American Journal of Analytical Chemistry
Vol. 3 No. 3 (2012) , Article ID: 18276 , 5 pages DOI:10.4236/ajac.2012.33036
Synthesis and Thermal Studies of Polyaniline Stannic Silicate and Its Role in the Removal of Toxic Metal Ions
Department of Chemistry, National Institute of Technology, Srinagar, Kashmir
Email: *sajadali16@gmail.com
Received November 28, 2011; revised December 29, 2011; accepted January 17, 2012
Keywords: Organic-Inorganic Hybrid Material; Polyaniline Stannic Silicate; Synthesis; Thermal Studies
ABSTRACT
A composite ion exchange material, polyaniline stannic silicate was prepared under different experimental conditions. Ion exchange material synthesized at pH 1.0 shows an ion exchange capacity 1.6 meq/g for Na+ ions. The exchanger has been characterized on the basis of X-ray and TGA. Ion exchange capacity, pH titration, elution and distribution studies were also carried out to determine the preliminary ion exchange properties of the material. The effect of temperature on the ion exchange capacity of the material at different temperatures has been studied. The sorption behavior of metal ions was also explored in different surfactant mediums. The practical utility of the material was explored by achieving quantitative separation of toxic metal ions in synthetic mixtures.
1. Introduction
Ion exchange materials have been proved very suitable tool to various environmental problems [1-3]. Although the organic ion exchangers have a wide applicability but a few limitations of the organic resins have been reported. One of the severest limitations of the organic resins is their poor thermal stability. Since organic ion exchangers were found to be unstable at high temperature & strong radiation, inorganic ion exchangers were taken as alternatives. However, the inorganic ion exchangers also have their own limitations e.g. non-suitable for column operation because of non-granulometric nature. In order to overcome the above limitations of organic resins and inorganic ion exchangers, many investigators have introduced organic-inorganic composite ion exchangers consisting properties of both the constituents and enhanced utility of inorganic materials and organic binding matrices [4]. Composite materials formed by developing organic polymers and inorganic particles are very important for the environmental point of view. The increasing level of heavy metals in the environmental has been of great interest because it represents a serious threat to human health, living resources and ecological system. The main sources of heavy metals are industrial effluents, wastewater fertilizer industries etc. Various methods have been developed for the removal of toxic metals from aqueous systems such as adsorption, reverse osmosis, ion exchange. But due to highly selective nature of ion exchange for metal ions, ion exchange is one of the best methods for the removal of heavy toxic metals ions. Cu and Pb are highly toxic and are associated with many health problems. The detection of trace amount of these elements in contaminated water has received considerable attention, as contamination by toxic elements is a major problem in many countries in the world.
In the present work, an attempt has been made for the synthesis, characterization, ion exchange behavior and analytical applications of composite cation exchanger, polyanailine stannic silicate.
2. Experimental
2.1. Reagents and Instruments
The main reagents used for the synthesis were aniline, stannic chloride pentahydrate, sodium silicate, and potassium persulphate (Loba Cheme Pvt Ltd (India)). All other chemicals and reagents used were of analytical grade.
The main instruments used during the study were UV-vis spectrophotometer (Elico EI 301E, India). FTIR spectrophotometer (Perkin-Elmer spectrum BX, USA), an automatic thermal analyzer (V2.2A DuPont 99000, a PW 1148/89 based X-ray diffractometer (Phillips, Holland), a water bath incubator shaker (MSW-275, India) and muffle furnace (G1-111, India).
2.2. Preparation of Reagent
The solutions of 0.1 M Stannic chloride pentahydrate and sodium silicate were prepared in demineralized water (DMW). Solutions of 10% (v/v) of double distilled aniline and 0.1 M potassium persulphate were prepared in 1.0 M HCl.
2.3. Preparation of Polyaniline Stannic Silicate
Polyaniline gels were prepared by mixing different volume ratio of 10% aniline and 0.1 M potassium persulphate with continuous stirring by a magnetic stirrer. Green colored polyaniline gels were obtained by keeping the solution below 10˚C for an hour. A precipitate of stannic (IV) silicate was prepared at room temperature (25˚C ± 2˚C) by adding mixture of 0.1 M sodium silicate solution gradually to an aqueous solution of 0.1 M stannic chloride pentahydrate in different volume ratios. The white precipitates were obtained. The pH of the mixture was adjusted to 1.0, 1.2 and 1.4 by adding HCl with continuous stirring. The gels of polyaniline were then added to the white inorganic precipitate of stannic (IV) silicate and mixed thoroughly with constant stirring. The resultant green color gels so obtained were kept for 24 h at room temperature (25˚C ± 2˚C) for digestion. The supernatant liquid was filtered off by suction. The gel was washed with demineralized water (DMW) till the filtrate was neutral. The product was dried in an oven at 50˚C. The dried material was broken into small granules on immersion in DMW. The granules were converted into H+ form by placing in 1 M HNO3 solution for 24 h. The excess acid from the material was removed after several washings with DMW and finally dried at 50˚C ± 2˚C. The particles of size 50 - 100 µm of the material were obtained by sieving. In this way a number of samples of polyaniline stannic silicate were prepared in different experimental conditions described in Table 1. It was decided to study sample S-5 in detail on the basis of highest ion exchange capacity for Na+ ions.
2.4. Column Ion-Exchange Capacity
To determine the ion-exchange capacity, column process was used. 1.0 g dry cation-exchanger in H+ form was packed in a column (1.0 cm id) fitted with glass wool at the bottom. Metal nitrates as eluents were used to elute the H+ ions completely from the cation-exchanger column. The effluent was titrated against a standard solution of 0.1 M NaOH.
2.5. Thermal Stability
In order to determine the effect of heating temperature on ion-exchange capacity, 1.0 g sample of the material (S-5) in H+ form was heated at different temperatures in a muffle furnace for 1 h and Na+ ion-exchange capacity was determined by standard column process as mentioned above. Thermo gravimetric analysis and differential thermal analysis studies (TGA-DTA) were performed at a heating rate of 10˚C min–1 in nitrogen atmosphere.
2.6. Distribution Studies
The distribution coefficients (Kd) for various metal ions were determined in a number of solvent systems. The effect of temperature on the distribution coefficient was also studied. Polyaniline stannic silicate (0.3 g) in H+ form was put into 100 mL conical flasks each containing 30 mL solution of 0.1 M concentration of metal ions. The mixture was continuously shaken for 4 h at 30˚C, 40˚C, 50˚C and 60˚C. The amount of metal ions present in the solution was determined by titrating it against disodium salt of EDTA (0.01 M) using standard procedures. Distribution coefficient values were calculated by using the following relationship:
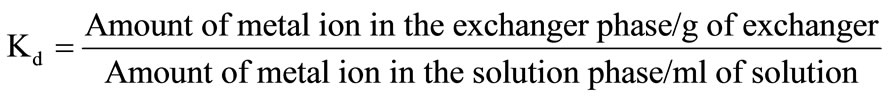

Table 1. Synthesis of polyaniline stannic silicate in different conditions.
In our case
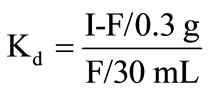
where I is the initial amount of the metal ion in the solution phase, F is the final amount of the metal ion in the solution phase.
2.7. Quantitative Separation of Metal Ions in Binary Synthetic Mixtures
Quantitative binary separations of some metal ions of analytical interest were achieved on polyaniline stannic silicate. 2.0 g of exchanger in H+ form was packed in a glass column (i.d = 0.5 cm) with a glass wool support at the end. The column was washed thoroughly with DMW. 2.0 mL binary mixture of the metal ions to be separated was poured on to the column and allow to flow at a rate of 0.3 - 0.5 mL∙min–1 till the solution level was just above the surface of the material. The column was then rinsed with DMW. Individual metal ions were eluted using appropriate eluting reagents. The flow rate of the eluent was maintained (0.3 - 0.5 mL∙min–1) throughout the elution process. The effluent was collected in 10 mL fractions and was titrated against the standard solution of 0.01 M di-sodium salt of EDTA.
3. Results and Discussion
In this study of the preparation and characterization of organic-inorganic composite cation exchange material, a number of samples of organic-inorganic electrically conducting composite polyaniline stannic silicate providing a new class of hybrid ion exchangers were prepared by the sol-gel mixing of organic conducting polymer polyaniline with the inorganic precipitate of stannic silicate (Table 1). Among them, sample S-5 possessed enhanced Na+ ion exchange capacity (1.6 meq/g) and better thermal stability. Polyaniline gel was prepared by oxidation coupling using K2S2O8 in acidic aqueous medium as given in the following reaction:
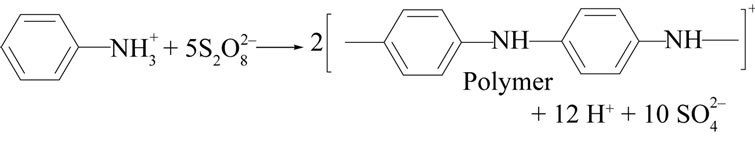
The effect of temperature on the reaction seems to be very pronounced. Aniline underwent oxidation coupling only below 10˚C very effectively, leading to formation of polyaniline with fairly good yield. The binding of polymer into matrix of Sn(IV) silicate can be shown as:
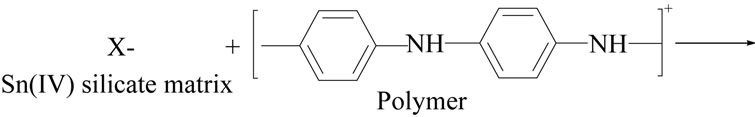

However sample 5 of polyaniline stannic silicate exhibited better granulometric and mechanical properties and reproducible behavior as is evident from Table 1. The samples prepared in various batches did not show any appreciable deviation in their ion exchange capacities. The ion exchange capacity of the composite cation exchanger for alkali metal ions and alkaline earth metal ions increases according to the decrease in the hydrated ionic radius increases, because the exchange now becomes more difficult (Table 2). It was observed that on heating the material at different temperatures, the physical appearance and ion exchange capacity of the dried sample material (S5) were changed as the temperature increased as shown in Table 3.
The TGA analysis curve (Figure 1) of the material showed continuous weight loss up to 150˚C, which may be due to the removal of external water molecule and a slow weight loss up to 200˚C was observed for the removal of interstitial and coordination water present in the material. Further, a continuous weight loss observed between 200˚C and 300˚C may be caused by physical transitions such as crystallization occurring during heating. Further weight loss between 300˚C and 500˚C may be due to the complete decomposition of the organic part of the material. When the temperature was raised beyond 500˚C, a smooth horizontal level represents the formation of corresponding oxides of metals. It has been observed that 50˚C is the optimum temperature for the highest uptake of metal ions studied (Table 4). The X-ray diffraction pattern of this material recorded in powdered form suggests the semi-crystalline nature of the material (Figure 2). In order to explore the potentiality of polyaniline stannic silicate in the separation of metal ions, distribution studies of metal ions were performed in different surfactant solvent systems. It is apparent from data in Table 5 that the material is selective for Cu2+. It has been found that Kd value of most of the metal ions decreases with the increase in concentration of the surfactant. The separation capability of the material has been demonstrated by achieving some analytically difficult binary separations (Zn2+-Cu2+, Zn2+-Pb2+ and Cd2+-Cu2+) of metal ions on its column. The salient features of these separations are summarized in Table 6.
4. Conclusion
Polyaniline Sn(IV) silicate, an organic-inorganic composite cation exchange material represents a new class of ion exchange materials that combines good characteristics of both organic and inorganic components within a
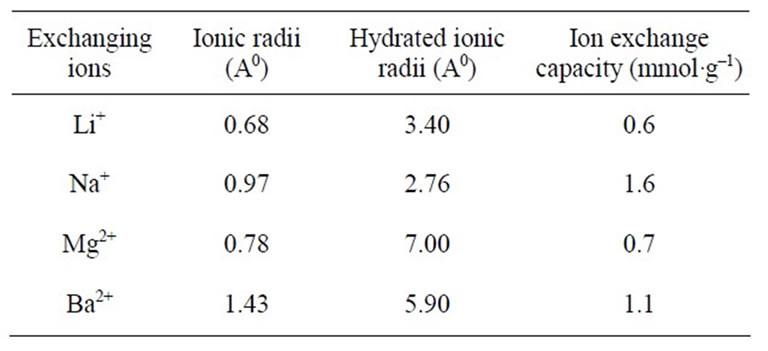
Table 2. Ion exchange capacity of polyaniline stannic silicate (sample S-5) for different metal ions.

Table 3. Effect of temperature on the ion-exchange capacity of polyaniline stannic silicate.
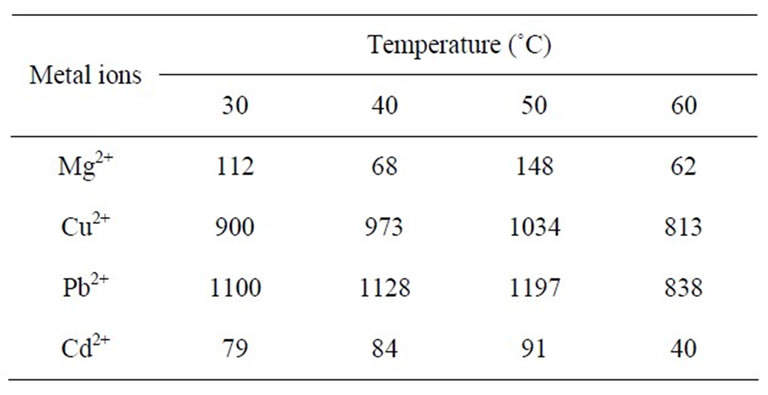
Table 4. Effect of temperature on Kd (mL∙g–1) of metal ions in 0.5% Tritoin-X100.
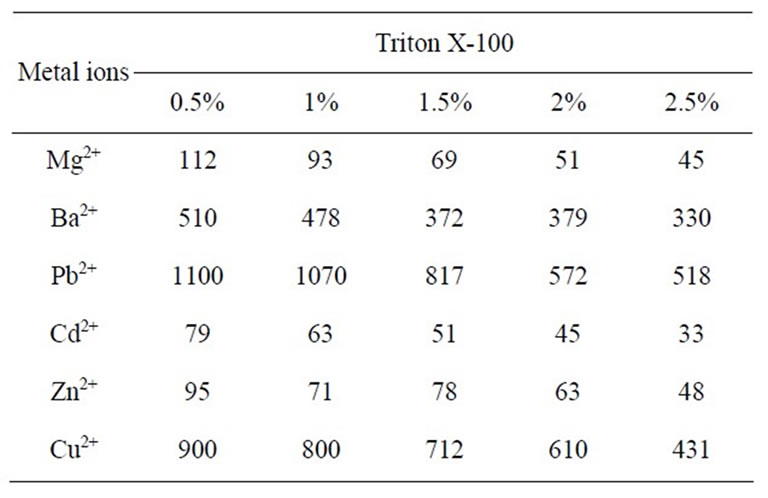
Table 5. Distribution coefficients of metal ions in Triton X-100 (Kd mL∙g–1) at 30˚C ± 2˚C.

Table 6. Quantitative separations of metal ions in binary mixtures on a column of polyaniline stannic silicate columns.
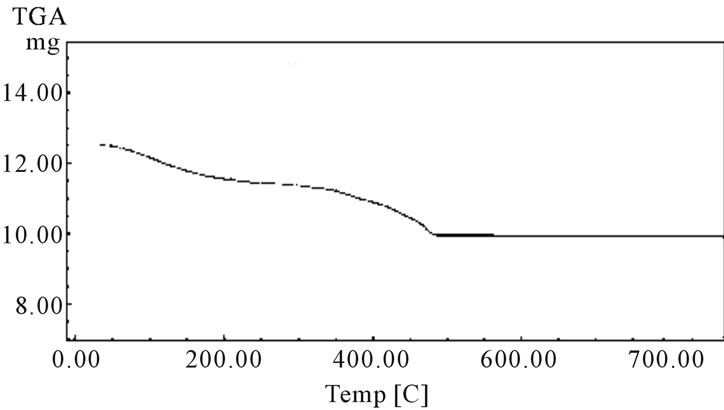
Figure 1. TGA curve of polyaniline stannic silicate.

Figure 2. X-ray diffraction pattern of polyaniline stannic silicate.
single composite. It is quite clear from the results that the composite cation exchanger has enhanced ion exchange capacity and thermal stabilities. The selective behavior of this composite cation exchanger is important from the environmental pollution chemistry point of view, where an effective separation method is needed for the removal of toxic metal ions.
REFERENCES
- H. Zhang, J. H. Pang, D. Wang, A. Li, X. Li and Z. Jiang, “Sulfonated Poly (Acrylene Ether Nitrate) and Its Composite with Phosphotungstic Acid as Materials for Proton Exchange Membranes,” Journal of Membrane Science, Vol. 264, No. 1-2, 2005, pp. 56-64. doi:10.1016/j.memsci.2005.04.021
- M. J. Shaw, P. N. Nesterenko, G. W. Dicinoski and P. R. Haddad, “Selectivity Behavior of a Bonded PhosphateCarboxylate Polymeric Ion Exchanger for Metal Cations with Varying Eluent Compositions,” Journal of Chromatography A, Vol. 997, No. 1-2, 2003, pp. 3-11. doi:10.1016/S0021-9673(03)00111-0
- A. A K. Niwas, R. Niwas and M. M Alam, “Ion-Exchange Kinetics of Styrene Supported Zr(IV) Tungstophosphate: An Organic Inorganic Type Cation Exchanger,” Indian Journal of Chemical Technology, Vol. 9, No. 12, 2002, p. 256.
- A. A. Khan, M. M. Alam and F. Mahmmad, “Ion-Exchange Kinetics and Electrical Conductivity Studies of Polyanilic Sn(IV) Tungstoarsenate; a New Semi-Crystallinic ‘Polymeric-Inorganic’ Composite Cation-Exchange Material,” Electrochimica Acta, Vol. 48, No. 17, 2003, pp. 2463-2472. doi:10.1016/S0013-4686(03)00272-X
NOTES
*Corresponding author.

