Open Journal of Rheumatology and Autoimmune Diseases
Vol.4 No.1(2014), Article ID:43244,5 pages DOI:10.4236/ojra.2014.41011
Expression of CD55, CD59, CD46 and CD35 in Peripheral Blood Cells from Rheumatoid Arthritis Patients
1Divison of Clinical Pathology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil; 2Division of Rheumatology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil.
Email: *rmaxavier@hcpa.ufrgs.br
Copyright (c) 2014 Amanda Kirchner Piccoli et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights (c) 2014 are reserved for SCIRP and the owner of the intellectual property Amanda Kirchner Piccoli et al. All Copyright (c) 2014 are guarded by law and by SCIRP as a guardian.
Received October 20th, 2013; revised November 20th, 2013; accepted November 28th, 2013
Keywords:Rheumatoid Arthritis; Complement System; Complement Regulatory Protein; Flow Cytometry
ABSTRACT
Evidences point that complement activation plays a role in rheumatoid arthritis (RA). In this context, expression of the CReg was investigated on white cells from peripheral blood of 30 RA patients and 30 healthy controls. Us- ing flow cytometric analyses the relative fluorescence intensities (MFI) of Cregs were determined. CD59 MFI was significantly increased in RA cells comparing to controls, respectively: lymphocytes 36.8 versus 27.07; monocytes 32.0 versus 21.37; and granulocytes: 84.6 versus 66.1 (p < 0.05). Interestingly, no difference was ob- served on the MFI to CD55, CD46 and CD35 in these cells. These data indicate an overexpression of CD59 in all the peripheral blood cells of RA patients, perhaps due to an increased synthesis for compensatory mechanisms because of complement activation, inflammatory status or other factors associated with the disease.
1. Introduction
Rheumatoid Arthritis (RA) is an autoimmune disease that affects 1% of the adult human population with females affected three times more than males [1]. RA is associated with persistent inflammatory polyarticular synovitis affecting mainly peripheral joints [2]. The thin synovium is normally a delicate intimal lining. In RA, it becomes thicker, resulting in swollen and tender joints because of the inflammatory infiltration that consists of macrophages, CD4+ T cells, B cells, dendritic cells, plasma cells, mast cells and granulocytes. As a result of these processes, the joint becomes deformed, unstable, inflamed, and painful, leading to great disability [3].
The complement system, when not regulated, can cause tissue damage. This condition can be induced by pro-inflammatory mechanisms, such as cytokines and chemokines. These are usually up-regulated in RA, indicating that these patients are at increased risk to damage mediated by the complement system. To counteract or contain self-damage, the complement system has a variety of regulators which can be membrane-bound or secretory proteins, which appear to be more or less efficient in distinct conditions. Normal cells resist complement-mediated lysis by several mechanisms such as specific membrane-bound proteins. Examples of these are the decay accelerating factor (CD55), the membrane inhibitor of reactive lysis or protectin (CD59), the membrane cofactor protein (CD46) and the complement receptor type I (CD35) [4,5].
Previous studies have analyzed only the expression of CD55, CD59, CD46 and CD35 in peripheral blood granulocytes and synovial fluid granulocytes [6-8], as well as CD35 expression in monocytes and lymphocytes from the peripheral blood and synovial fluid of patients with RA [8-10]. The aim of this study is to analyze the expression profile of CD55, CD59, CD46, and CD35 complement regulatory (CReg) proteins on the surface of leukocytes by flow cytometry in blood donors and patients with RA.
2. Materials and Methods
2.1. Subjects
Thirty consecutive patients that fulfilled the American College of Rheumatology classification criteria [11] for RA were included. The controls were blood donors without history of disease and were matched by age and sex. The RA patients were seen during their routine follow-up visits in the RA clinics of the Hospital de Clínicas de Porto Alegre. The exclusion criteria were concomitant presence of leukaemia, primary lymphoproliferative diseases, or overlap with an autoimmune disease. This study was performed with approval of the ethics committee of the Hospital de Clínicas de Porto Alegre/ Brazil, and all subjects gave their written informed consent.
2.2. Flow Cytometric Analysis of CD55, CD59, CD46 and CD35 Membrane in Leukocytes
The flow cytometric analysis was processed as reported elsewhere [12]. Briefly, 100 μL of whole blood were stained with Fluorescein isothiocyanate (FITC) or Phycoerythrin (PE) conjugated monoclonal antibody (CD55 PE, CD59FITC, CD35PE and CD46FITC (BD Biosciences, San Diego, CA, USA)). After incubation, 1.0 mL of FACSlyse (BD Biosciences, San Diego, CA, USA) was added and lysis was allowed for 10 min at room temperature. Samples were washed once and re-suspended in PBS.
Cells were analyzed on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences, San Diego, CA, USA). Membrane fluorescence intensity was estimated by the relative mean fluorescence intensity (MFI). Cells labeled with isotype-controls were used to set the fluorescence thresholds for positivity fluorescence.
2.3. Statistics
Varibles with normal distribution were presented with mean and standard deviation. The statistical significance of the differences observed between the distinct subsets of leukocytes (means) were calculated using Student’s t-test. Correlation of disease activity (DAS28) and CReg expression was evaluated using Spearman correlation coefficient. p values < 0.05 were considered to be associated with statistical significance.
3. Results
The description of the 30 patients and 30 healthy controls and the treatments are summarized on Table 1. Twelve of the 30 RA patients (40%) had leukocytosis whereas seven of the 30 RA patients (23.3%) had monocytosis, four (13.3%) had granulocytosis and two (6.7%) had lymphocytosis. Seven of the 30 RA patients (23.3%) showed lymphopenia.
To evaluate the proportion and MFI of CD55, CD59, CD46 and CD35 cells in RA patients and healthy controls, CellQuest software was used and the cells were performed as previous work [12].
Flow Cytometry Analyses from the Blood Cells
The CD59 MFI in RA cells was significantly increased compared to controls cells: granulocytes (84.60 vs. 66.10), monocytes (32.00 vs. 21.37) and lymphocytes (36.8 vs. 27.07) (Figure 1).
The proportions of CD59+ (91.84%), CD46+ (99.78%), CD35+ (98.50%) in RA monocytes were also significantly increased. Together with the proportions of CD59+ (91.84%) in RA lymphocyte. Meanwhile, CD55, CD46 and CD35 MFI did not show significant differences between RA and controls, as summarized on Table 2.
Regarding association with disease activity (DAS28), we observed increased CD59 expression on lymphocytes, monocytes and granulocytes from patients with higher activity (data not shown), however there was no significance correlation (CD59 MFI Lymphocytes, p = 0.288; CD59 MFI Monocytes, p = 0.757 and CD59 MFI Granulocytes, p = 0.208).
4. Discussion
In this study we found a CD59 increased expression in all cells in the peripheral blood of patients with rheumatoid arthritis: on lymphocytes, monocytes and granulocytes. In contrast with our findings, Jones et al. [7] observed no difference between MFI of CD59 on granulocytes from peripheral blood of RA patients compared with healthy controls. However they evaluated a rather small number of patients (n = 18), and the study might not have had enough power to detect a difference. These authors did not evaluate monocytes and lymphocytes.
Furthermore, Konttinen et al. [13] have described that RA synovial cells do not express CD59, and that CD59 expression is weak in many synovial stromal and endothelial cells. In addition, CD59 expression seems to be partially lost in many acute arthritis in these cells. The finding of a lack of CD59 expression in synovial cells in RA and acute arthritis might imply in an inefficient control of MAC activation in synovial tissue. This could predispose to synovial cell damage or to various stimulatory effects by MAC. Factors inducing this low expression are not known.
In another study, Crockard et al. [6] have noted that CD35 expression was upregulated on synovial fluid gra-
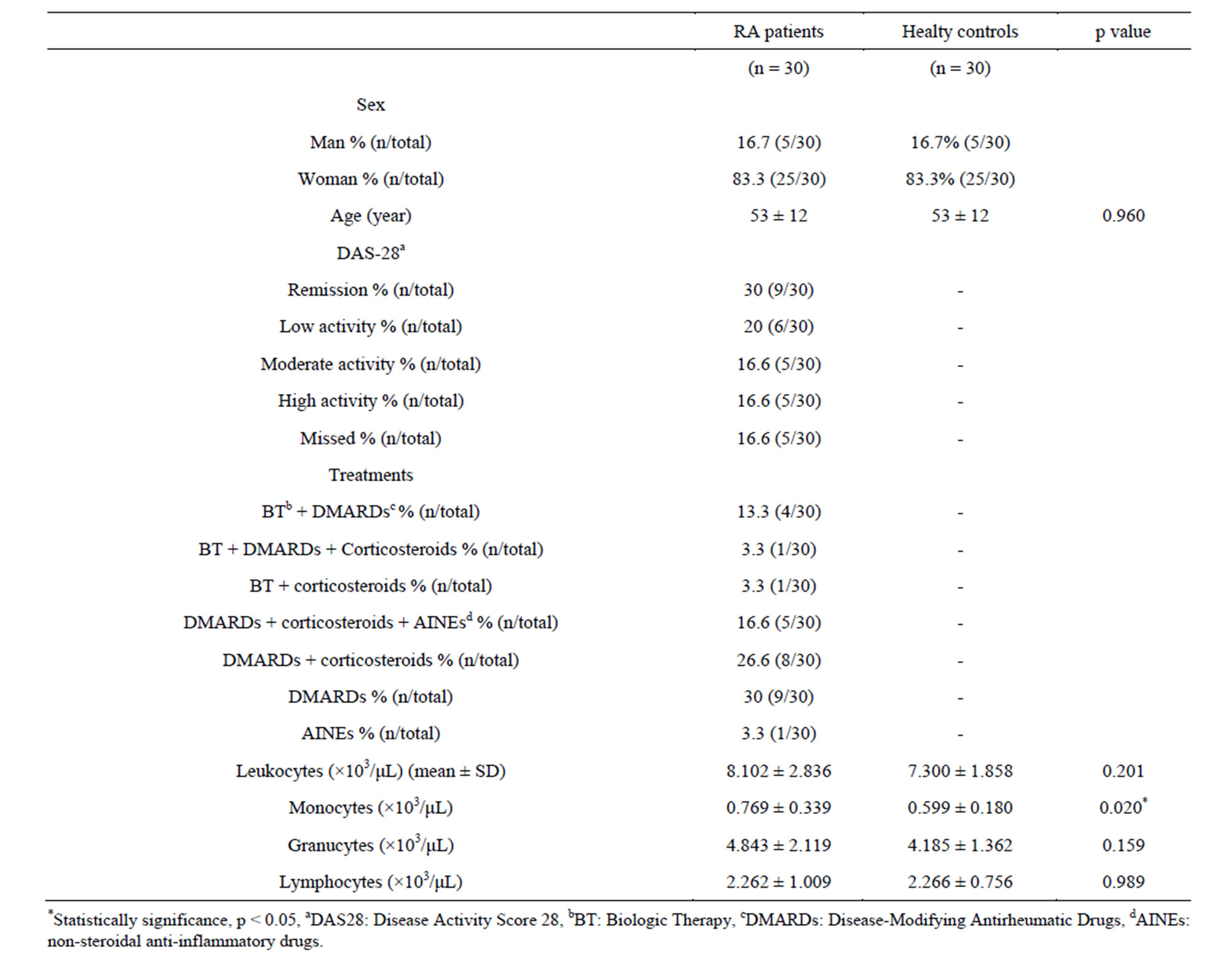
Table 1. Description of RA patients and healthy controls.
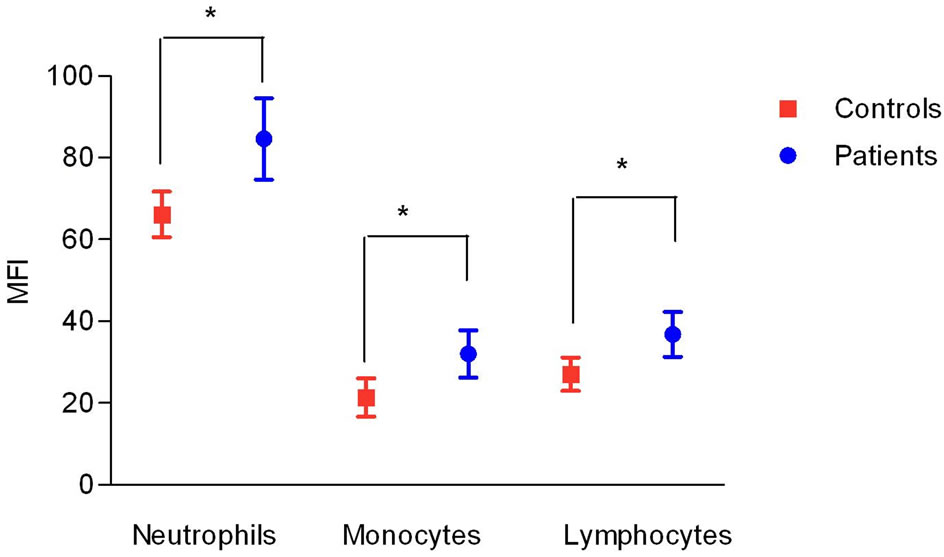
Figure 1. Expression of CD59 in peripheral blood cells in RA patients (patients) and healthy control subjects (controls). Data shown as mean and stard deviation. (*) = p < 0.05 t test.
nulocytes compared with peripheral blood granulocytes. Indeed, McCarthy et al. showed that when granulocytes are activated in vitro CD35 expression is increased. Concerning monocytes, Gadd et al. [10] found CD35 to be upmodulated in cells from synovium and blood. Also, Torsteinsdóttir et al. [9] reported that the expression of CD35 in RA monocytes was elevated, in accordance to our data, although we did not found a significant difference. On the other hand, Jones et al. [7], observed a significant reduction in the expression of CD35, CD55 and CD46 on peripheral blood granulocytes in RA patients. It has been discussed that the difference in the expression of these molecules leads to increased adhesiveness, resistance to complement and an increased capacity of neutrophils to remove immune complexes. We did not observe any difference in the proportion and MFI for CD55, CD46 and CD35 in peripheral blood granulocytes.
Moreover, we found that the absolute value of monocytes with positive CD59 was significantly higher than controls (data not shown). This increase of expression of
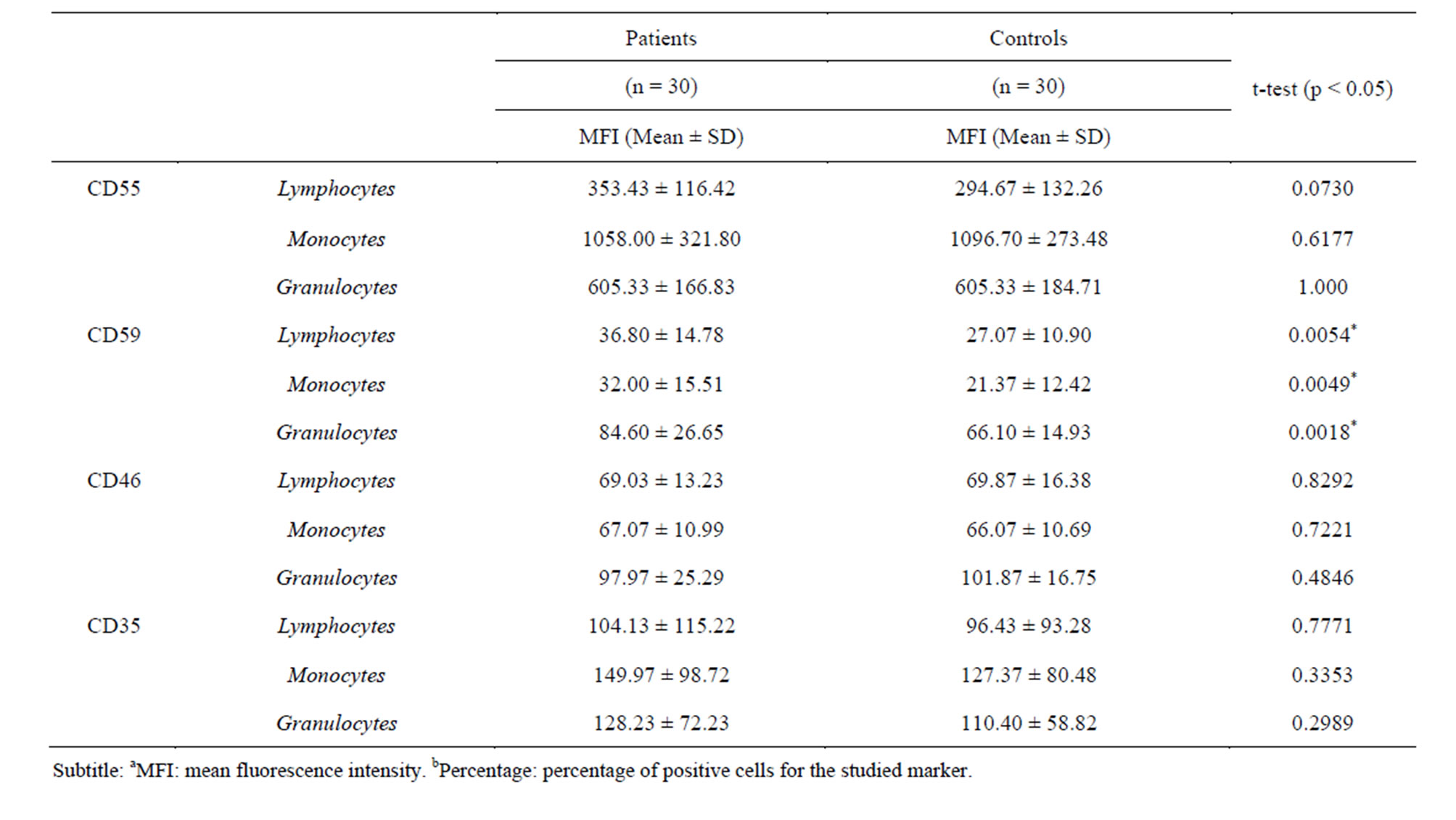
Table 2. Expression of the CReg in the patients and controls.
this regulatory protein could be due to the monocytosis present in RA patients (p = 0.02, data not shown) and we do not find any other report in the literature. The reason by which these CReg are higher expressed in monocytes from RA patients, and the possible correlation with monocytosis need to be further explored.
About CD46 we did not observe any statistically significant difference in CD46 expression in granulocytes, monocytes and lymphocytes from peripheral blood of patients with RA compared with healthy controls. By contrast, Jones et al. [7] found a statistically significant decrease in the expression of CD46 on granulocytes from peripheral blood. We did not find another study for this protein in peripheral blood leukocytes in RA to elucidate these opposite findings. Recent work has suggested that CD46 can play a role in modifying adaptative immunity, suppressing T-regulatory cells and as a scavenge of damaged cells [14].
The mechanisms involved in the upregulation of CD59 expression are still unclear. CD59, a 18 kDa glycoprotein anchored to the cell membrane by glycosylphosphatidylinositol (GPI) linkage, is constitutively expressed on haematopoietic and resident tissue cells throughout the human body. The main function of CD59 derives from its capacity to interact with the C8 and C9 components of the cytolytic MAC to block the conversion of C9 from a hydrophilic to an amphipathic molecule, thus preventing insertion of C9 into the membrane [15]. It is suggested that CD59 may be involved in several immunological phenomena, such as: interaction with proteins of lymphocytes, monocytes and granulocytes, necessary for activation of these cells [16,17]; exert synergic function in T cell adhesive interactions [18]; play a role in cell interaction between monocytes and T cells [19]; and may suppress the production of pathogenic antibodies [20].
In summary, we believe that our findings may help explain the mechanisms of CRegs expression modified in RA patient. And also we did not found any other report at the literature showing CD59 increase in all peripheral blood cells in RA. Several explanations can be proposed to explain to the CD59 increased expression in leukocytes in RA: 1) increased synthesis for compensatory mechanisms because of complement activation; 2) leukocytes activation and/or 3) disease activity. Whether these changes are contributory to the disease or merely a consequence of the chronic inflammatory state remains unclear. However, the data suggest that CReg might be working systemically to suppress the disease activity associated with uncontrolled complement activation in RA.
REFERENCES
- C. A. Smith and F. C. Arnett, “Epidemiologic Aspects of Rheumatoid Arthritis. Current Immunogenetic Approach,” Clinical Orthopaedics and Related Research, Vol. 265, 1991, pp. 23-35.
- M. Okroj, D. Heinegard, R. Holmdahl and A. M. Blom, “Rheumatoid Arthritis and the Complement System,” Annals of Medicine, Vol. 39, No. 7, 2007, pp. 517-530. http://dx.doi.org/10.1080/07853890701477546
- J. J. Goronzy and C. M. Weyand, “Rheumatoid Arthritis,” Immunological Reviews, Vol. 204, 2005, pp. 55-73. http://dx.doi.org/10.1111/j.0105-2896.2005.00245.x
- P. F. Zipfel and C. Skerka, “Complement Regulators and Inhibitory Proteins,” Nature Reviews Immunology, Vol. 9, No. 10, 2009, pp. 729-740.
- E. Wagner and M. M. Frank, “Therapeutic Potential of Complement Modulation,” Nature Reviews, Vol. 9, No. 1, 2010, pp. 43-56.
- A. D. Crockard, J. M. Thompson, S. J. Mcbride, J. D. Edgar, T. A. Mcneill and A. L. Bell, “Markers of Inflammatory Activation: Upregulation of Complement Receptors Cr1 and Cr3 on Synovial Fluid Neutrophils from Patients with Inflammatory Joint Disease,” Clinical Immunology and Immunopathology, Vol. 65, No. 2, 1992, pp. 135-142. http://dx.doi.org/10.1016/0090-1229(92)90216-B
- J. Jones, I. Laffafian, A. M. Cooper, B. D. Williams and B. P. Morgan, “Expression of Complement Regulatory Molecules and Other Surface Markers on Neutrophils from Synovial Fluid and Blood of Patients with Rheumatoid Arthritis,” British Journal of Rheumatology, Vol. 33, No. 8, 1994, pp. 707-712. http://dx.doi.org/10.1093/rheumatology/33.8.707
- D. Mccarthy, M. J. Taylor, J. Bernhagen, J. D. Perry and A. S. Hamblin, “Leucocyte Integrin and Cr1 Expression on Peripheral Blood Leucocytes of Patients with Rheumatoid Arthritis,” Annals of the Rheumatic Diseases, Vol. 51, No. 3, 1992, pp. 307-312. http://dx.doi.org/10.1136/ard.51.3.307
- I. Torsteinsdottir, N. G. Arvidson, R. Hallgren and L. Hakansson, “Monocyte Activation in Rheumatoid Arthritis (Ra): Increased Integrin, Fc Gamma and Complement Receptor Expression and the Effect of Glucocorticoids,” Clinical and Experimental Immunology, Vol. 115, 1999, pp. 554-560. http://dx.doi.org/10.1046/j.1365-2249.1999.00817.x
- S. J. Gadd, T. Felzmann, O. Majdic, D. Maurer, P. Petera, W. J. Chen and A. L. Et, “Phenotypic Analysis of Functionally Associated Molecules on Peripheral Blood and Synovial Fluid Monocytes from Arthritis Patients,” Rheumatology International, Vol. 12, No. 4, 1992, pp. 153-157. http://dx.doi.org/10.1007/BF00274935
- F. C. Arnett, S. M. Edworthy, D. A. Bloch, D. J. Mcshane, J. F. Fries, N. S. Cooper, et al., “The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 31, No. 3, 1988, pp. 315-324. http://dx.doi.org/10.1002/art.1780310302
- A. P. Alegretti, T. Mucenic, J. Merzoni, G. A. Faulhaber, L. M. Silla and R. M. Xavier, “Expression of CD55 and CD59 on Peripheral Blood Cells from Systemic Lupus Erythematosus (Sle) Patients,” Cellular Immunology, Vol. 265, No. 2, 2010, pp. 127-132. http://dx.doi.org/10.1016/j.cellimm.2010.07.013
- Y. T. Konttinen, A. Ceponis, S. Meri, A. Vuorikoski, P. Kortekangas, T. Sorsa, et al., “Complement in Acute and Chronic Arthritides: Assessment of C3c, C9, and Protectin (Cd59) in Synovial Membrane,” Annals of the Rheumatic Diseases, Vol. 55, No. 12, 1996, pp. 888-894. http://dx.doi.org/10.1136/ard.55.12.888
- C. Kemper, J. W. Verbsky, J. D. Price and J. P. Atkinson, “T-Cell Stimulation and Regulation: With Complements from Cd46,” Immunologic Research, Vol. 32, No. 1-3, 2005, pp. 31-43. http://dx.doi.org/10.1385/IR:32:1-3:031
- J. Petranka, J. Zhao, J. Norris, N. B. Tweedy, R. E. Ware, P. J. Sims, et al., “Structure-Function Relationships of the Complement Regulatory Protein, Cd59,” Blood Cells, Molecules & Diseases, Vol. 22, No. 3, 1996, pp. 281-296. http://dx.doi.org/10.1006/bcmd.1996.0111
- F. Lund-Johansen, J. Olweus, F. W. Symington, A. Arli, J. S. Thompson, R. Vilella, et al., “Activation of Human Monocytes and Granulocytes by Monoclonal Antibodies to Glycosylphosphatidylinositol-Anchored Antigens,” European Journal of Immunology, Vol. 23, No. 11, 1993, pp. 2782-2791. http://dx.doi.org/10.1002/eji.1830231110
- G. T. Venneker and S. S. Asghar, “Cd59: A Molecule Involved in Antigen Presentation as Well as Downregulation of Membrane Attack Complex,” Experimental and Clinical Immunogenetics, Vol. 9, No. 1, 1992, pp. 33-47.
- M. Deckert, J. Kubar and A. Bernard, “Cd58 and Cd59 Molecules Exhibit Potentializing Effects in T Cell Adhesion and Activation,” Journal of Immunology, Vol. 148, No. 3, 1992, pp. 672-677.
- P. E. Korty, C. Brando and E. M. Shevach, “Cd59 Functions as a Signal-Transducing Molecule for Human T Cell Activation,” Journal of Immunology, Vol. 146, No. 12, 1991, pp. 4092-4098.
- M. Mizuno, “A Review of Current Knowledge of the Complement System and the Therapeutic Opportunities in Inflammatory Arthritis,” Current Medicinal Chemistry, Vol. 13, No. 14, 2006, pp. 1707-1717. http://dx.doi.org/10.2174/092986706777441959
NOTES
*Corresponding author.

