Journal of Sustainable Bioenergy Systems
Vol. 2 No. 4 (2012) , Article ID: 26024 , 7 pages DOI:10.4236/jsbs.2012.24020
The Impacts of Sugarcane Expansion on Wildlife in the State of São Paulo, Brazil
1Isotopic Ecology Lab, CENA, University of São Paulo, Piracicaba, Brazil
2University of Sagrado Coração, Rua Irmã Arminda, Bauru, Brazil
3ESEC Tupinambás, ICMBio, Rua Antônio Cândido, São Sebastião, Brazil
4Conservation Science Group, Department of Zoology, University of Cambridge, Cambridge, U.K
Email: *lmverdade@usp.br
Received October 20, 2012; revised November 25, 2012; accepted December 3, 2012
Keywords: Biofuel; Ethanol; Wildlife; Sugarcane; Wildlife Diseases; Agricultural Landscapes
ABSTRACT
Brazil has become one of the top producers and exporters of food, fibre, and bionergy in the world.The expansion of livestock and soybean production in the Amazon basin appears to be independent of the sugarcane expansion in southeastern Brazil whereas the impact of sugarcane expansion over areas of soybean plantation in previous Cerrado biome is still unknown. However, the expansion of sugarcane production in the state of São Paulo has been shown to cause an increase in the local abundance of rodents and result in the emergence of infectious diseases such as hantaviruses and leptospirosis in humans. In addition, with an increase in the use of agrochemicals there will be an increase in euthrophication of watercourses and soil pollution. Considering that São Paulo has a population of approximately 40 million people, these local impacts are relevant. Environmental law should be improved and enforced in Brazil to ensure that sugarcane production is not only economically profitable but also environmentally responsible.
1. Introduction
Brazil has become one of the top producers and exporters of food, fibre, and bionergy in the world. In 2007, the country had 275 million ha of its territory (30%) converted to agricultural areas, of which around 211 million ha are covered with pastures and 57 million with agriculture [1]. Livestock in Brazil is dominated by low technology input pasture supporting approximately only one animal per hectare in average [2].
The agricultural area is dominated by export crops, with around 23 million ha of soybean, 12 million of corn and 8 million of sugarcane. On the other hand, the staple crops of Brazil—beans, rice and manioc—cover a much smaller area of 3.2, 2.6 and 1.8 million ha, respectively. While the area of staple crops is declining, soybean and especially sugarcane have been growing rapidly in the last 30 years. The rapid expansion of sugarcane can be explained by a combination of government policies aimed at the development of sustainable renewable energy, energy security and rural development. As a consequence, estimates made by the Brazilian government and the local ethanol industry indicate that areas covered with sugarcane may reach 14 million hectares by 2016 [3].
Increase of sugarcane area is occurring primarily in South-eastern Brazil, particularly in the state of São Paulo, where most of the sugarcane industrial plants and mills are located (Figure 1 [4]). A second vector of sugarcane expansion is the centre-western region of the country, where the Cerrado (the Brazilian savannah) is located. The original biomes currently converted to sugarcane plantations are the Atlantic forest and the Cerrado. Recently this expansion has occurred mostly over pastures and soybean plantations [3]. However, it has been speculated that a “compensatory” expansion of pastures and soybean plantations in the Brazilian Amazon based on deforestation may occur [5-8].
Extensive livestock production in Brazil is characterized by low technology and low input, while major crops like sugarcane and soybean are characterized by high technology and high input agriculture. The intensive use of machinery coupled with the heavy use of mineral fertilizers, insecticides and herbicides, besides annual burning of sugarcane fields to facilitate harvesting result in a series of environmental impacts [9,10]. These impacts potentially affect the local fauna [11,12].
The main goal of this article is to synthesize a series of recent studies conducted in Brazil, on major changes observed in the local fauna, and additionally, the possible scenarios of sugarcane expansion in São Paulo state, and
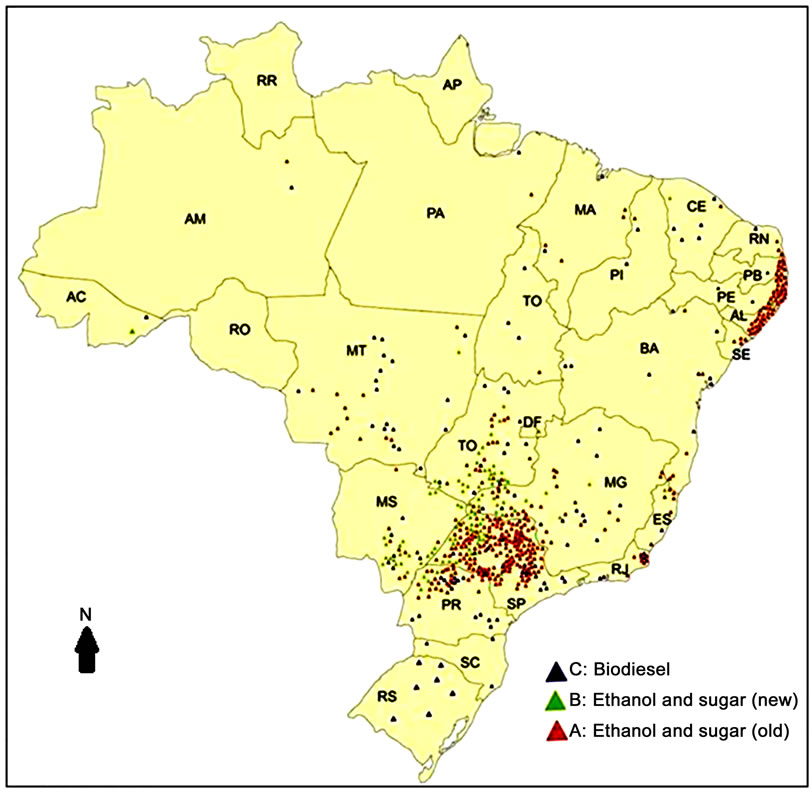
Figure 1. Sugarcane mills in Brazil (source: [73]).
the possible effects of such land use change.
Sugarcane Expansion in the Cerrado
The Cerrado is the second largest Brazilian biome originally covering an area of approximately 200 million ha over the Brazilian Plateau between the Amazon forest and the Atlantic forest [13]. This biome is formed by distinct ecosystems from savannah to woodland being considered a biodiversity hotspot [14] with a rich biodiversity from the Pleistocene [15] to the present [16]. However, an intensive process of agriculture expansion, especially soybean, took place in the Brazilian Cerrado since the 1970’s [17]. The impact of this massive land use change is still unknown. However, signs of game species decline and the consequent decadence of certain native ethnic groups such as the Nambiquaras have been described quite earlier in the mid 1930’s [18].
The impact of the current agricultural land use change from soybean to sugarcane plantations should be prioritized for studies on biodiversity conservation as it can affect not only agricultural fields but also border areas of native vegetation [19]. In order to understand the possible changes on the patterns of distribution and abundance of biodiversity in such conditions, we should first carry out biodiversity inventories in areas where the original ecosystems of the Cerrado biome have been replaced by an agricultural landscape whose matrix is soybean. Then, we should evaluate α- and β-diversity changes when/if the landscape matrix shifted to sugarcane plantations [20, 21].
2. Materials and Methods
2.1. Sugarcane Expansion in São Paulo and Indirect Deforestation in the Amazon
The state of São Paulo is located in the Southeastern of Brazil (19˚46'45" - 25˚18'43"S; 44˚09'38" - 53˚06'35"W), has an area of approximately 248.210 km2, and a population of about 42 million (21.5% of the population of Brazil) [27]. Deforestation in São Paulo occurred predominantly from the 1700s to the 1970s [22,23]. Currently, only approximately 13% (3,457,301 ha) of São Paulo is still covered by remnants of its original biomes, the Atlantic forest and the Cerrado, over approximately 120 thousand fragments, with only 25% (892,552 ha) of their total area in conservation units [24-26].
Since the 1970s, the total area in Brazil covered by pastures for livestock production expanded from 154.1 to 177.7 million hectares [27]. During the same period, soybean plantations for agroindustry expanded from 5.5 to 21.7 million hectares, representing an increase of 16.2 million hectares [28,29]. During this period, sugarcane plantations, primarily for ethanol production, expanded from approximately 1.5 to 9.0 million hectares [2,30].
The major concern that expansion of livestock and soybean production in the Amazon basin results in increased deforestation rates [7,28,31,32,] seems justifiable. However, this process appears to be independent of the sugarcane expansion in São Paulo. Pastures and soybean landscapes are currently spread in Brazil over an area 19.7 and 2.4 times larger, respectively, than the area covered by sugarcane plantations. Their combined expansion since the 1970s, when Brazil began using ethanol, is 4.4 times larger than the current area covered by sugarcane plantations, and 2.5 times larger than the area projected for 2016. In fact, most livestock and soybean expansions are already occurring in the southern Amazon independently of the expansion of sugarcane in São Paulo [6,33, 34].
These data suggest that there is no connection between sugarcane expansion in São Paulo and expansion of livestock and soybean production and their result in deforestation, in the Amazon. On the other hand, livestock expansion in the Amazon appears to be related to a seconddary effect of illegal timber exploitation where deforestation is followed by exotic pastures implantation under low technology regime and low input livestock production [35].
3. Results and Discussion
3.1. Impacts of Agricultural Land Use Change on Biodiversity in São Paulo
Biodiversity loss is associated with forest fragmentation [36-38], which is usually associated with agriculture expansion as occurred in São Paulo state. Poaching also occurs in São Paulo because of insufficient law enforcement despite rigid state regulations [39,40]. In addition, local watercourses are polluted due to insufficient sewage treatment [41], what is also deleterious for wildlife [42,43]. As a consequence, São Paulo currently has 320 vertebrate species listed as locally endangered [44].
On the other hand, agricultural landscapes can have some conservation value, as they maintain considerable biodiversity [45-47]. Agricultural landscapes in São Paulo still have approximately two-thirds of the original species of mediumto large-sized mammals [48,49] and approximately 60% of the original bird species [50]. However, only one-third of the original rodent and marsupial species are still found in agricultural landscapes from São Paulo possibly due to their limited ability to disperse [51]. Sugarcane plantations have a smaller α-diversity but a significantly higher abundance of rodents than other local ecosystems, either natural or agricultural [51], because sugarcane plantations can produce as much as 120 tons·year·ha−1 of biomass [52], providing a considerable amount of food for C4-plant eaters.
Small rodents [51] and their (meso) predators [48,50] take advantage of this tremendous food supply despite the use of controlled fires prior to harvest. The fire ban, which has been proposed by the sugarcane industry to be fully implemented by 2017 [53], may further increase population densities of small rodents. The dominant species in sugarcane plantations, Calomys tener and Necromys lasiurus, are potential hosts for Hantavirus, which has been previously introduced in central-southern Brazil [54]. As sugarcane plantations are usually close to suburban areas, hantaviruses might become a major issue for public health as a consequence of sugarcane expansion in São Paulo. Diseases such as leptospirosis and typhus are also associated with small rodents [55]. Chemical rat control in urban and rural environments involves highly toxic substances. While the use of such substances is a tempting opportunity for the agrochemical industry, its use in large scale in São Paulo could be disastrous as rodenticides are extremely toxic [56] and are commonly misused in tropical crops [57].
Capybaras (Hydrochoerus hydrochaeris), the largest living rodents, have experienced dramatic population growth along with the expansion of sugarcane plantations in central-eastern São Paulo [58]. This population boom has been associated with the local resurgence of spotted fever [59].
There seems to be a predominance of Cerrado birds in local pastures due to limited weed control [50]. These species do not seem to adapt well to sugarcane plantations. Therefore, species abundance of birds tends to decline as sugarcane replaces pastures. Birds also tend to be sensitive to agrochemicals [60], which may contribute even further to their decline in areas where sugarcane fields replace pastures.
Local livestock production is generally extensive, with a low investment rate and low productivity [61]. On the other hand, industrial sugarcane plantations are production-intensive [62], with a relatively high input of agrochemicals [7,9,11]. Therefore, the replacement of pastures by sugarcane may result in soil erosion and the eutrophication of watercourses caused by an increase in total suspended solids (TSS) and nutrients, in particular nitrogen and phosphorus [7,10].
The diversity of stream fish in agricultural landscapes of São Paulo is higher where riparian forests remain, but this is usually not the case in landscapes that contain sugarcane fields or pastures [63]. However, many fish species tend to be sensitive to water quality. High levels of heavy metals have been found in commercial fish and fresh water turtles from the Piracicaba River [42,43], which encompasses a drainage area that is widely covered by sugarcane plantations
3.2. Market Regulations and Land Use Policy
Brazil has recently become the largest exporter of meat and the second-largest soybean producer in the world [28, 34,64]. Like ethanol, these commodities are part of a strong agroindustry sector where economic values are significantly more relevant than environmental values related to biodiversity conservation [34,61]. Such economic values are efficiently lobbied for political ends [65,66]. However, environmental values have increased political and economic relevance [53], as there is a growing market for “organic” or “environmentally friendly” agricultural products in both developed and developing countries. Ethanol is one such product. As an example, the paper industry is this far the only large agroindustrial sector that closely follows Brazilian environmental law because of market certification regulations [67,68].
In São Paulo, where most of the ethanol and paper industries are based, the environmental law requires that at least 20% of rural properties keep or recover local native ecosystems, which is called “Legal Reserve” (LR). In addition, native vegetation should be maintained along watercourses (from 30 to 100-m wide on each margin, depending on watercourse width) and in areas having a slope >30% [69]. These areas are called “Areas of Permanent Protection” (APP). There is currently a strong political movement to decrease APP dimensions and to make the use of LR more flexible which might result in massive biodiversity loss in agricultural landscapes [70]. If current environmental laws are maintained and enforced, the state of São Paulo will double its area of native vegetation. However, LR and APP are necessary to assure not only biodiversity conservation [71] in agricultural landscapes but also water quality for human consumption and hydroelectric generation, which is still the most important source of Brazilian energetic matrix [9]. Environmental law should be improved and enforced in Brazil to ensure that sugarcane production is not only economically profitable but also environmentally responsible.
The Biota Program from São Paulo Science Foundation (FAPESP), a long-term research program on biodiversity documentation, conservation and sustainable use resulted in considerable improvement of the state of São Paulo environmental public policy including the zoning for sugarcane expansion [72]. As a complement, a longterm monitoring network program on biodiversity is planned to be established in pristine areas of the Atlantic Forest and the Cerrado as well as in agricultural landscapes including those with a sugarcane matrix. This will implement a means to monitor and mitigate the various impacts of changing and intensification of land use on local biodiversity [72].
4. Conclusions
The expansion of livestock and soybean production in the Amazon basin appears to be independent of the sugarcane expansion in southeastern Brazil whereas the impact of sugarcane expansion over areas of soybean plantation in previous Cerrado biome is still unknown. However, local impacts of the sugarcane expansion in the state of São Paulo on wildlife (i.e., an increase in rodents abundance) and the environment (i.e., eutrophication of water courses), as well as on public health (i.e., a possible increase on wildlife associated diseases such as hantaviruses and leptospirosis and a possible increase in contamination by agrochemicals) are relevant. Such impacts should be considered by local policy makers.
5. Acknowledgements
This study was financed by FAPESP—Biota Program (Proc. No. 2006/60954-4) and CNPq (Proc. No. 557872/ 2008-1). Phil Wilkinson critically reviewed this manuscript.
REFERENCES
- G. Saprovek, A. Barretto, I. Klug, L. Papp and J. Lino, “A Revisão do Código Florestal Brasileiro,” Novos Estudos, Vol. 88, No. 1, 2010, pp. 181-205.
- Secretaria de Comunicação Pessoal, “Brazil Insights Series: Agriculture and Livestock,” 2010. http://www.brasil.gov.br/para/press/reference-texts/brazil-insights-series-agriculture-and-livestock
- UNICA—União da Indústria Canavieira, “Estatística de produção de açúcar no Brasil,” 2008. http://www.unica.com.br
- UDOP—União dos Produtores de Bioenergia, “Usinas e Destilarias: Mapas,” 2010. http://www.udop.com.br/index.php?item=mapa_bra
- P. M. Fearnside, “Deforestation in Brazilian Amazônia: History, Rates and Consequences,” Conservation Biology, Vol. 19, No. 3, 2005, pp. 680-688. doi:10.1111/j.1523-1739.2005.00697.x
- M. H. Costa, S. N. M. Yanagi, P. J. O. P. Souza, A. Ribeiro and E. J. P. Rocha, “Climate Change in Amazô- nia Caused by Soybean Cropland Expansion, as Compared to Caused by Pastureland Expansion,” Geophysical Research Letters, Vol. 34, No. 7, 2007, pp. 125-136. doi:10.1029/2007GL029271
- L. A. Martinelli and S. Filoso, “Expansion of Sugarcane Ethanol Production in Brazil: Environmental and Social Challenges,” Ecological Applications, Vol. 18, No. 4, 2008, pp. 885-898. doi:10.1890/07-1813.1
- D. M. Lapola, R. Shaldach, J. Alcamo, A. Bondeau, J. Koch, C. Koelking and J. A. Priess, “Indirect Land-Use Changes Can Overcome Carbon Savings from Biofuels in Brazil,” PNAS, Vol. 107, No. 8, 2010, pp. 3388-3393. doi:10.1073/pnas.0907318107
- K. Yu, R. D. DeLaure, R. Tao and R. L. Beine, “Nonpoint Source of Nutrients and Herbicides Associated with Sugarcane Production and Its Impact on Louisiana Coastal Water Quality,” Journal of Environmental Quality, Vol. 37, No. 6, 2008, pp. 2275-2283. doi:10.2134/jeq2008.0082
- T. W. Simpson, L. A. Martinelli, R. W. Sharpley and R. W. Howarth, “Impact of Ethanol Production on Nutrient Cycles and Water Quality: the United Sates and Brazil as Case Studies”, In: R. W. Howarth and S. Bringezu, Eds., Biofuels: Environmental Consequences and Interactions with Changing Land Use, Proceedings of the Scientific Committee on problems of the Environment (SCOPE), International Biofuels Project Rapid Assessment, Cornell University, Ithaca, 2009, pp. 153-167.
- O. E. Sala, D. Sax and H. Leslie, “Biodiversity Consequences of Biofuel Production”, In: R. W. Howarth and S. Bringezu, Eds., Biofuels: Environmental Consequences and Interactions with Changing Land Use. Proceedings of the Scientific Committee on problems of the Environment (SCOPE), International Biofuels Project Rapid Assessment, Cornell University, Ithaca, 2009, pp. 127-137.
- L. Bies, “The Biofuels Explosion: Is Green Energy Good for Wildlife?” Wildlife Society Bulletin, Vol. 34, No. 4, 2006, pp. 1203-1205. doi:10.2193/0091-7648(2006)34[1203:TBEIGE]2.0.CO;2
- M. B. Arruda, “Ecossistemas Brasileiros,” IBAMA, Brasília, 2001.
- G. A. B. Fonseca, R. Cavalcanti, A. Rylands and A. Paglia, “Cerrado,” In: R. A. Mittermeier, G. P. Roblkes, M. Hoffmann, J. Pilgrim, T. Brooks, C. G. Mittermeier, J. Lamoreux and G. A. B. Fonseca, Eds., Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions, CEMEX, Mexico City, 2004, pp. 93-97.
- C. Cartelle, “Pleistocene Mammals of the Cerrado and Caatinga of Brazil,” In: J. F. Eisenberg and K. H. Redford, Eds., Mammals of the Neotropics, The Central Neotropics: Ecuador, Peru, Bolivia and Brazil, The University of Chicago Press, Chicago, 1999, pp 27-46.
- J. Marinho-Filho, F. Rodrigues and M. Guimarães, “Vertebrados da Estação Ecológica de Águas Emendadas: História Natural e Ecologia em um Fragmento de Cerrado do Brasil Central,” IBAMA, Brasília, 1998.
- J. Tollefson, “The Global Farm,” Nature, Vol. 466, No. 7306, 2010, pp. 554-556. doi:10.1038/466554a
- C. Lévi-Strauss, “Tristes Tropiques,” Librarie Plon, Paris, 1955.
- W. T. Stamps, T. V. Dailey, N. M. Gruenhagen and M. J. Linit, “Soybean Yield and Resource Conservation Field Borders,” Agriculture, Ecosystems and Environment, Vol. 124, No. 1, 2008, pp. 142-146. doi:10.1016/j.agee.2007.08.004
- D. P. Faith, “Quantifying Biodiversity: A Phylogenetic Perspective,” Conservation Biology, Vol. 16, No. 1, 2002, pp. 248-52. doi:10.1046/j.1523-1739.2002.00503.x
- L. M. Verdade, L. M. Rosalino, C. Gheler-Costa, N. M. Pedroso and M. C. Lyra-Jorge, “Adaptation of Mesocarnivores (Mammalia: Carnivora) to Agricultural Landscapes of Mediterranean Europe and Southeastern Brazil: A Trophic Perspective”, In: L. M. Rosalino and C. Gheler-Costa, Eds., Middle-Sized Carnivores in Agricultural Landscapes, Nova Science Publishers, New York, 2010, pp. 1-38.
- W. Dean, “Rio Claro: Um Sistema Brasileiro de Grande Lavoura 1820-1920,” Paz e Terra, Rio de Janeiro, 1977.
- W. Dean, “With Broadax and Firebrand: The Destruction of the Brazilian Atlantic Forest,” California University Press, Berkeley, 1995.
- F. J. N. Kronka, M. A. Nalon, C. K. Matsukuma, M. M. Kanashiro, M. S. S. Ywane, L. M. P. R. Lima, J. R. Guillaumon, A. M. F. Barradas, M. Pavão, L. A. Manetti and S. C. Borgo, “Mapeamento e quantificação do reflorestamento no estado de São Paulo,” 2000. http://www.sp.br.emb-japan.go.jp/portugues/img/simp_kronka.pdf
- SMA/IF—Secretaria de Meio Ambiente do Estado de São Paulo/Instituto Florestal, “Inventário Florestal da Vegetação Natural do Estado de São Paulo,” Imprensa Oficial, São Paulo, 2005.
- M. C. Ribeiro, J. P. Metzger, A. C. Martensen, F. J. Ponzoni, M. M. Hirota, “The Brazilian Atlantic Forest: How Much Is Left, and How Is the Remaining Forest Distributed? Implications for Conservation,” Biological Conservation, Vol. 142, No. 6, 2009, pp. 1141-1153. doi:10.1016/j.biocon.2009.02.021
- IBGE—Instituto Brasileiro de Geografia e Estatística, “Mapeamento Agrícola do Estado de São Paulo,” 2009. http://www.ibge.br
- J. M. Dros, “Administrando os Avanços da Produção de Soja. Dois Cenários da Expansão do Cultivo de Soja na América do Sul,” AID Environemt, Amsterdã, 2004.
- CONAB—Companhia Nacional de Abastecimento, “Limites da Expansão da Soja no Brasil,” 2004. http://conab.gov.br/publicacoes/indicadores
- G. Fischer, E. Teixeira, E. T. Hizsnyik and H. Velthuizen, “Land Use Dynamics and Sugarcane Production,” In: P. Zuurbier and J. V. Vooren, Eds., Sugarcane Ethanol: Contributions to Climate Change Mitigation and The Environment, Wageningen Academic Press, Netherlands, 2008, pp. 29-62.
- P. M. Fearnside, “The Roles and Movements of Actors in the Deforestation of Brazilian Amazonia,” Ecology and Society, Vol. 13, No. 1, 2008, pp. 1-18.
- A. M. M. Ferreira and E. Salati, “Forças de Transformação do Ecossistema Amazônico,” Estudos Avançados, Vol. 19, No. 54, 2005, pp. 25-44. doi:10.1590/S0103-40142005000200003
- D. C. Morton, R. S. DeFries, Y. E. Shimabukuro, L. O. Anderson, E. Arai, F. del Bon Espirito-Santo, R. Freitas and J. Morisette, “Cropland Expansion Changes Deforestation Dynamics in the Southern Brazilian Amazon,” PNAS, Vol. 103, No. 39, 2006, pp. 14637-14641. doi:10.1073/pnas.0606377103
- D. C. Nepstad, C. M. Stickler and O. T. Almeida, “Globalization of the Amazon Soy and Beef Industries: Opportunities for Conservation,” Conservation Biology, Vol. 20, No. 6, 2006, pp. 1523-1532. doi:10.1111/j.1523-1739.2006.00510.x
- P. M. Fearnside, “Can Pasture Intensification Discourage Deforestation in the Amazon and Pantanal Regions of Brazil?” In: C. H. Wood and R. Porro, Eds., Deforestation and Land Use in the Amazon, University Press of Florida, Gainesville, 2002, pp. 283-364.
- M. V. Lomolino and D. R. Perault, “Assembly and Disassembly of Mammal Communities in a Fragment Temperate Rain Forest”, Ecology, Vol. 81, No. 6, 2000, pp. 1517-1532.
- T. E. Lovejoy, R. O. Bierregard, A. B. Rylands, J. R. Malcolm, C. E. Quintela, L. H. Harper, K. S. Brown, G. V. N. Powell, H. O. R. Schubart and M. B. Hay, “Edge and Other Effects of Isolation on Amazon Forest Fragments,” In: M. E. Soule, Ed., Conservation Biology, Sinauer Press, Cambridge, 1986, pp. 257-285.
- R. Pardini, “Effects of Forest Fragmentation on Small Mammals in an Atlantic Forest landscape,”Biodiversity and Conservation, Vol. 13, No. 13, 2004, pp. 2567-2586. doi:10.1023/B:BIOC.0000048452.18878.2d
- L. M. Verdade, “A Exploração da Fauna Silvestre no Brasil: Jacarés, Sistemas e Recursos Humanos,” Biota Neotropica, Vol. 4, No. 2, 2004, pp. 1-12.
- L. Cullen Junior, R. E. Bodmer and C. Valladares-Pádua, “Effects of Hunting in Habitat Fragments of the Atlantic Forests, Brazil,” Biological Conservation, Vol. 95, No. 1, 2000, pp. 49-56. doi:10.1016/S0006-3207(00)00011-2
- L. A. Martinelli, A. M. Silva, P. B. Camargo, L. R. Moretti, A. C. Tomazelli, D. M. L. Silva , E. G. Fisher, K. Sonoda and M. S. Salomão, “Levantamento das Cargas Orgânicas Lançadas nos Rios do Estado de São Paulo”, Biota Neotropica, Vol. 2, No. 2, 2002, pp. 1-18.
- C. I. Piña, V. A. Lance, B. O. Ferronato, I. Guardia, T. S. Marques, L. M. Verdade, “Heavy Metal Contamination in Phrynops geoffroanus (Schweigger, 1812) (Testudines: Chelidae) in a River Basin, São Paulo, Brazil,” Bulletin of Environmental Contamination and Toxicology, Vol. 83, No. 6, 2009, pp. 771-775. doi:10.1007/s00128-009-9866-6
- A. M. C. Meche, B. E. S. N. Martins, C. J. Lofrano, M. Merchant, L. M. Verdade, “Determination of Heavy Metals by Inductively Coupled Plasma-Optical Emission Spectrometry in Fish from the Piracicaba River in Southern Brazil,” Microchemical Journal, Vol. 94, No. 2, 2009, pp. 171-174. doi:10.1016/j.microc.2009.10.018
- SMA—Secretaria de Meio Ambiente do Estado de São Paulo, “Fauna ameaçada do Estado de São Paulo,” Secretaria de Estado do Meio Ambiente, São Paulo, 2008.
- G. C. Daily, P. R. Ehrlich and G. A. Sanchez-Azofeifa, “Countryside Biogeography: Use of Human-Dominated Habitats by the Avifauna of Southern Costa Rica,” Ecological Applications, Vol. 11, No. 1, 2001, 1-13. doi:10.1890/1051-0761(2001)011[0001:CBUOHD]2.0.CO;2
- J. Ranganathan, R. J. R. Daniels, M. D. S. Chandran, P. R. Ehrlich and G. Daily, “Sustaining Biodiversity in Ancient Tropical Countryside,” PNAS, Vol. 105, No. 46, 2008, pp. 17852-17854. doi:10.1073/pnas.0808874105
- C. A. Harvey, N. I. J. Tucker and A. Estrada, “Live Fences, Isolated Trees, and Windbreaks: Tools for Conserving Biodiversity in Fragmented Tropical Landscapes,” In: G. Schroth, G. A. B. Fonseca, C. A. Harvey, C. Gascon, H. L. Vasconcelos and A. M. N. Izac, Eds., Agroforestry and Biodiversity Conservation in Tropical Landscapes, Island Press, Washington, 2004, pp. 261-289.
- G. Dotta and L. M. Verdade, “Medium to Large-Sized Mammals in Agricultural Landscapes of South-Eastern Brazil,” Mammalia, Vol. 75, No. 4, 2011, pp. 345-352. doi:10.1515/MAMM.2011.049
- M. C. Lyra-Jorge, G. Ciocheti, V. R. Pivello and S. T. Meirelles, “Carnivore Mammals in a Fragmented Landscape in Northeast of São Paulo State, Brazil,” Biodiversity Conservation, Vol. 17, No. 7, 2008, pp. 1573-1580. doi:10.1007/s10531-008-9366-8
- M. Penteado, “Distribuição e Abundancia de Avifauna na Bacia Hidrográfica do Rio Passa Cinco,” Ph.D. Dissertation, Universidade de São Paulo, Piracicaba, 2006.
- C. Gheler-Costa, C. A. Vettorazzi, R. Pardini and L. M. Verdade, “The Distribution and Abundance of Small Mammals in Agroecosystems of Southeastern Brazil,” Mammalia, Vol. 76, No. 2, 2012, pp. 185-191. doi:10.1515/mammalia-2011-0109
- M. J. T. Norman, C. J. Pearson and P. G. E .Searle, “The Ecology of Tropical Food Crops,” Cambridge University Press, Cambridge, 1995. doi:10.1017/CBO9781139172479
- W. A. N. Amaral, J. P. Marinho, R. Tarasantchi, A. Beber and E. Giuliani, “Environmental Sustainability of Sugarcane Ethanol in Brazil”, In: P. Zuurbier and J. V. Vooren, Eds., Sugarcane ethanol: Contributions to climate change mitigation and the environment, Wageningen Academic Press, Netherlands, 2008, pp 113-138.
- L. T. M. Figueiredo, G. M. Campos and F. B. Rodrigues, “Síndrome Pulmonar e Cardiovascular por Hantavirus: Aspectos Epidemiológicos, Clínicos, do Diagnostico Laboratorial e do Tratamento,” Revista Sociedade Brasileira Medicina Tropical, Vol. 34, No. 1, 2001, pp. 15-27. doi:10.1590/S0037-86822001000100003
- P. Daszak, A. A. Cunningham and A. D. Hyatt, “Emerging Infectious Diseases of Wildlife: Threats to biodiversity and Human Health,” Science, Vol. 287, No. 5452, 2000, pp. 443-449. doi:10.1126/science.287.5452.443
- A. P. Buckle, “Rodent Control Methods: Chemical,” In: A. P. Buckle and R. H. Smith, Eds., Rodent Pests and Their Control, CAB International, Wallingford, 1994, pp. 127-160.
- L. A. Fiedler and M. W. Fall, “Rodent Control in Practice: Tropical Field Crops,” In: A. P. Buckle and R. H. Smith, Eds., Rodent Pests and Their Control, CAB International, Wallingford, 1994, pp. 313-338.
- K. M. P. M. B. Ferraz, S. F. B. Ferraz, J. R. Moreira, H. T. Z. Couto and L. M. Verdade, “Capybara (Hydrochoerus hydrochaeris) Distribution in Agroecosystems: A CrossScale Habitat Analysis”, Journal of Biogeography, Vol. 34, No. 2, 2007, pp. 223-230. doi:10.1111/j.1365-2699.2006.01568.x
- M. B. Labruna, “Ecology of Rickettsia in South America,” Annals of the New York Academy of Sciences, Vol. 1166, No. 1, 2009, pp. 156-166. doi:10.1111/j.1749-6632.2009.04516.x
- H. Oelke, “Limitations of Estimating Bird Populations Because of Vegetation Structure and Composition,” In: C. J. Ralph and J. M. Scott, Eds., Estimating Numbers of Terrestrial Birds: Studies in Avian Biology, Allen Press, Kansas, 1981, pp. 316-321.
- I. C. Macedo and E. A. Seabra, “Mitigation of GHG Emission Using Sugarcane Bioethanol,” In: P. Zuurbier and J. V. Vooren, Eds., Sugarcane ethanol: Contributions to Climate Change Mitigation and the Environment, Wageningen Academic Press, Netherlands, 2008, pp. 95-111.
- W. Lywood, J. Pinkney and S. Cockerill, “The Relative Contributions of Changes in Yield and Land Area to Increasing Crop Output,” Global Change and Biodiversity—Bioenergy, Vol. 1, No. 5, 2009, pp. 360-369.
- P. Gerhard, “Comunidade de Peixes de Riachos em Fun- ção da Paisagem na Bacia do Rio Corumbataí, Estado de São Paulo,” Ph.D. Dissertation, Universidade de São Paulo, Piracicaba, 2005.
- P. M. Fearnside, “Soybean Cultivation as a Threat to the Environment in Brazil,” Environmental Conservation, Vol. 28, No. 1, 2001, pp. 23-38. doi:10.1017/S0376892901000030
- P. Zuurbier and J. V. Vooren, “Introtuction to Sugarcane Ethanol Contributions to Climate Change Mitigation and the Environment,” In: P. Zuurbier and J. V. Vooren, Eds., Sugarcane Ethanol: Contributions to Climate Change Mitigation and the Environment, Wageningen Academic Press, Netherlands, 2008, pp. 19-28.
- W. E. Tyner, “The Global Impacts of US and EU Biofuels Policies,” In: P. Zuurbier and J. V. Vooren, Eds., Sugarcane Ethanol: Contributions to Climate Change Mitigation and the Environment, Wageningen Academic Press, Netherlands, 2008, pp. 181-197.
- W. P. Lima and M. J. B. Zakia, “As Florestas Plantadas e a Água: Implemantando o Conceito da Microbacia Hidrográfica Como Unidade de Planejamento,” Rima, São Carlos, 2006.
- R. M. A. Rapassi, M. A. A. Tarsitano, J. C. R. Pereira and C. A. M. Araujo, “Cultura do Eucalipto na Região de Suzanápolis, Estado de São Paulo: Análise Econômica,” Informações Econômicas, Vol. 38, No. 4, 2008, pp. 7-13.
- D. C. Victoria, M. C. Hott, E. E. Miranda and O. T. Oshiro, “Delimitação de Áreas de Preservação Permanente em Topos de Morros Para o Território Brasileiro,” Revista Geográfica Acadêmica, Vol. 2, No. 2, 2008, pp. 66-72.
- J. P. Metzger, T. M. Lewinsohn, C. A. Joly, L. M. Verdade, L. A. Martinelli and R. R. Rodrigues, “Brazilian Law: Full Speed in Reverse?” Science, Vol. 329, No. 5989, 2010, pp. 277-278. doi:10.1126/science.329.5989.276-b
- J. P. Metzger, “O Código Florestal Tem Base Científica?” Conservação e Natureza, Vol. 8, No. 1, 2010, pp. 1-17.
- C. A. Joly, R. R. Rodrigues, J. P. Metzger, C. F. B. Haddad, L. M. Verdade, M. C. Oliveira and V. S. Bolzani, “Biodiversity Conservation Research Training and Policy in São Paulo,” Science, Vol. 328, No. 5984, 2010, pp. 1358-1359. doi:10.1126/science.1188639
- UDOP—União dos Produtores de Bioenergia, “Usinas e Destilarias: Mapas,” 2010. http://www.udop.com.br/index.php?item=mapa_bra
NOTES
*Corresponding author.

