Open Journal of Anesthesiology
Vol.3 No.2(2013), Article ID:29615,5 pages DOI:10.4236/ojanes.2013.32026
Effects of Intraoperative Administration of Dexmedetomidine on the Percentage of T-Lymphocyte Subsets and Natural Killer Cells in Patients with Colorectal Cancer*
![]()
1Department of Anesthesiology, Xuzhou Medical College, Xuzhou, China; 2Department of Anesthesiology, Liaocheng People’s Hospital and Liaocheng Clinical School of Taishan Medical University, Liaocheng, China.
Email: #zwzhang68@sina.com
Received January 25th, 2013; revised February 28th, 2013; accepted March 12th, 2013
Keywords: Dexmedetomidine; T-Lymphocyte Subsets; Natural Killer Cell; Colorectal Cancer Surgery
ABSTRACT
Study Objective: To observe the effect of dexmedetomidine (DEX) on T-lymphocyte subsets and natural killer (NK) cells in the peripheral blood of perioperative patients with colorectal cancer. Design: A random double-blind control clinical study. Setting: A university hospital. Patients: Forty patients with colorectal cancer, ASA I-П. Interventions: All patients were randomly divided into a DEX group (n = 20) and a control group (n = 20). Before induction of anesthesia, epidural catheters were placed in the L1-L2 or T12-L1 intervertebral spaces. The DEX group received 1 μg/kg of DEX (200 μg/50 ml) intravenously for 15 min prior to the surgery, which was then infused at a rate of 0.5 μg/kg/h until 30 min before the end of the surgery. The control group received an intravenous infusion of saline (50 ml) instead of DEX during the same periods as the DEX group. All patients received routine anesthesia and postoperative analgesia. Measurements: Blood samples from all patients were collected at the following time points: before anesthesia (T0), 24 h after surgery (T1), 48 h after surgery (T2) and 72 h after surgery (T3). Changes in T-lymphocyte subsets (CD3+, CD4+, CD8+, CD4+/CD8+) and NK cells were determined by flow cytometry. Main Results: Compared with the control group, the percentages of CD3+ and CD4+ cells and the CD4+/CD8+ ratio in the DEX group increased significantly from T1 to T3 (p < 0.05), while the percentage of CD8+ cells decreased significantly at T1 and T2 (p < 0.05). Meanwhile, the concentration of NK cells in the control group decreased significantly from T1 to T3 (p < 0.05), while it increased significantly in the DEX group from T1 to T3 (p < 0.05). Conclusions: Intraoperative use of DEX can reduce perioperative immunosuppression and favor the immune function of patients with colorectal cancer.
1. Introduction
Surgical resection is common treatment for colorectal cancer, especially in developing countries. However, the response to surgical stress plays a paradoxical role in cancer treatment. On one hand, it augments the healing process by initiating clotting, tissue healing and favorable inflammation. On the other hand, the response may translate to high catabolism, insulin resistance, overactivity of host defense and even organ dysfunction. In addition, immunosuppression caused by surgical stress may also accelerate the proliferation or metastasis of tumor cells after surgery and hinder the recovery of cancer patients. The immunosuppression presents as lymphopenia, reduced secretion of cytokines and inhibited cytotoxicity of lymphocytes and natural killer (NK) cells. NK cells are of great significance for tumor rejection, and it is known that surgery, pain, anxiety and other factors can inhibit the activity of NK cells. It has been observed that NK cell activity can be inhibited for up to 7 d after surgery [1].
Studies have shown that NK cell activity can be inhibited by morphine, ketamine, thiopental, isoflurane and sevoflurane in a dose-dependent manner [2]. Dexmedetomidine is a strong potent and highly selective α2-adrenergic receptor agonist. Clinical practices demonstrate that it has sedative as well as analgesia effects and dosages dependence. It can be used alone or combined with midazolam, propofol or opioids resulting in decreasing the needs of other sedatives and analgesic drugs. Dexmedetomidine has been used in many clinical fields, such as anxiliary examination, various surgeries, ICU etc., with good sedative and analgesic effect. It has no incompatibility with other anesthesia, analgesic and sedative drugs. Perioperative use of DEX reduces the dose of postoperative consumption of patients controlled epidural analgesia (PCEA) bolus (local anesthetics) [3]. In vitro studies have shown that propofol can competitively inhibit the binding of lymphocyte function-associated antigen 1 and intercellular adhesion molecule-1 on the surface of T cells and therefore inhibit interleukin IL-2 secretion and T-cell proliferation, while DEX and midazolam do not [4]. Thus, we hypothesized that intraoperative DEX would relieve the immunosuppressive effects caused by surgical stress and benefit the rehabilitation of cancer patients. The main purpose of this study was to investigate the effects of DEX on T-lymphocyte subsets and NK cell proportions in perioperative patients undergoing colorectal surgery, which may improve how perioperative patients are currently managed.
2. Materials and Methods
2.1. Inclusion Criteria
The procedures were approved by Medical Ethics Committee of Liaocheng People’s Hospital, and written informed consent was obtained from all patients and their families. Forty patients with colorectal cancer admitted into our hospital from May 1, 2011, to August 31, 2011, were involved in this study. Inclusion criteria were general anesthesia for resection of colorectal cancer; expected operation duration of more than 2 h; aged 18 to 64 years; body mass index <30 kg/m2; ASA level I to П; and no blood transfusion during surgery or within 1 week postoperative. Patients with liver and/or kidney dysfunction, cardiovascular diseases, or any contraindication to spinal anesthesia and patients treated with immune system regulators preoperatively were excluded. The patients were randomly divided into a control group and a dexmedetomidine treatment group (DEX group), 20 cases for each group. All treatments followed the double-blind principle.
2.2. Surgical Procedure
None of the patients received preoperative medication. Before induction of anesthesia, the epidural catheter was placed into the L1-L2 or T12-L1 intervertebral space through a midline puncture, and arterial blood pressure was monitored by radial arterial puncture. For the DEX group, dexmedetomidine (1 μg/kg) (Jiangsu Hengrui Medicine, China; batch number: 10061534) (200 μg/50 ml) was injected intravenously 15 min before the surgery and then infused at a rate of 0.5 μg/kg/h until 30 min before the end of surgery. For the control group, an equal volume (50 ml) of saline was injected intravenously 15 min before surgery, which was then infused until 30 min before the end of the surgery. All infusion pumps were prepared by nurses and blind to anesthetists. Then for all patients fentanyl (1.5 - 2.0 μg/kg), propofol (1 - 2 mg/kg) and vecuronium (0.1 mg/kg) were infused intravenously. The patients were made to hyperventilate for 3 min to facilitate endotracheal intubation. The patients’ lungs were ventilated with a Primus Dräger Ventilator (Dräger, Lübeck, Germany) to maintain PaCO2 between 35 and 40 mmHg. The depth of anesthesia was monitored and maintained at a bispectral index value of 40 to 50 by adjusting the inhalation of sevoflurane concentration. Thirty minutes before skin incision, a ropivacaine-sufentanil solution (0.2% ropivacaine, sufentanil 1 μg/ml) was given through the epidural catheter, initially with a single 8-ml bolus and then as a continuous infusion at a rate of 6 ml/h. If there were signs of incomplete analgesia, the patient was to receive a second loading dose of 6 ml of the ropivacaine—sufentanil solution. If sustained signs of incomplete analgesia were observed, fentanyl was to be injected to ensure intraoperative analgesia and the patient would be excluded from our study.
Postoperative epidural analgesics of patients were delivered by a Hospira Gemstar infusion pump (Hospira, Inc., Lake Forest, IL). The basal infusion rate was set at 6 ml/h, with a bolus of 4 ml and limited doses at 15 ml/h, administered by the acute pain service (APS) team. The dose and infusion rate were adjusted based on a visual analogue scale by the APS team. Once the epidural analgesia was no longer required, the epidural catheter was removed. If the epidural catheter seemed to fall off or became displaced, the epidural catheter was to be removed. The patient would be given intravenous analgesia instead and excluded from our study.
The age, body weight, height, operation duration and sex of each patient were recorded. Peripheral blood was sampled at the following time points: before administration of dexmedetomidine (or placebo) (T0), 24 h after surgery (T1), 48 h after surgery (T2) and 72 h after surgery (T3). The proportions of T-lymphocyte subsets (CD3+, CD4+, CD8+ and CD4+/CD8+) and NK cells (CD56) in peripheral blood were determined using FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). The results of flow cytometry were analyzed with Cellquest software (Becton Dickinson, San Jose, CA).
2.3. Statistical Analysis
Measurement data are presented as means ± standard deviation. Values of the same group at different time points were compared according to single-factor analysis of variance, and different groups were compared using independent samples t-test. A p value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, USA).
3. Results
3.1. Patient Characteristics
There were no statistically significant differences between the two groups of patients with regard to age, weight, height, sex ratio and operation duration (Table 1).
3.2. Comparison of T-Lymphocyte Subsets
Before anesthesia, the percentages of CD3+ and CD4+ cells and the CD4+/CD8+ ratio in the control and DEX groups showed no significant differences. Compared with those at T0, the percentages of CD3+ and CD4+ cells and the CD4+/CD8+ ratio at T1 through T3 in the control group were significantly decreased (p < 0.05), while the percentages of CD3+ and CD4+ cells and the CD4+/CD8+ ratio of the DEX group were significantly decreased at T1 (p < 0.05), the percentage of CD4+ cells decreased significantly at both T1 and T2 (p < 0.05). Compared with the control group, the percentages of CD3+ and CD4+ cells and the CD4+/CD8+ ratio of the DEX group increased significantly at T1 to T3 (p < 0.05), while CD8+ cells decreased at T1 and T2 (p < 0.05) (Figures 1-4).
3.3. Comparison of NK Cell Percentages in Peripheral Blood
There was no significant difference in the NK cell percentages between the control and DEX groups before anesthesia. Compared with T0, the NK cell percentage decreased at T1 to T3 in the control group (p < 0.05) and decreased significantly at T1 and T2 in the DEX group (p < 0.05). Overall, the NK cell percentage in the DEX group was significantly higher than that of the control group from T1 to T3 (p < 0.05) (Figure 5).
4. Discussion
Colorectal cancer is the most common gastrointestinal malignancy with increasing incidence and mortality rates year after year [5]. It is known that immunosuppression contributes to tumorigenesis and progression of cancers [6]. The mechanism of anti-tumor immunity with regard to solid tumors relies on cellular immunity [7], which is divided into two categories: specific cellular immunity and nonspecific cellular immunity. The former is mainly mediated by T lymphocytes, while the latter primarily involves NK cells and occasionally monocytes and macrophages. T lymphocytes and NK cells make up thefirst defense barrier against tumorigenesis in the body. The balance among T-cell subsets is also pivotal for homeostasis of the immune system. Among the T-cell subsets, CD4+ T cells are predefined as helper T cells, which support anti-tumor immunity, while CD8+ T cells are suppressor T cells, which inhibit the immune response. A dynamically balanced CD4+/CD8+ ratio represents the stability of cellular immune functions. A reduced CD4+/ CD8+ ratio is indicative of immune suppression, which may contribute to tumor proliferation. The amount of CD3 T cells reflects the total number of T cells, and an increase in CD3+ T cells driven by CD4+ T cells indicates stronger immunity. NK cells also play an important role in antitumor immunity because they do not require activation to kill cells that are deficient in MHC class I [8]. Thus, the proportions of T-cell subsets and the amount of NK cells can reflect the status of cellular immunity in the body. Our study found that after surgery, cellular immune function was inhibited in patients with colorectal cancer. Compared with the control group, the group that received intra-operative DEX treatment had significantly improved proportions of NK cells and T lymphocytes. This suggests that the cellular immune function of DEXtreated patients was protected, which would benefit a patient’s rehabilitation.
DEX is a novel, highly specific α2-adrenergic receptor agonist, the α2-/α1-adrenoceptor selectivity ratio of which is 1620:1. The potency of DEX is much greater than that of clonidine in the nervous system. DEX appeared on the US market in March 2000 and has been mainly used for sedation and analgesia of ICU patients. It has also been reported to be used for neurosurgical craniotomy, anesthesia, and postoperative analgesic [9]. It was observed that high doses (1 µg/kg/h) of DEX during gynecological operations reduced the demand for epidural analgesia drugs at 24 h after surgery, improved the pain scoring and relieved postoperative immunosuppression [3]. Wu et al. got similar results in a study of patient-controlled intravenous analgesia after total hip replacement surgery in elderly patients [10]. As far as the impact of DEX on
Table 1. Patient characteristics.
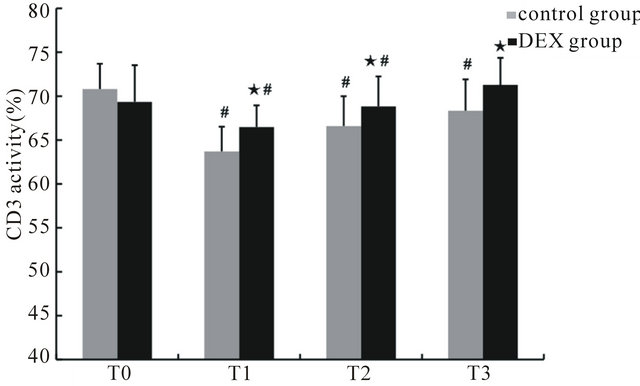
Figure 1. The percentage of CD3+ cells in peripheral blood at different time points. ★Compared to control group, p < 0.05; #compared to T0, p < 0.05.
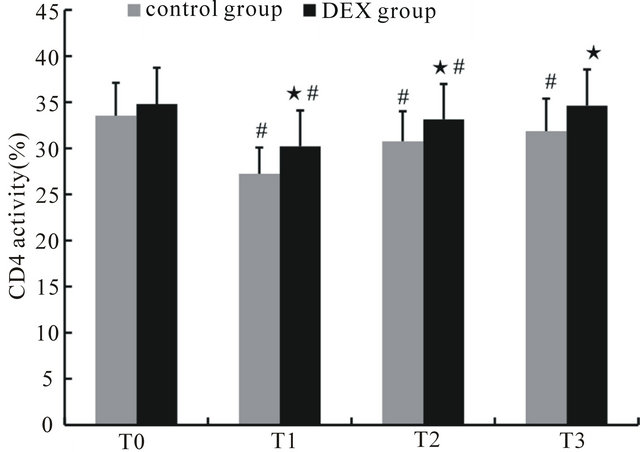
Figure 2. The percentage of CD4+ cells in peripheral blood at different time points. ★Compared to control group, p < 0.05; #compared to T0, p < 0.05.

Figure 3. The percentage of CD8+ cells in peripheral blood at different time points. ★Compared to control group, p < 0.05.
the immune system is concerned, however, it has only been demonstrated in animal experiments that DEX does
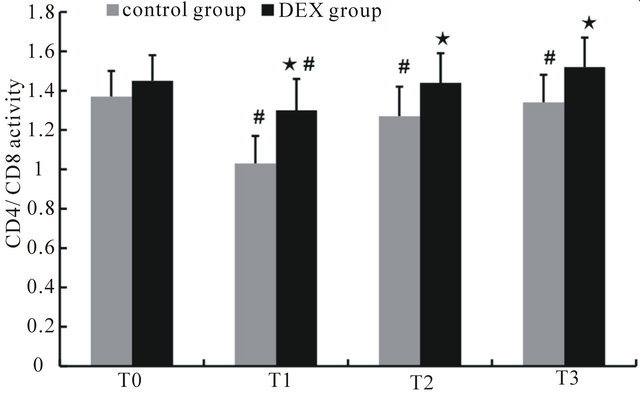
Figure 4. The changes in the CD4+/CD8+ ratio at different time points. ★Compared to control group, p < 0.05; #compared to T0, p < 0.05.
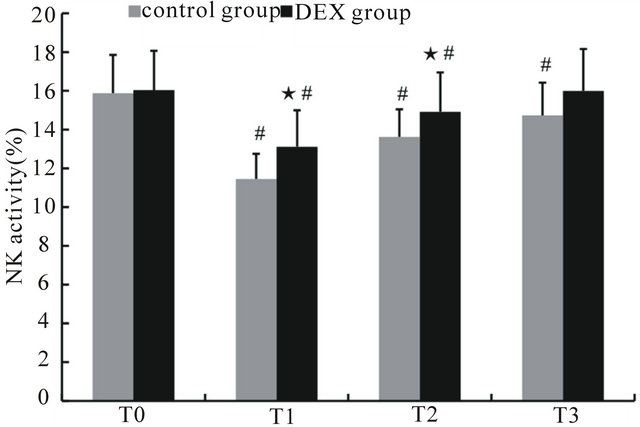
Figure 5. The percentage of NK cells in peripheral blood at different time points. ★Compared to control group, p < 0.05; #compared to T0, p < 0.05.
not affect neutrophil chemotaxis, phagocytosis or the production of superoxide anion, and it can maintain the activity of NK cells the after surgery or general anesthesia; furthermore, DEX limited endotoxin-induced inflammatory response, reduced regional accumulation of tumor necrosis factor, IL-6 and neutrophils and thereby reduced the incidence of hypotension [11]. Our study confirmed the protective role of DEX in the immune system of patients with colorectal cancer who underwent general anesthesia and surgery, consistent with the prior research. We speculate that the immune protective mechanism of DEX is probably related to reductions of the anesthetic dose and the anesthesiaand surgery-induced sympathetic reflex. This requires further study to be confirmed.
In conclusion, intra-operative use of DEX can reduce peri-operative immuno-suppression and favor the immune function of patients with colorectal cancer.
5. Acknowledgements
This work is supported by department of science and technology of Liaocheng city grant. We appreciate the valuable comments from other members of our anesthesiology department and central laboratories.
6. Author Contribution
TH Zhao carried out the data analysis, wrote the manuscript, and interpreted the data. Z Liu, AL Yu and TH Zhao carried out the immunoassays. ZW Zhang participated in the sequence alignment, conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
REFERENCES
- A. Espi, J. Arenas, E. Garcia-Granero, E. Marti and S. Lledo, “Relationship of Curative Surgery on Natural Killer Cell Activity in Colorectal Cancer,” Diseases of the Colon & Rectum, Vol. 39, No. 4, 1996, pp. 429-434. doi:10.1007/BF02054059
- B. Welden, G. Gates, R. Mallari and N. Garrett, “Effects of Anesthetics and Analgesics on Natural Killer Cell Activity,” AANA Journal, Vol. 77, No. 4, 2009, pp. 287-292.
- N. Ohtani, Y. Yasui, D. Watanabe, M. Kitamura, K. Shoji and E. Masaki, “Perioperative Infusion of Dexmedetomidine at a High Dose Reduces Postoperative Analgesic Requirements: A Randomized Control Trial,” Journal of Anesthesia, Vol. 25, No. 6, 2011, pp. 872-878. doi:10.1007/s00540-011-1239-8
- K. Yuki, S. G. Soriano and M. Shimaoka, “Sedative Drug Modulates T-Cell and Lymphocyte Function-Associated Antigen-1 Function,” Anesthesia & Analgesia, Vol. 112, No. 4, 2011, pp. 830-838. doi:10.1213/ANE.0b013e31820dcabb
- D. M. Parkin, F. Bray, J. Ferlay and P. Pisani, “Global Cancer Statistics,” CA: A Cancer Journal for Clinicians, Vol. 55, No. 2, 2005, pp. 74-108. doi:10.3322/canjclin.55.2.74
- A. M. Wolf, D. Wolf, M. Steurer, G. Gastl, E. Gunsilius and B. Grubeck-Loebenstein, “Increase of Regulatory T Cells in the Peripheral Blood of Cancer Patients,” Clinical Cancer Research, Vol. 9, No. 2, 2003, pp. 606-612.
- E. Barbera-Guillem, M. B. Nelson, B. Barr, et al., “Lymphocyte Pathology in Human Colorectal Cancer. Experimental and Clinical Therapeutic Effects of Partial B Cell Depletion,” Cancer Immunology, Immunotherapy, Vol. 48, No. 10, 2000, pp. 541-549. doi:10.1007/PL00006672
- L. Pilla, P. Squarcina, J. Coppa, et al., “Natural Killer and NK-Like T-Cell Activation in Colorectal Carcinoma Patients Treated with Autologous Tumor-Derived Heat Shock Protein,” Cancer Research, Vol. 65, No. 9, 2005, pp. 3942-3949. doi:10.1158/0008-5472.CAN-04-3493
- I. Rozet, “Anesthesia for Functional Neurosurgery: The Role of Dexmedetomidine,” Current Opinion in Anaesthesiology, Vol. 21, No. 5, 2008, pp. 537-543. doi:10.1097/ACO.0b013e32830edafd
- Z. L. Wu, Z. F. Zhou, L. X. Xu and S. Z. She, “Effect of Dexmedetomidine on Patient-Controlled Intravenous Analgesia with Fentanyl in Elderly Patients after Total Hip Replacement,” Nan Fang Yi Ke Da Xue Xue Bao, Vol. 31, No. 4, 2011, pp. 701-704.
- T. Taniguchi, Y. Kidani, H. Kanakura, Y. Takemoto and K. Yamamoto, “Effects of Dexmedetomidine on Mortality Rate and Inflammatory Responses to Endotoxin-Induced Shock in Rats,” Critical Care Medicine, Vol. 32, No. 6, 2004, pp. 1322-1326. doi:10.1097/01.CCM.0000128579.84228.2A
NOTES
*Conflict of interest: The authors declare no conflict of interest.
#Corresponding author.

