
ABA: Abscisic Acid;
bp: Base Pair;
EGFP: Enhanced Green Fluorescent Protein;
JA: Jasmonic Acid;
PP2C: Protein Phosphatase 2C;
SA: Salicylic Acid.
NOTES
*Corresponding author.

