American Journal of Plant Sciences
Vol.3 No.7(2012), Article ID:20683,4 pages DOI:10.4236/ajps.2012.37110
Improved Clonal Propagation of Alpinia calcarata Rosc., a Commercially Important Medicinal Plant and Evaluation of Chemical Fidelity through Comparison of Volatile Compounds
![]()
1Biotechnology and Bioinformatics Division, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Thiruvananthapuram, Kerala, India; 2Phytochemistry and Phytopharmacology Division, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Thiruvananthapuram, Kerala, India; 3Department of Plant Science, Central University of Kerala, Kasargod, Kerala, India.
Email: *cgsudha@yahoo.co.in
Received February 24th, 2012; revised March 30th, 2012; accepted May 25th, 2012
Keywords: Alpinia calcarata; Clonal Propagation; Essential Oil; GC-MS
ABSTRACT
An efficient and improved clonal propagation of Alpinia calcarata, a commercially important medicinal plant was established on Murashige and Skoog medium. The axillary shoot proliferation was achieved with maximum 5.2 ± 0.7 shoots in 92.8% of rhizome explants in medium with 2.0 mg/L 6-benzylamiopurine (BAP) and 0.2 mg/L indole-3-acetic acid (IAA). Axillary shoot buds (60%) upon subculture for 8 weeks in the same medium produced multiple shoot initials (12.1 ± 0.4) mediated with meristemoids (4.0 ± 0.5) and callus. A gradual reduction in the concentration of BAP or elimination of IAA was required for rapid induction of normal plants devoid of callus from propagules during subsequent subculture. Single clump of 3 - 4 multiple shoot initials during second subculture on medium with 1.0 mg/L BAP and 0.1 mg/L IAA yielded an average of 21 shoots which was best among different propagules tried. The shoot multiplication rate was further enhanced to 32 shoots when the similar propagules passed to third subculture on medium with 1.0 mg/L BAP alone. Clumps of multiple shoot initials upon subculture on medium with 1.0 mg/L BAP alone exhibited 10 fold multiplication rates. Use of liquid medium in culture bottles with polypropylene caps supported fast growth of the shoots and spontaneous root formation on 50% of the shoots. Shoots transferred to half-strength MS liquid medium with 0.2 mg/L of IAA and IBA was optimum for maximum roots (8.14 ± 1.34) in 100% shoots. The rooted plants were hardened in mist chamber showed 95% survival and well established in the field. The acclimatized plants showed rhizome formation after 4 - 6 weeks of growth under shade house. Volatile chemicals profile of the leaves, rhizome and root of the in vitro and conventionally propagated plants analyzed by gas chromatography-mass spectrometry were qualitatively and quantitatively similar. The analysis of growth characteristics of 36 months old in vitro and conventionally propagated plants showed a 50% increment of rhizome fresh biomass with prolific root and leaf growth in the former than the latter ones. The protocol described herein will have practical applications for the large scale production of phytochemically uniform plants for commercial cultivation of A. calcarata.
1. Introduction
Alpinia calcarata Rosc. (Zingiberaceae), is a commercially important aromatic medicinal plant, native to India and China [1]. The rhizomes of the plant are used extensively in the traditional systems of medicine in Asian countries. The rhizomes are reported to have antimicrobial [2], antinociceptive [3] and anti-inflammatory [4] activities. Apart from these bioactivities, the rhizome exhibits insecticidal activity [5]. In India, the dried rhizomes form a major ingredient of several Ayurvedic drug formulations such as Rasnadhi Kazhayam, Rasnadhi Choornam, Rashnadhi Thailam and Ashawagandharishtam [6].
The aromatic compounds, 1,8-cineole and β-pinene were reported as the major constituents in leaf, flower and rhizome oil and α-fenchyl acetate from root oil of the plant [7]. It has been estimated that approximately 1.70 tons of dried rhizome of A. calcarata were required for the northern districts of Kerala state in India [8]. The plant is propagated conventionally by rhizome cuttings which are insufficient for a commercial scale production to meet the present day demand. Moreover, it is impractical and uneconomic to utilize the rhizomes for cultivation purposes as it constitute the raw material for the drug preparation. Furthermore, improvement through conventional breeding will be difficult due to its rare flowering and lack of seed set. The isolated cultivation of the plant will not meet the present day demand within the country. The optimum stage of harvest for maximum yield of rhizomes of the plant is 36 - 46 months during which the suitable propagules can be separated for cultivation. Prolonged maturity period is yet another barrier for the continuous availability of propagules for cultivation.
In vitro propagation techniques through resident meristems favour the production of true to type plants. It is a well accepted and proven technology to generate genuine raw materials of medicinal plants. Qualitative and quantitative analysis of secondary metabolites are advantageous for the micropropagation of medicinal plants and it has been reported in many medicinal plant species [9-11].
Production of plants in vitro from small to commercial scale has forced many researchers to think about important issues such as cost efficiency, higher productivity, automation and optimization of minor environment [12]. Although there is a previous report on preliminary information on micropropagation of A. cacarata [13] there has been no study for the establishment of a cost effective rapid production of uniform plants. By considering the importance of large scale cultivation of A. calcarata, the present study focuses to establish an efficient and cost effective rapid clonal propagation and assessment of chemical fidelity of the in vitro raised plants.
2. Materials and Methods
Actively growing axillary shoot buds derived from the rhizomes of 12 month-old stock plants raised in the herbal garden of our institute were used as explants. The outer 3 - 4 scale leaves and cut basal surfaces of the explants were removed after thorough wash under running tap water. They were immersed in 2% Teepol detergent (v/v) for 30 min. with continuous swirling. Traces of detergents were removed by thorough washing under tap water and rinsed thrice in distilled water. Surface decontamination was performed by immersing the explants in 15% (v/v) Steriliq commercial bleach for 15 min followed by 0.1% (w/v) mercuric chloride (HgCl2) for 8 - 10 min. Each step of sterilization was followed by 5 - 6 times rinses in sterile distilled water. The explants (0.8 - 1.2 cm) were prepared followed by the removal of the exposed outer 3 - 4 scale leaves and cut surfaces and dipped in the sterile distilled water. They were blotted on sterile filter paper prior to inoculation onto 15 ml aliquots of solidified Murashige and Skoog medium [14] dispensed in 25 × 150 mm culture tubes. The first step of shoot bud induction experiment was concentrated to determine the best cytokinin among 6-benzylaminopurine (BAP), Kinetin (KN), Thidiazuron (TDZ) used at concentration of (0.1 - 5.0 mg/L) and the second step was to find out the combined action of optimum concentration of BAP (2.0 mg/L) and auxins, indole-3 acetic acid (IAA), ∞ naphthalene acetic acid (NAA), and indole-3 buteric acid (IBA) at concentration of 0.05 - 0.5 mg/L. The pH of the medium was adjusted to 5.8 before autoclaving at 121˚C and 108 Pa for 18 min. All the cultures were incubated at 24˚C ± 2˚C under 16-h photoperiod at a photon flux density of 20 - 50 µmol·m−2·s−1 from day light fluorescent tubes for 8 weeks. Each treatment was conducted twice using 20 replicates.
Shoot multiplication was achieved by subculturing the regenerated axillary shoot buds (propagules) after 8 weeks of initiation. Outer 2 - 3 leaf sheaths were carefully removed from the propagules before inoculation on to the same solid medium supplemented with BAP alone (1.0 - 3.0 mg/L) or with 0.2 mg/L IAA in 250 ml Erlenmeyer flask and incubated for 8 weeks. During the 2nd subculture, the propagules were segregated into solitary axillary shoot buds with swollen base, clumps of multiple shoot initials (mls) and meristemoides (mrs) and they were cultured separately onto the same medium supplemented with BAP alone (0.5 - 1.0 mg/L) or with 0.1 mg/L IAA in 250 ml Erlenmeyer flask and incubated for 6 week. Thereafter, the solitary axillary shoot buds alone were subjected to the removal of leaf sheaths prior to inoculation. From the 3rd subculture onwards, well developed shoots were used for root initiation and clumps of minimum 3 - 4 multiple shoot initials and meristemoids were subcultured as propagules.
Shoot clumps of 3 - 4 developing shoot initials and meristemoids were subcultured on MS liquid and solid medium with 1.0 mg/L BAP in culture bottles with polypropylene caps from the 4th subculture onwards. Thereafter clumps of multiple shoot initials were subcultured regularly on the same liquid and meristemoids in solid medium, dispensed in culture bottles at 6 weeks interval to scale up shoot cultures. However, meristemoids were also maintained on solid medium with 0.1 mg/L BAP in 250 ml Erlenmeyer flasks as stock cultures. The well grown shoots (3.0 - 4.0 cm) from 6 weeks-old shoot cultures were used for root initiation on half-strength MS (full-strength myo-inositol and sucrose) liquid and solid medium with varied concentrations of auxins (IAA, IBA, NAA) alone or in combinations in 15 ml aliquots in 25 × 150 mm culture tubes. Shoots inoculated in liquid medium was supported with aseptic coarse filter paper discs. Six weeks after root initiation, the plants were carefully removed and immersed in 0.3% Indofil M-45 for 15 - 20 minutes followed by 3 - 4 washes under tap water. They were transferred to 5 × 5 cm clay pots containing sand: soil: cow dung (1:1:1) and well irrigated and hardened for 3 - 4 weeks in mist chamber. After 4 weeks, acclimatized plants were transferred to perforated black Polythene covers (13 × 20 cm) with same fresh potting mixture and weaned for 4 weeks under shade house before transferring to the field condition. During subculture in liquid medium, the shoots obtained with spontaneously initiated roots were directly used for acclimatization and those without roots were inoculated for root induction in the optimized medium.
2.1. Isolation and Analysis of Essential Oil
Fresh leaves, rhizome and roots (200 g each) of 36 month-old field established in vitro and conventionally propagated plants were hydro-distilled for 4 h. using Clevenger type apparatus to recover the essential oil. The oil was analyzed for the volatile chemical profile using gas chromatography-flame ionization detector (GC-FID) and gas chromatography-mass spectrometry (GC-MS). The GC-FID analysis was carried out on a Varian CP- 3800 gas chromatograph equipped with a flame ionization detector (FID) and a CP Sil 8CB fused silica capillary column (30 m × 0.32 mm, film thickness-0.25 μm). The GC/MS analysis was done on a Hewlett Packard 6890 gas chromatograph fitted with a cross-linked 5% phenyl methyl siloxane HP-5 MS capillary column (30 m × 0.32 mm, film thickness-0.25 μm) coupled with a 5973 series selective mass detector. Essential oil (1.0 μL) was injected under splitless injection condition. Helium was used as the carrier gas at 1.4 ml/ min constant flow mode, with injector temperature 220˚C and oven temperature 60˚C to 246˚C (3˚C/min). Mass spectra at electron impact (EI+) mode were taken at 70 eV and ion source temperature was 250˚C. The constituents were identified by MS library search (Wiley, 275), relative retention indices (RRI) calculated using homologues of n-alkanes as standards [15] and literature [16].
2.2. Analysis of Growth Characteristics
Growth characteristics in terms of length and number of roots, biomass of rhizome, length and width of leaves of randomly selected 10 plants were recorded after 36 month of growth in the field. Rhizome and root characters were measured after a wash to remove the soil and other debris.
2.3. Statistical Analysis
Data were statistical analyzed by analysis of variance (ANOVA) and means were compared using multiple range test (P ≤ 0.05) using SPSS/PC + Version 4.0 (SPSS Inc, Chicago IL, USA).
3. Results and Discussion
Maximum frequency of contamination free explants (85%) was obtained after HgCl2 treatment for 7 - 8 min and further exposure was lethal to the tissue. In general, contamination was reported at high rate on explants from rhizomes of zingibers were used [17,18]. Low rate of contamination in the present system might be due to the active growth stage of the explants, appropriate steps for surface sterilization and removal of enough outer leaf sheaths.
Irrespective of the type and concentrations of the cytokinins, each axil of the explants elicited solitary shoot bud without the intervening callus formation. Among various cytokinins tried, BAP was more active than TDZ and KN to initiate normal axillary shoot bud rapidly. The explants cultured on MS medium with 2.0 mg/L BAP initiated single shoot bud after 14 - 16 days from each axils (Figure 1(a)). The maximum number of shoots (4.3 ± 5.2) obtained in 68.3% of explants grown on this medium was optimal for shoot induction (Figure 2). TDZ and KN showed best responses at 1.0 mg/L and 3.0 mg/L respectively. The shoot buds initiated on medium with all concentrations of KN were thin and unhealthy. On the other hand, one of the axillary buds initiated on more than 85% explants cultured on medium containing TDZ was grown vigorously (Figure 1(b)). In such explants, the other shoot buds showed poor growth or not protruded properly. The shoot buds initiated on medium with higher concentrations of all cytokinins were thick and stunted besides the diminishing status of all characters noticed. The adverse effect of higher concentrations of cytokinin was met with many zingibers studied in vitro [19,20]. Along with shoot buds, root initiation was also noticed from the axils of the explants grown on medium with all cytokinins. Simultaneous root and shoot formation from rhizomes during initiation in cytokinin supplemented medium was similarly reported in turmeric, ginger [21] and in A. galangal [17]. The prominent shoot organogenic efficiency of BAP in the present study was well documented in other members of Zingiberaceae [20, 22,23]. However, studies on Zingibers indicate that the requirement of cytokinins is found to be varying within the species as in Curcuma longa [23,24] and Zingiber officinale [21,25] and species to species as in C. aromatica [26] and C. zedoary [18]. The possible reason for the altered requirement of PGRs might be due to the growth condition of the plant from which the explants were harvested, the endogenous status of hormones and biochemicals of the explants used. This is supported by the view that plant growth is directly affected with mineral availability and has complex growth regulatory mechanisms [27] in which plant growth regulators have role in the control mechanisms [28].
Supplementation of auxins with optimal concentration of 2.0 mg/L BAP showed rapid induction of shoot buds and enhanced frequency of response marginally compared to the medium with 2.0 mg/L BAP alone (Table 1). Incorporation of 0.2 mg/L IAA with 2.0 mg/L BAP was optimum to initiate shoot buds (5.6 ± 0.7) in 92% of the explants after 8 weeks which was significantly more (P < 0.05) compared to other treatments (Table 1). These shoot buds were robust and attained 4.0 ± 0.4 cm after 8 weeks (Figure 1(c)). At all higher concentrations of
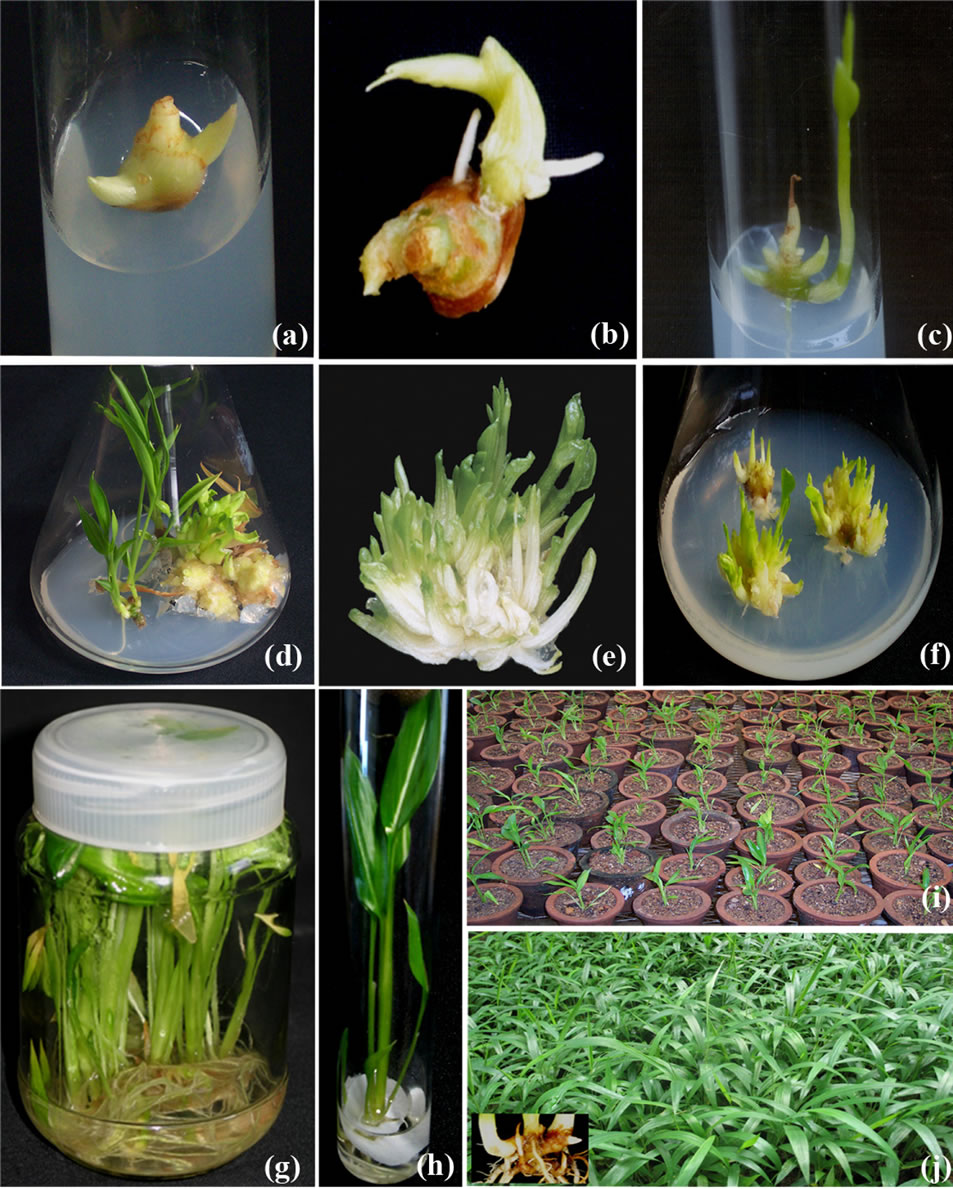
Figure 1. Micropropagation of Alpinia calcarata on MS medium (a) Axillary shoot bud initiation on explants grown on medium with 2.0 mg/l BAP after 14 - 16 days; (b) Vigorous growth of axillary shoot bud on medium with 1.0 mg/l TDZ; (c) Fully grown axillary shoots initiated on explants in the optimal medium with 2.0 mg/l BAP and 0.2 mg/l IAA after 8 weeks; (d) Response of aseptic explants with multiple shoot initials, meristemoids, solitary axillary shoots in medium with 2.0 mg/l BAP and 0.2 mg/IAA; (e) Prolific multiple shoot initials; (f) Shoot initiation from meristemoids during 3rd subculture in medium with 1.0 mg/l BAP; (g) Root initiation on shoots on half-strength liquid medium with 0.2 mg/l IAA; (h) Shoot multiplication in liquid medium with 1.0 mg/l BAP; (i) Acclimatized 4 week old plants in mist chamber; (j) 8-Weeks old plants in shade house.
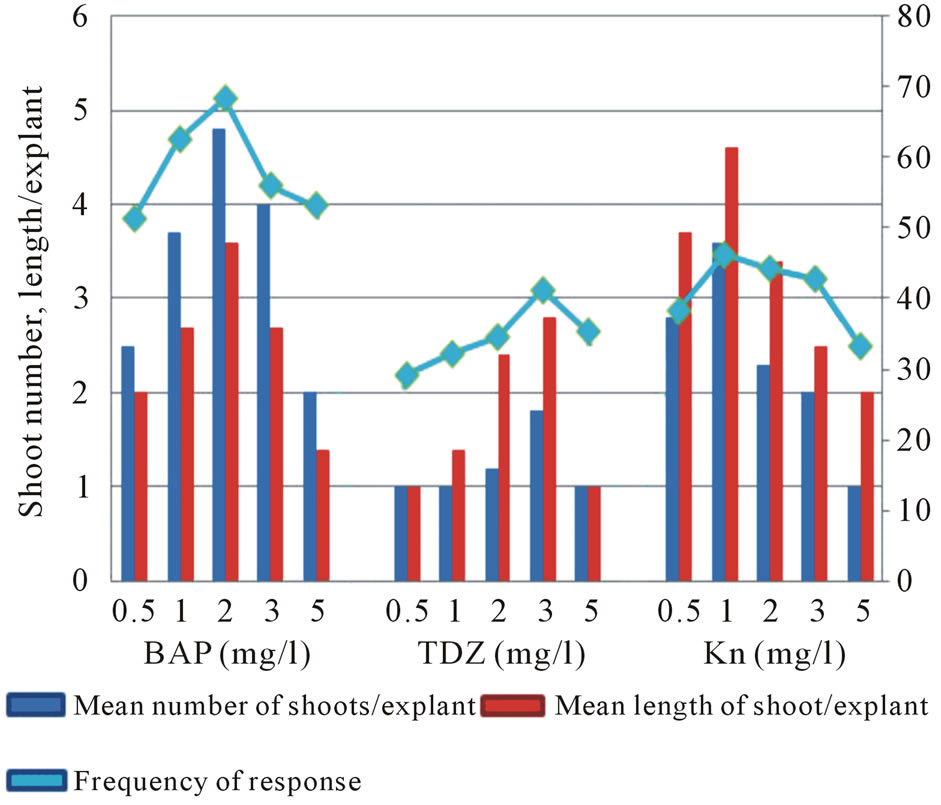
Figure 2. Effect of BAP, TDZ and KN on axillary shoot initiation on MS medium; Observation: after 8 weeks.
auxins, length of the shoot decreased and failed to enhance the number of shoots. The explants cultured on medium with BAP-auxin combinations initiated callus at varying degree and it was prominent in NAA supplemented medium. Comparatively, a favorable effect of IAA over other auxins in the present study is in consonance with the report in A. calcarata [13] and Curcuma haritha [29]. The inefficiency of NAA and IBA along with BAP in the present system was similar to the recent observations in Kaempferia galanga [30].
During first subculture the explants responded with the formation of solitary axillary shoot, clumps of multiple shoot initials or meristemoids intervened with callus. Sixty percentages of the propagules subcultured on medium with 2.0 mg/L BAP and 0.2 mg/L IAA produced maximum multiple shoot initials (12.1 ± 0.4) mediated with meristemoids (4.0 ± 0.5) which was significantly more (P ≤ 0.05) compared to other treatments (Table 2). The shoot cultures grew well and attained maximum growth after 8 weeks (Figure 1(d)). On the other hand, 40% of the explants grown in the same medium initiated solitary shoot buds alone from each axil (7.2 ± 0.3). None of the propagules grown on medium with BAP alone produced meristemoids or multiple shoot initials. Trial experiments indicated that a gradual reduction in the concentration of BAP or elimination of IAA was required during the subsequent subculture for the uninterrupted induction of normal plants devoid of callus.
During second and third subculture, three different propagules (1. solitary axillary shoot, 2. clumps of 3 - 4 shoot initials and 3. meristemoids) were used and data are shown in Table 3. Single clump of multiple shoot initials cultured on medium with 1.0 mg/L BAP and 0.1 mg/L IAA yielded an average of 21 shoots which was the
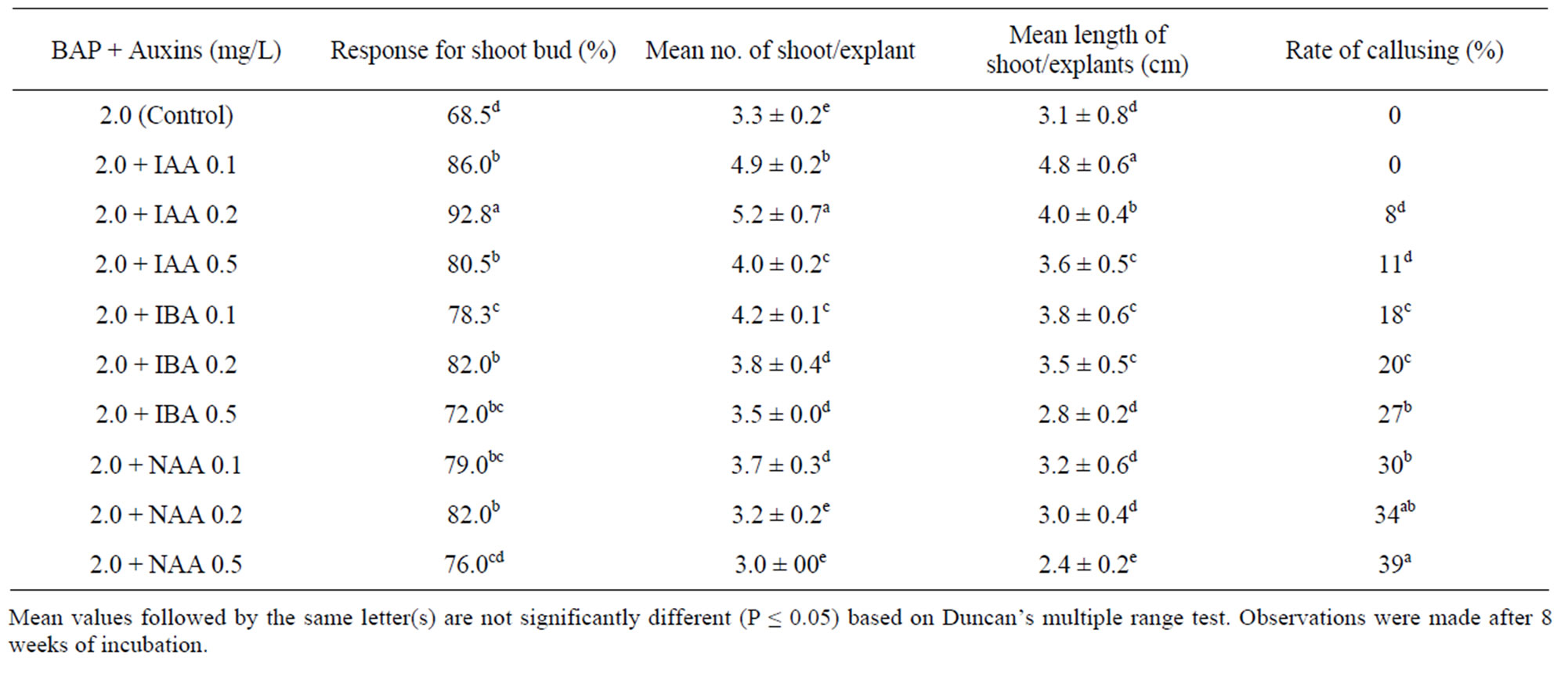
Table 1. Effect of BAP and auxins on MS medium on axillary bud initiation on rhizome explants from field grown plants of A. calcarata.
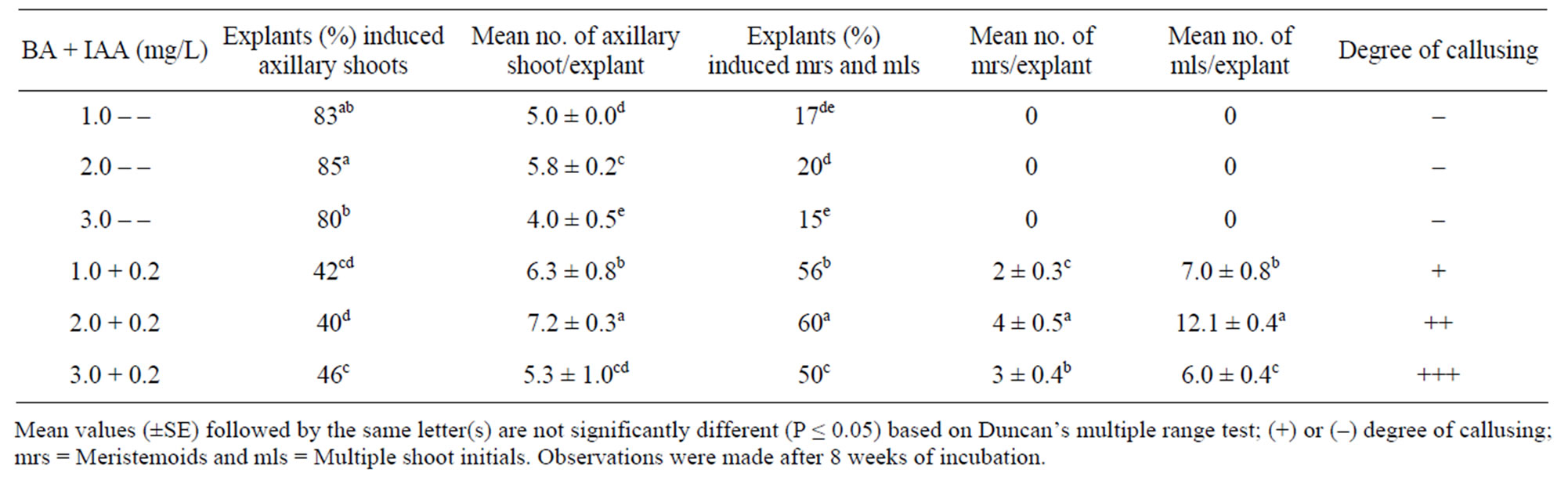
Table 2. Effect of BAP-IAA on MS medium on shoot multiplication of A. calcarata during first subculture.
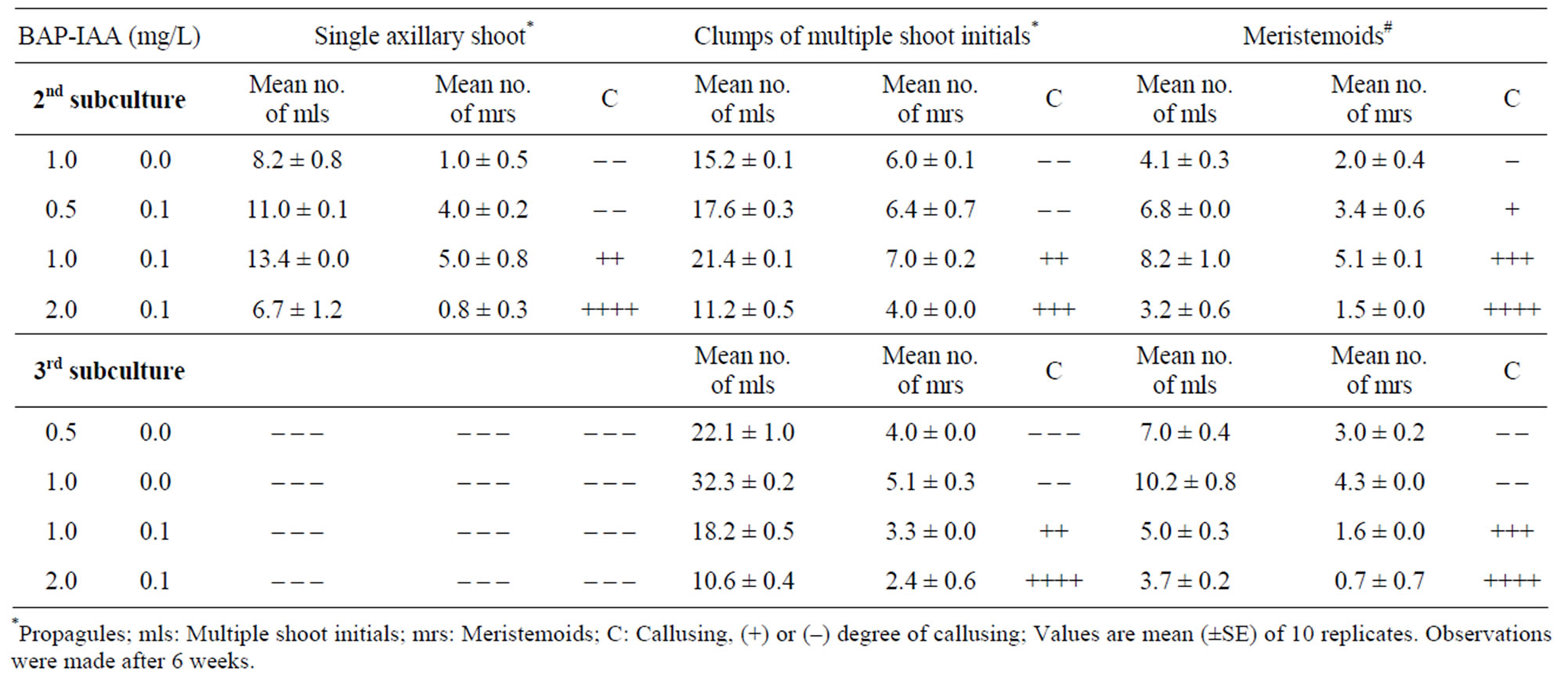
Table 3. Shoot multiplication potential of different propagules grown in MS medium with BAP-IAA.
best among different propagules. The rate of multiplication was further enhanced to 32 shoots when the same propagules passed to 3rd subculture on medium with 1.0 mg/L BAP alone (Table 3; Figure 1(e)). The meristemoids upon subculture yielded an average of 10 shoot initials within 2 weeks (Figure 1(f)). Higher concentrations of BAP or incorporation of IAA showed unorganized meristemoids and a tendency of callusing during subsequent subcultures. During the 3rd subculture onwards, 1.0 mg/L BAP alone favoured the production of normal shoots from clumps of multiple shoot initials and exhibited 10 fold multiplication rates. By following the subculture procedure using clumps of multiple shoot initials approximately 500 shoots were obtained from a single axillary shoot bud explant after 7 months.
During the 4th subculture, clumps of multiple shoot initials grown on liquid medium in culture bottles showed rapid and robust growth with enhanced production of new shoot buds than that observed on solid medium (data not shown). Spontaneous root induction was also noticed in more than 50% shoots grown in liquid medium (Figure 1(g)). The shoots without roots were separated from the clumps and induced roots using optimized rooting medium as in other zingibers [18,29]. The enhanced growth of shoots in liquid medium might be due to the easy and rapid intake of nutrients [31] and partial immersion of the propagules. The beneficial effect of liquid medium and spontaneous rooting in the present system is in accordance with the findings of other zingibers [24,32] and favourable for a cost effective micropropagation.
Meristemoids grown in solid medium were differentiated rapidly into shoot initials while those in liquid medium showed hyperhydricity and symptoms of vitrification which restricted the development of normal shoot buds. The growth of the meristemoids grown in agar medium in culture bottles was rapid compared to the same medium in 250 ml Erlenmeyer flasks. Humidity will be more in culture bottles with polypropylene caps, compared to flasks with cotton plugs, which circumstanced the rapid differentiation of shoot initials. The slow differentiation of the meristemoids in Erlenmeyer flasks was very ideal to keep them as stock cultures. Rapid shoot initiation in solid medium and shoot multiplication in liquid medium in the present study was in concomitant with the reported on C. longa [33].
The use of liquid media induced healthy roots without callus intervention and reduced the duration of root induction from 15 days to 8 days compared to the solid medium. There was no significant difference between solid and liquid medium on other parameters measured. The data on root initiation on liquid medium was analyzed and shown in Table 4. The shoots inoculated on half-strength MS liquid medium with 0.2 mg/L IAA with 0.2 mg/L of IBA showed maximum roots (8.14 ± 1.34) with an average length of (6.05 ± 0.77) cm in 100% of the plantlets which was significantly more (P < 0.05) compared to other treatments (Table 4; Figure 1(h)). The roots initiated by NAA alone were detached from the shoots during acclimatization due to callusing and showed poor rate of survival. This unfavorable condition
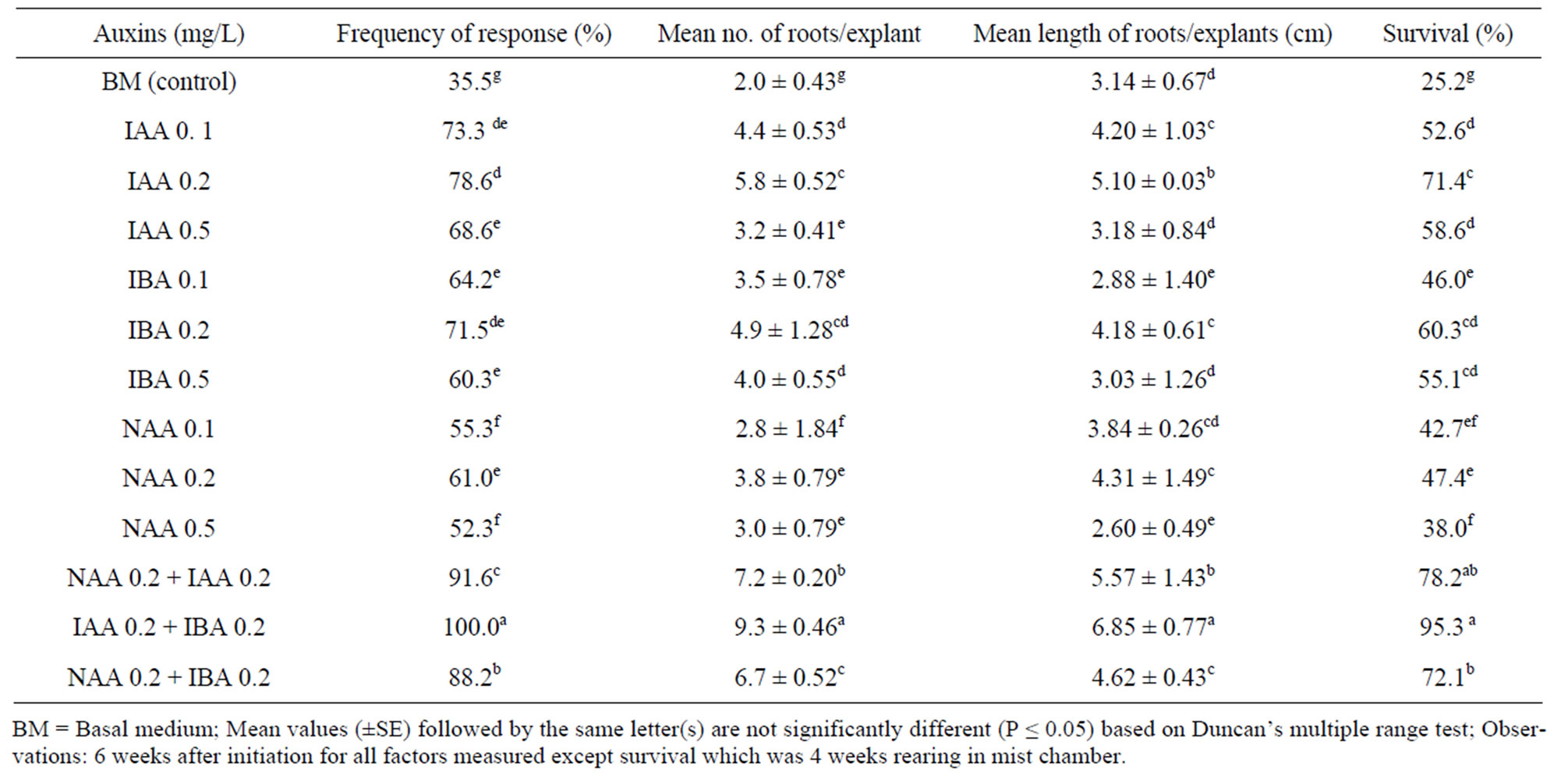
Table 4. Effect of different concentrations and combinations of auxins on half-strength MS liquid medium on rooting of in vitro shoots of A. calcarata.
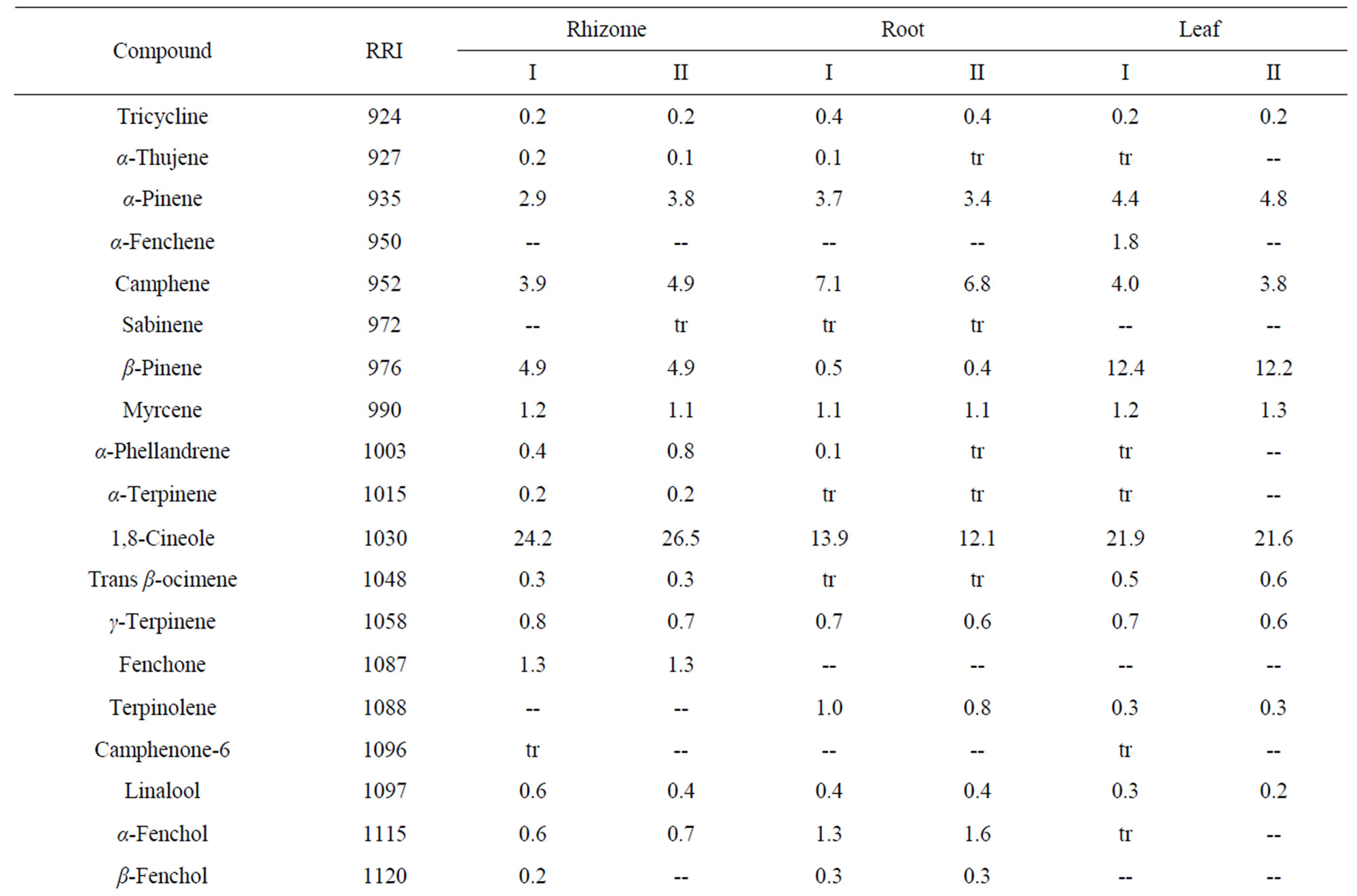
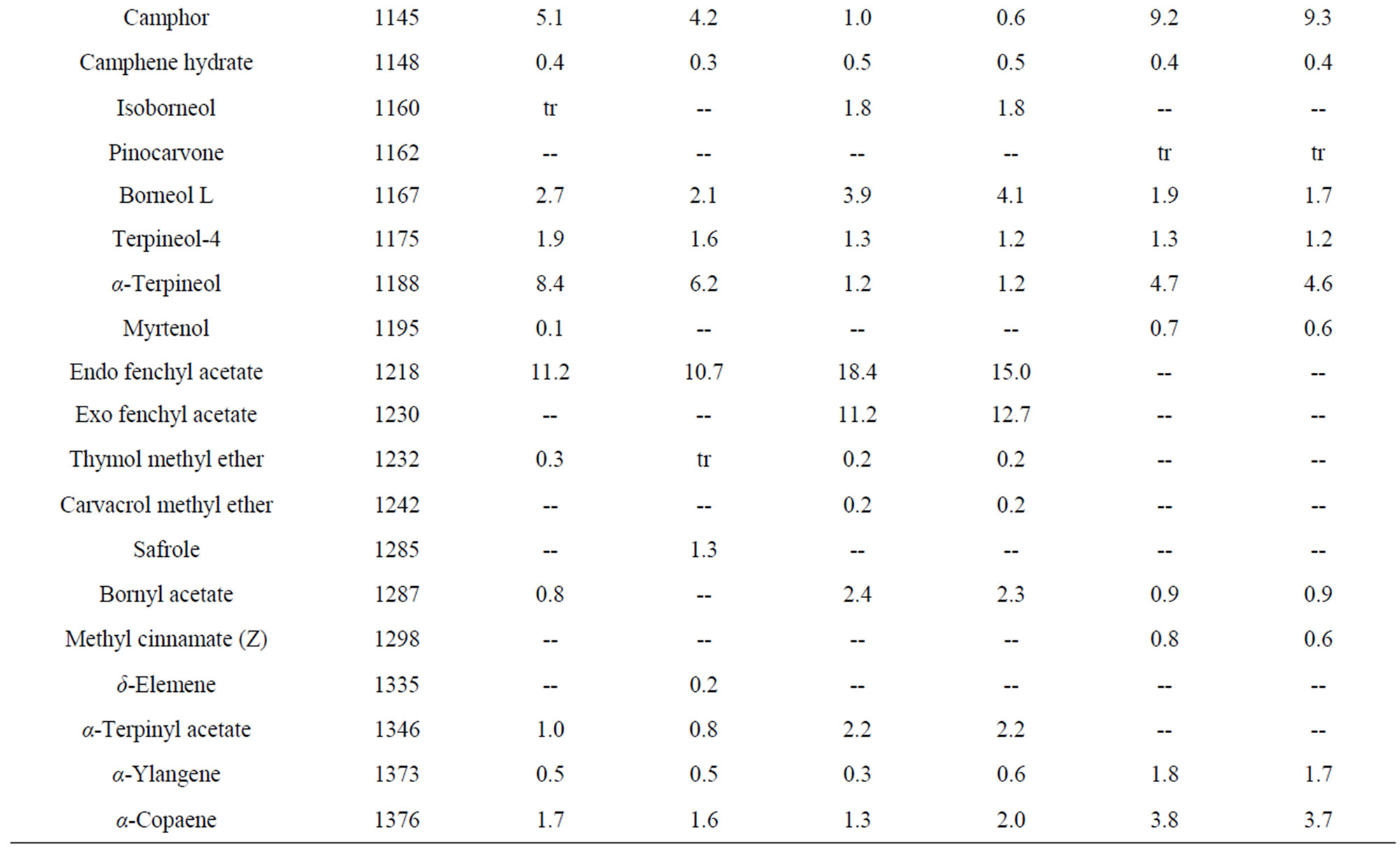
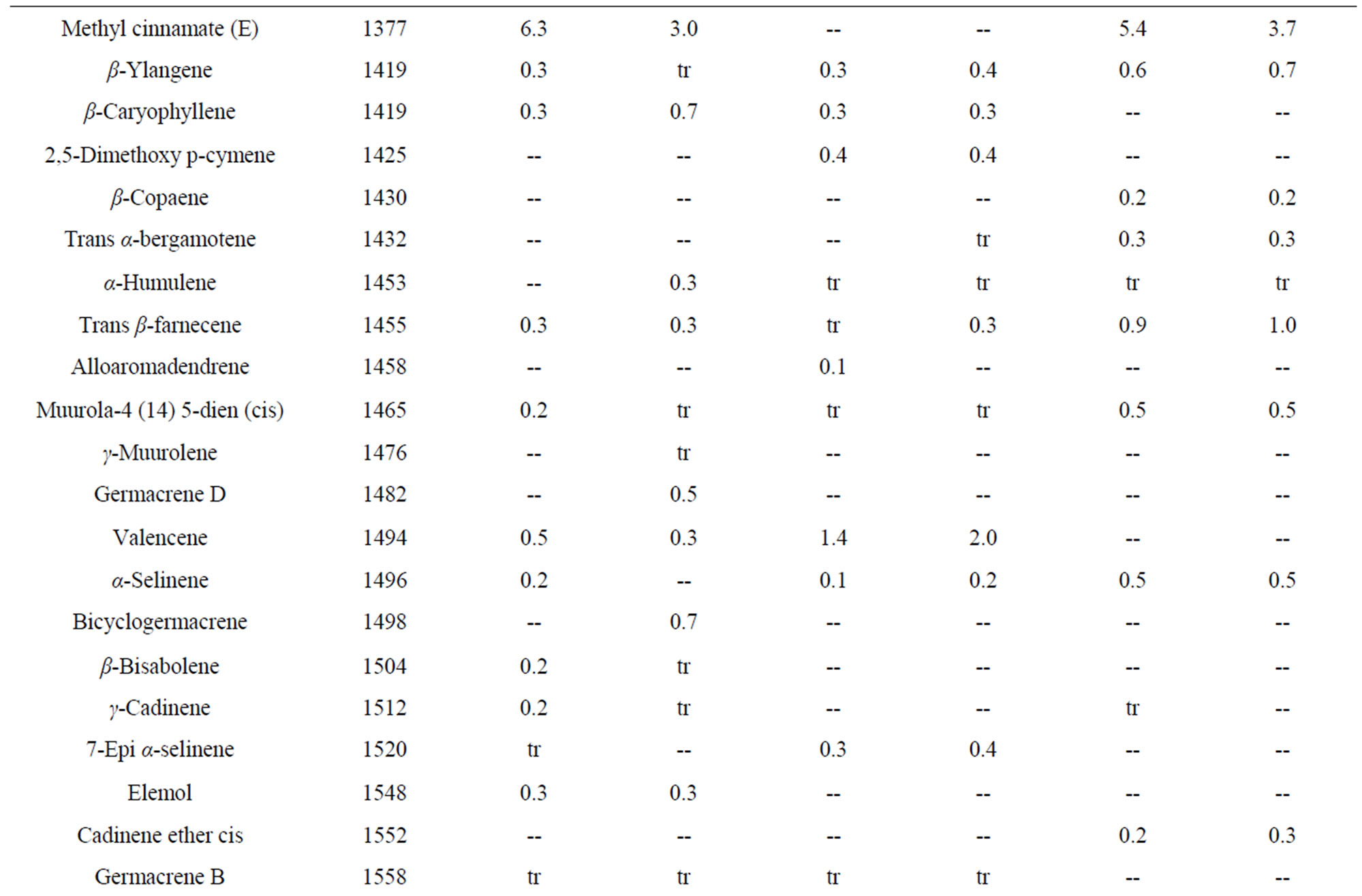
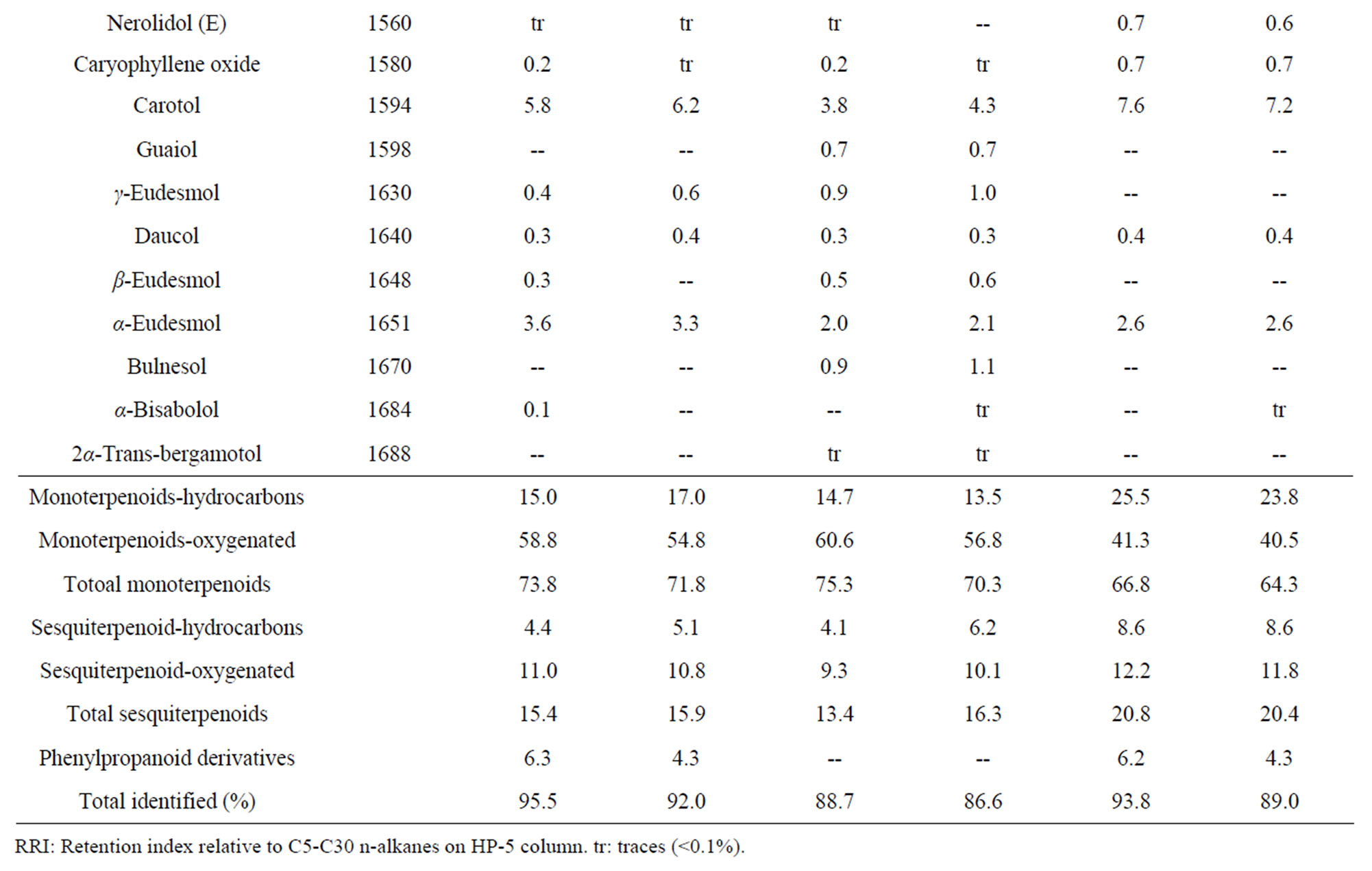
Table 5. Essential oil constituents of rhizome, root and leaf of Alpinia calcarata (sample I: micropropaogated plants and sample II: conventionally propagated plants).
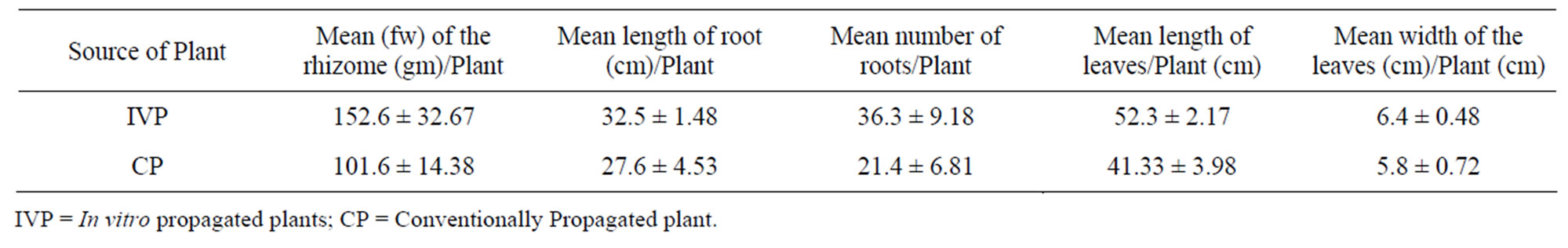
Table 6. Growth characteristics of in vitro and conventionally propagated 36 months-old field-grown plants of A. calcarata.
was alleviated when NAA was combined with IAA or IBA and it promoted the frequency of rooting and survival. The use of liquid medium has minimized the damage of roots while transplanting. The rooted plants from the optimal medium transferred to 5 × 5 cm clay pots containing sand: soil: cow dung (1:1:1) and reared under the mist chamber showed 95% survival (Figure 1(i)). The acclimatized plants were grown rapidly under shade house (Figure 1(j)) and exhibited rhizome formation after 4 - 6 weeks of weaning (Figure 1(j inset)).
The volatile chemical compounds of the in vitro and conventionally propagated plants were identical. Analysis of the essential oil using GC-MS revealed 70 compounds comprising 86.6% to 95.5% of the oil (Table 5). The major compounds of the samples analyzed constituted monoterpenoids, sesquiterpenoids and phenyl propanoid derivatives and among these, monoterpenoids were predominant and the results corroborates with the earlier report [7]. The major compounds of the leaf oil were 1,8-cineole, camphor and carotol while 1,8-cineole, endo fenchyl acetate, α-terpineol, methyl cinnamate (E) and carotol were the major compounds in rhizome oil. The root oil showed endo fenchyl acetate, 1,8-cineole and exo fenchyl acetate as the major constituents. The volatile chemical profiling has been reported as a relatively easy assessment of the metabolite profiling of in vitro regenerated plants [34] and the study confirms the chemical fidelity of the in vitro propagated plants, which is a prerequisite for micropropagated medicinal plants.
The growth characteristics of the randomly selected in vitro and conventionally propagated plants analyzed showed an enhanced growth of all characters measured on former than latter ones (Table 6). A 50% increment of rhizome fresh biomass was obtained from in vitro propagated plants. The enhanced growth of the rhizome of in vitro propagated plants was due to the healthy root system developed under in vitro condition which facilitated the best absorption of nutrients from the soil.
4. Conclusions
In view of the commercial importance and scarcity of the continuous availability of the planting material due to the prolonged maturity period, the present study offers an efficient and improved clonal propagation protocol for Alpinia calcarata and revealed the chemical fidelity of the in vitro raised plants for the first time. The present protocol has significance for the commercial propagation purposes and for the study of secondary metabolites. Use of liquid medium for both shoot multiplication and root induction eliminated a major expensive constituent of gelling agent (Agar). Compared to solid medium, liquid medium can be disbursed into more number of culture bottles than Erlenmeyer flasks. Use of culture bottles with polypropylene caps is very inexpensive compared to borosilicate Erlenmeyer flasks. Apart from these, use of liquid medium is favorable to reduce the cost of labour and electricity. These factors are supportive for a costeffective protocol and it supports the development of automation technology in future for large scale propagation of this commercially important medicinal plant. Our results also suggest that the explants preparation and application of appropriate PGRs and its concentrations during initiation and each subculture are key factors to enhance the multiplication rate which would be applicable to other zingibers.
5. Acknowledgements
The authors acknowledge the Western Ghats Cell, Planning and Economic Affairs Department, Govt. of Kerala for financial support and the Director JNTBGRI for providing facilities.
REFERENCES
- J. K. Mangaly and M. Sabu, “A Taxonomic Revision of South Indian Alpinia Roxb. (Zingiberaceae),” Rheedea, Vol. 2, No. 1, 1992, pp. 38-51.
- J. P. Robinson, V. Balakrishnan, S. Raj and S. J. Britto, “Antimicrobial Activity of Alpinia calcarata Rosc. and Characterization of New α, β-Unsaturated Carbonyl Compound,” Advances in Biological Research, Vol. 3, No. 5-6, 2009, pp. 185-187.
- L. S. R. Arambewela, L. D. A. M. Arawwawala and W. D. Ratnasoorya, “Antinoceceptive Activities of Aqueous and Ethanolic Extract of Alpinia calcarata Rhizomes in Rats,” Journal of Ethnopharmacology, Vol. 95, No. 2-3, 2004, pp. 311-316. doi:10.1016/j.jep.2004.07.015
- L. D. A. M. Arawwawala, L. S. R. Arambewela and W. D. Ratnasooriya, “Alpinia calcarata Roscoe: A Potent Antiinflammatory Agent,” Journal of Ethnopharmacology, Vol. 139, No. 3, 2012, pp. 889-892. doi:10.1016/j.jep.2011.12.036
- T. R. Dutta, R. Ahemed, S. R. Abbas and M. K. V. Rao, “Plants Used by Andaman Aborigins in Gathering Rock Honey,” Economic Botany, Vol. 39, No. 2, 1985, pp. 130- 138. doi:10.1007/BF02907833
- M. Sabu, “Zingiberaceae and Costaceae of South India,” Indian Association of Angiosperm Taxonomists, Calicut, 2006, p. 52.
- P. N. Kaul, R. B. R. Rajeswara, K. Singh, A. K. Bhattacharya, G. R. Mallavarapu and S. Ramesh, “Volatile Constituents of Essential Oils Isolated from Different Parts of Alpinia calcarata Rosc.,” Journal of Essential Oil Research, Vol. 17, No. 1, 2005, pp. 7-9. doi:10.1080/10412905.2005.9698814
- N. Sasidharan and P. K. Muraleedhara, “Survey on the Commercial Exploitation and Consumption of Medicinal Plants by the Drug Industry in Northern Kerala,” Research Report No. 193, Kerala Forest Research Institute, Thrissur, Kerala, 2000.
- S. Mohanty, R. Parida, S. Singh, R. K. Joshi, E. Subudhi and S. Nayak, “Biochemical and Molecular Profiling of Micropropagated and Conventionally Grown Kaempferia galanga,” Plant Cell Tissue and Organ Culture, Vol. 106, No. 1, 2010, pp. 39-46. doi:10.1007/s11240-010-9891-5
- M. Singh and R. Chaturvedi, “Improved Clonal Propagation of Splilanthes acmella Murr. for Production of Scopoletin,” Plant Cell Tissue and Organ Culture, Vol. 103, No. 2, 2010, pp. 243-253. doi:10.1007/s11240-010-9774-9
- M. S. Rathore and N. S. Shekhawat, “Micropropagation of Pueraria tuberose (Roxb. Ex Willd.) and Determination of Puerarin Content in Different Tissues,” Plant Cell Tissue and Organ Culture, Vol. 99, No. 3, 2009, pp. 327- 334. doi:10.1007/s11240-009-9608-9
- P. K. Pati, J. Kaur and P. Singh, “A Liquid Culture System for Shoot Proliferation and Analysis of Pharmaceutically Active Constituents of Catharanthus roseus (L.) G. Don.,” Plant Cell Tissue and Organ Culture, Vol. 105, No. 3, 2011, pp. 299-307. doi:10.1007/s11240-010-9868-4
- K. T. Agretious, K. P. Martin and M. Hariharan, “In Vitro Clonal Multiplication of Alpinia calcarata Rosc.,” Phytomorphology, Vol. 46, No. 2, 1996, pp. 133-138.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Culture,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
- H. Van Den Dool and P. D. Kratz, “A Generalization of the Retention Index System Including Linear Temperature Programmed Gas Liquid Partition Chromatography,” Journal of Chromatography, Vol. 11, 1963, pp. 463-471. doi:10.1016/S0021-9673(01)80947-X
- R. P. Adams, “Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry,” 4th Edition, Allured Pub. Co., Carol Stream, 2007.
- M. Borthakur, J. Hazarika and R. S. Sing, “A Protocol for Micropropagation of Alpinia galangal,” Plant Cell Tissue and Organ Culture, Vol. 55, No. 3, 1999, pp. 231-233. doi:10.1023/A:1006265424378
- N. H. Loc, D. T. Duc, T. H. Kwon and M. S. Yang, “Micropropagation of Zedoary (Curcuma zedoaria Roscoe) —A Valuable Medicinal Plant,” Plant Cell Tissue and Organ Culture, Vol. 81, No. 1, 2005, pp. 119-122. doi:10.1007/s11240-004-3308-2
- K. K. Behera, D. Pani and S. Sahoo, “Effect of Plant Growth Regulator on in Vitro Multiplication of Turmeric (Curcuma longa L. cv. Ranga),” International Journal of Biological Technology, Vol. 1, No. 1, 2010, pp. 16-23.
- S. K. Shukla, S. Shukla, V. Koche and S. K. Mishra, “In Vitro Propagation of Tikhur (Curcuma angustifolia Roxb.): A Starch Yielding Plant,” Indian Journal of Biotechnology, Vol. 6, 2007, pp. 274-276.
- S. M. Balachandran, S. R. Bhat and K. P. S. Chandel, “In Vitro Clonal Multiplication of Turmeric (Curcuma spp.) and Ginger (Zingiber officinale Rosc.),” Plant Cell Reports, Vol. 8, No. 9, 1990, pp. 521-524. doi:10.1007/BF00820200
- S. P. Geetha, C. Manjula, C. Z. John, D. Minoo, B. K. Nirmal and P. N. Ravindran, “Micropropagation of Kaempferia spp. (K. galanga L. and K. rotunda L.),” Journal of Spices and Aromatic Crops, Vol. 6, No. 2, 1997, pp. 129-135.
- K. Nasirujjaman, M. S. Uddin, S. Zaman and M. A. Reza, “Micropropagaion of Turmeric (Curcuma longa Linn.) through in Vitro Rhizome Bud Culture,” Journal of Biological Sciences, Vol. 5, No. 4, 2005, pp. 490-492. doi:10.3923/jbs.2005.490.492
- S. Prathanturarug, N. Soonthornchareonnon, W. Chuakul, Y. Phaidee and P. Saralamp, “Rapid Micropropagation of Curcuma longa Using Bud Explants Pre-Cultured in Thidiazuron-Supplemented Liquid Medium,” Plant Cell Tissue and Organ Culture, Vol. 80, No. 3, 2005, pp. 347- 351. doi:10.1007/s11240-004-1020-x
- T. R. Sharma and B. M. Singh, “High Frequency in Vitro Multiplication of Disease-Free Zingiber officinale Rosc.,” Plant Cell Reports, Vol. 17, No. 1, 1997, pp. 68-72. doi:10.1007/s002990050354
- S. Nayak, T. Kaur, S. Mohanty, G. Ghosh, R. Choudhury, L. Acharya and E. Subudhi, “In Vitro and ex Vitro Evaluation of Long-Term Micropropagated Turmeric As Analysed through Cytophotometry, Phytoconsituents, Biochemical and Molecular Markers,” Plant Growth Regulation, Vol. 64, No. 1, 2010, pp. 91-98. doi:10.1007/s10725-010-9541-2
- M. S. Rathore and N. S. Shekhawat,) “Micropropagation of Pueraria tuberose (Roxb. Ex Willd.) and Determination of Puerarin Content in Different Tissues,” Plant Cell Tissue and Organ Culture, Vol. 69, No. 4, 2009, pp. 327- 333. doi:10.1007/s11240-009-9608-9
- K. T. Kuppusamy, C. L. Walcher and J. L. Nemhauser, “Cross Regulatory Mechanism in Hormone Signaling,” Plant Molecular Biology, Vol. 69, No. 4, 2009, pp. 375- 381. doi:10.1007/s11103-008-9389-2
- M. Bejoy, M. Dan and M. P. Anish, “Factors Affecting the in Vitro Multiplication of the Endemic Zingiber Curcuma haritha Mangaly and Sabu,” Asian Journal of Plant Sciences, Vol. 5, No. 5, 2006, pp. 847-853. doi:10.3923/ajps.2006.847.853
- R. Parida, S. Mohanty, A. Kuanar and S. Nayak, “Rapid Multiplication and in Vitro Production of Leaf Biomass in Kaempferia galanga through Tissue Culture,” Electronic Journal of Biotechnology, Vol. 13, No. 4, 2010, Fulltext- 12.
- F. L. Marga, R. N. Prasad and A. Roychowdhury, “Agar Fraction Could Protect Apple Shoots Cultured in Liquid Medium against hyperhydricity,” Plant Cell Tissue and Organ Culture, Vol. 49, No. 1, 1997, pp. 1-5. doi:10.1023/A:1005832115954
- C. Stanly, A. Bhatt and C. L. Keng, “A Comparative Study of Curcuma zedoaria and Zingiber zerumbet Plantlet Production Using Different Micropropagation Systems,” African Journal of Biotechnology, Vol. 9, No. 28, 2010, pp. 4326-4333.
- N. D. Salvi, L. George and S. Eapen, “Micropropagation and Field Evaluation of Micropropagated Plants of Turmeric,” Plant Cell Tissue and Organ Culture, Vol. 68, No. 2, 2002, pp. 143-151. doi:10.1023/A:1013889119887
- I. M. Fortunato and P. Avato, “Plant Development and Synthesis of Essential Oils in Micropropagated and Mycorrhiza Inoculated Plants of Origanum vulgare L. ssp. Hirtum (Link) Ietswaart,” Plant Cell Tissue and Organ Culture, Vol. 93, No. 2, 2008, pp. 139-149. doi:10.1007/s11240-008-9353-5
NOTES
*Corresponding author.

