Journal of Biosciences and Medicines
Vol.03 No.11(2015), Article ID:61336,9 pages
10.4236/jbm.2015.311011
“Dermal Nitroglycerin Patch” in Treatment of Preterm Labour
Kunjan Shah, B. D. Gupta, Raksha Sharma
NIMS Jaipur, Rajasthan, India

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 2 September 2015; accepted 20 November 2015; published 23 November 2015

ABSTRACT
Although preterm birth is the delivery before 37 + 0 weeks of gestation, the majority of prematurity-related complications occur before 33 + 0 weeks of gestation. The need of today is to select the best agent out of the broad spectrum of available tocolytic agents, for providing maximum benefit to unfortunate sufferers of prematurity. The study aims to assess the role of “Dermal Nitroglycerin Patch” in treatment of preterm labour. Method: Study conducted in Department of Obstetrics & Gynecology, NIMS Medical College & Hospital, Jaipur from July 2014-December 2014. A prospective randomized study of 50 women of preterm labor was enrolled after informed consent. Patients were given dermal nitroglycerin patch as tocolytic agent according to study protocol. Result: 1) Results of NTG PATCH are comparable with other tocolytic drugs in terms of successful tocolysis; 2) Maternal and fetal outcome is favourable in cases of NTG PATCH USE in preterm labour; 3) Side effect profile was also better with NTG Patch. Conclusion: This randomized prospective study lends support to the proportion that Transdermal Nitroglycerine (NTG Patch) may be promising safe, effective, well tolerated, cost effective and non invasive method of tocolysis.
Keywords:
Transdermal Nitroglycerine (NTG Patch), Preterm Labor

1. Introduction
In the era of modern obstetrics, where there has been a rapid advancement in all specialties including obstetrical practice, even today the preterm labor still remains an enigma for the obstetrician. The incidence of preterm birth ranges from 10% - 15% of all deliveries in India whereas in developed countries it lies between 5% - 10% [1] .
Currently, the therapeutic foundation for treating preterm labour involves the use of tocolysis. It is now well proved that when prolongation of pregnancy is not hazardous either to mother or to fetus, the best place for immature fetus is still inside the uterus. The initial goal of tocolytic therapy is prolongation of pregnancy even for a short period of time to have advantage of steroid therapy as well as the NICU care [2] . Although preterm birth is defined as delivery before 37 + 0 weeks of gestation, the majority of prematurity-related adverse outcomes relate to birth before 33 + 0 weeks of gestation.
Henceforth a multi-centric trial of tocolytic drugs is the NEED OF TODAY to select the best possible agent out of the broad spectrum of available tocolytic agents, for providing maximum benefit to unfortunate victims of undesired preterm labour.
Keeping this in view, the present study was performed:
“To assess the role of Dermal Nitroglycerine Patch in Treatment of Preterm Labour.”
1.1. Aim
The study aims to assess the role of “Dermal Nitroglycerin Patch” (10 mg) in treatment of preterm labour.
1.2. Objectives
・ To determine maternal outcome in terms of successful tocolysis thereby prolonging the duration of period of gestation required for fetal lung maturation.
・ To assess fetal outcome in terms of fetal distress, APGAR score < 7 at 5 minutes, resuscitation required, re- spiratory distress syndrome, NICU admission, mechanical ventilation required and neonatal death in the antenatal pregnant population with preterm labour pain at NIMS Hospital, Shobha Nagar, Jaipur, Rajasthan.
・ To assess side effects, safety, efficacy, tolerance and acceptance of either drug.
2. Material and Method
This study was carried out in the Department of Obstetrics and Gynecology, NIMS Medical College and Hospital, Shobha Nagar, Jaipur, Rajasthan from July 2014 to December 2014, over the period of 6 months.
2.1. Sample Size
A total of 50 pregnant women of age group between 17 years to 40 years with preterm labour, were enrolled in the study.
2.2. Inclusion Criteria of Patients
50 pregnant women with single tone pregnancy with preterm labour pain between the gestational age 24 - 37 completed weeks with threatened preterm labour pains or established preterm labour according to definition of A.C.O.G (1997) [3] (Uterine contraction of 4 in 20 minutes or 8 in 1hour plus progressive change in the cervix, cervical dilation > 1 cm, cervical effacement 80% or greater) were included in the study.
Ø Exclusion criteria of patients:
1) Women with active labour [cervical dilation > 4 cm].
2) Systemic diseases like diabetes mellitus, cardiac diseases, liver or renal diseases.
3) Obstetric complications like prolonged premature rupture of membrane (>24 hours), severe pregnancy induced hypertension, eclampsia, ante partum hemorrhage, hydramnios, oligoamnios, hyperthyroidism.
4) Fetal complications like chorioamnionitis, IUGR, congenital anomaly, fetal distress, intrauterine death.
5) Multiple gestation. Hypotension (Systolic BP < 90 mm of Hg).
6) Sensitivity or contraindication to tocolytics.
3. Method
Written consent was taken from all patients included in the study. Drug administration was done as per attached flow chart for monitoring (Chart 1).
Patient with preterm labour pain with gestational age < 34 weeks were given inj. Betamethasone 12 mg IM stat dose and same dose was repeated after 24 hours to improve lung maturity [4] . Every patient admitted with preterm labour pain were given inj. Buscopan I.M stat dose to exclude patients with false labour pain and oral Amoxycillin 500 mg 8 hourly and Tab. Metronidazole 400 mg 8 hourly for 5 days as antibiotic prophylaxis
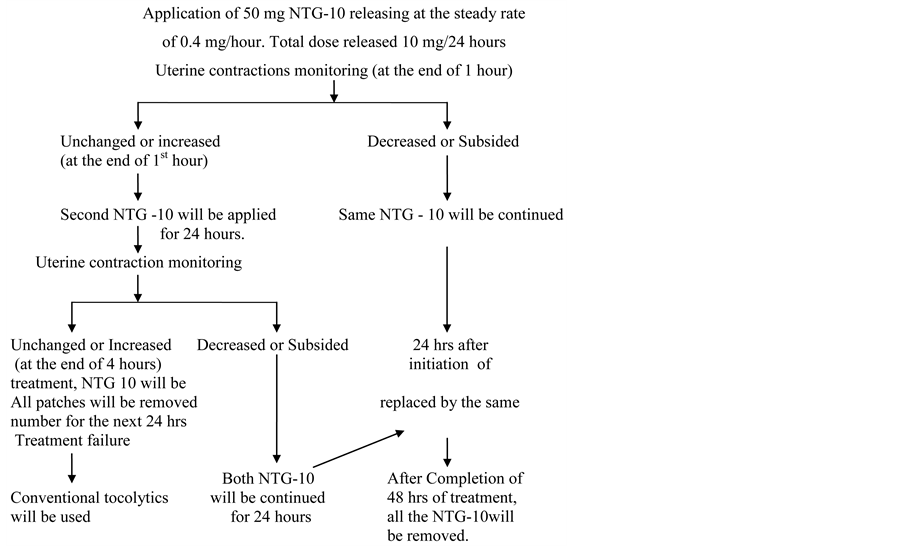
Chart 1. Showing treatment protocol of preterm labour with use of NTG PATCH.
against subclinical infection.
A) If contractions subsided, patients were discharged and assessed antenatally every week until delivery.
B) If delivery took place, placenta examined fully. Neonate evaluated for gestational age, birth weight, congenital anomalies, APGAR score etc.
Analysis of Data
To describe nominal data, simple percentages were used. Mean and standard deviations were used to describe normally distributed quantitative data from the subjects. Software used for analyzing data SPSS 21.
・ Treatment was considered successful, if there was abolition of uterine contractions, no progression of cervical dilatation, completion of course of maternal steroid and also if contractions did not reoccur within 48 hours of cessation of therapy.
・ Treatment was deemed failure, if uterine relaxation was not achieved or completion of steroid dose was not achieved despite maximal dose or patient or fetus developed some significant side effects that necessitated discontinuation of therapy.
4. Results
Table 1 shows the group distribution of enrolled patients according to age.
Parity distribution of the enrolled cases were according to Table 2.
Association is being noted between patient with history of preterm labour pain and the same patient presented with preterm labour pain at the same or earlier gestational age in present pregnancy (Table 3). 12 (24%) patients had history of preterm labour pain in previous pregnancy. Patients with history of preterm labour pain are more at risk of preterm pain in present delivery also.
Also risk of preterm labour was higher in patient with recurrent pregnancy losses (Table 3). Five (10%) patients gave history of RPL.
Patients with active labour (>4 cm dilation of cervix) were excluded from study. Table 4 shows the cervical status at the time of admission in patients with preterm labour.
98% women had attained successful tocolysis who received NTG as tocolytic (Table 5). Statistically this
Table 1. Age distribution of enrolled cases.
Table 2. Distribution of enrolled cases according to parity.
Table 3. Distribution according to obstetric history.
Table 4. Status of cervix at the time of admission in patient with preterm labour.
Table 5. Successful tocolysis and success rate of completion of maternal steroids.
value significant means that NTG has high tocolytic action FOR SUCCESSFUL TOCOLYSIS. By means of successful tocolysis means that it is possible to delay delivery of patients for at least 48 hours to complete the course of maternal steroids.
There were 18 patients in study group with gestational age < 34 weeks. 94.44% of women (17 out of 18) of gestational age < 34 weeks with preterm labour pain completed the course of steroid (Inj. Betamethasone 12 mg intramuscular 2 doses given 24 hours apart), Clinically this is very significant. Only 1 patient could not achieve complete steroid dose in NTG group due to extreme prematurity. Clinically NTG patchis more effective in prolongation of pregnancy for >48 hours and thereby achieving successful tocolysis (Table 5, Graph 1).
It was observed that only 2% women delivered within 48 hours of start of tocolytic therapy (Table 6).
The duration of prolongation of pregnancy exceeded for >7 days in 76% women of study group. Clinically it is observed that pregnancy can be prolonged for 18.25 days which shows that clinically NTG Patch is better (Table 6).
In the current study it was observed that 64% women delivered at the gestational age of more than 37 weeks. Only 8% patients delivered before 34 weeks in study group. It is found that there is significant effect of NTG patch as tocolytic agent (Table 7).
Cesarean was performed due to obstetrical indications. Table 8 shows the mode of delivery in enrolled cases.
In the present study headache occurred in 70% women of study Group. The severity of headache in was found to be mild in 70% of women and moderate in 30% of women which was relieved by Tab. Paracetamol 500 mg 1 tab stat. However, none of the women suffered from severe headache (Table 9).
Palpitation was observed in 4% of the women. The effect of NTG on pulse and blood pressure was not significant. The side effects were not so severe that required drug discontinuation (Table 9).
APGAR score was >7 in 92% in study group. 8% babies had APGAR < 7 (Table 10).
All those babies who had APGAR < 7, required resuscitation. Only 8% neonates had APGAR Score < 7 and required resuscitation (Table 11).
2% neonates developed respiratory distress syndrome.
In study group only one baby required mechanical ventilation due to development of severe RDS. The same baby died because of very premature birth and in this baby also, completion of course of steroid was not possible as successful tocolysis was not achieved in this baby (Table 11).
Neonatal complications were much lower in study group (Graph 2).
5. Discussion & Summary
Prevalence of preterm birth has increased during the last decades and it is a real public health concern. Tocolysis remains the predominant modality for the treatment of preterm labour. None of the currently available tocolytic agents are ideal.
This in depth study, on the basis of its findings concludes that:
1) Results of NTG PATCH are comparable with other tocolytic drugs in terms of successful tocolysis.
2) Maternal outcome in terms of successful tocolysis & thereby prolonging the duration of period of gestation can be successfully achieved by using NTG patch in appropriate selected cases of preterm labour.
3) Fetal outcomes in terms of fetal distress & NICU admission are favourable in cases of NTG PATCH USE in preterm labour.
4) APGAR score < 7 at 5 minutes, development of RDS, requirement of mechanical ventilation & in terms of neonatal morbidity & mortality, the effect of NTG is better & well-tolerated.
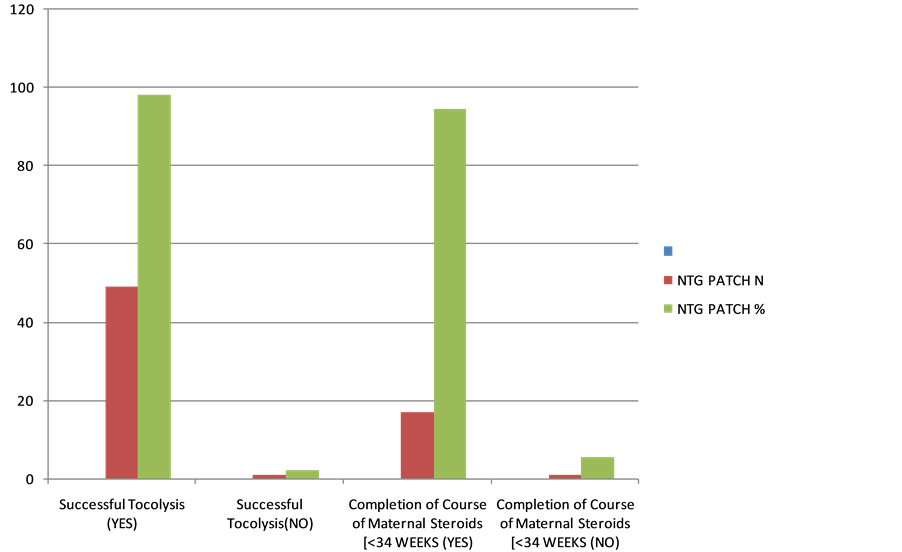
Graph 1. Successful tocolysis and success rate of completion of maternal steroids in study group.
Table 6. Duration of prolongation of pregnancy in the study group.
Table 7. Gestational age at which delivery occurred.
Table 8. Distribution of cases according to mode of delivery.
Table 9. Side effects of drug.
Table 10. Apgar score.
Table 11. Neonatal outcome.
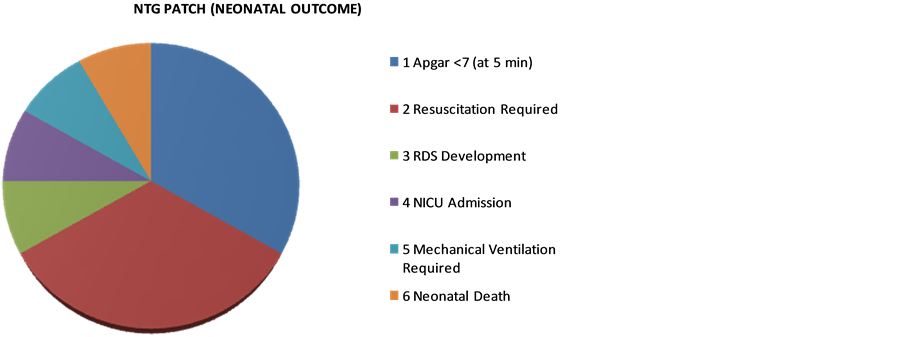
Graph 2. Neonatal outcome.
5) NTG patch use in preterm labour can help in reducing perinatal mortality.
6) Major common side effect of NTG Patch is headache which can be easily controlled & no major maternal or fetal side effects has been observed with NTG patch in preterm labour cases.
7) NTG Patch is better tolerated & acceptable due to its transdermal drug delivery system.
8) NTG Patch is also COST EFFECTVE.
9) Present study found a favourable outcome with NTG PATCH.
The ideal tocolytic agent is one which is effective in prolonging pregnancy but has no side effects for the woman or fetus.
In the present study, success rate and efficacy of NTG Patch is comparable with the studies of Kumar Aruna [5] , Sachan Rekha [6] and Krishna et al. [7] .
6. Conclusion
NTG or similar organic nitrate compounds appear to be at least as effective as the commonly used tocolytics today. NTG patch may prove to be the drug of choice for acute uterine relaxation in cases of preterm labour. This randomized prospective comparative study lends support to the preposition that Transdermal Nitroglycerine may be promising, safe, effective, well tolerated, cost effective and non invasive method of tocolysis.
Recommendation
NTG Patch as a TOCOLYTIC Agent: Since NTG PATCH is proved to be an effective and safe tocolytic agent in present study, tocolysis with NTG PATCH is recommended to all eligible patients in preterm labour. The availability of nitroglycerine as a transdermal patch opens new therapeutic perspectives, since this approach has few side effects and is convenient to use.
Limitations
The results from the present study needs to be further strengthened and validated by multilocational and bigger sample sized research studies to assess optimal dosage, dosage regimes, overall efficacy and fetal safety.
Cite this paper
KunjanShah,B. D.Gupta,RakshaSharma, (2015) “Dermal Nitroglycerin Patch” in Treatment of Preterm Labour. Journal of Biosciences and Medicines,03,82-90. doi: 10.4236/jbm.2015.311011
References
- 1. Chopra, S. (1997) Preterm Labour: Use of Tocolytics for Prolongation of Pregnancy. Obstetric and Gynecology Today, 2, 28-30.
- 2. Abramovici, A., Cantu, J. and Jenkins, S.M. (2012) Tocolytic Therapy for Acute Preterm Labour. Obstetrics and Gynecology Clinics of North America, 39, 77-87.
http://dx.doi.org/10.1016/j.ogc.2011.12.003 - 3. Cunnigham, F.G., Kenneth, K.J., Bloom, S.L., Hauth, J.C., Rouse, D.J. and Spong, C.Y. (2007) Williams Obstetrics. 23rd Edition, McGraw Hill Medical Publishing Division, New Delhi, 804-831.
- 4. Crowley, P. (2000) Prophylactic Corticosteroids for Preterm Birth. Cochrane Database of Systematic Reviews, 2, Article ID: CD000065.
- 5. Kumar, A., Bharadwaj, B. and Namita, P. (2001) Glyceryl Trinitrate Patch in Management of Pretermlabour. Journal of Obstetrics and Gynaecology of India, 51, 55-57.
- 6. Sachan, R., Gupta, P., Chaudhary, S. and Agarwal, R. (2012) Clinical Evaluation of Transdermal Nitroglycerine in Preterm Labor in Tertiary Care Teaching Hospital in North India. International Journal of Scientific and Research Publications, 2, 1-8.
- 7. Krishna, U. and Damle, D.S. (1996) Nitroglycerine Skin Patch Application for Tocolysis in Premature Onset of Labour. Journal of Obstetrics and Gynaecology Today, 1, 305-307.


