Advances in Materials Physics and Chemistry
Vol.2 No.4(2012), Article ID:26027,7 pages DOI:10.4236/ampc.2012.24040
The Synthesis of Gold Nanoparticles Using Amaranthus spinosus Leaf Extract and Study of Their Optical Properties*
1Biotech Hub, Centre for the Environment, Indian Institute of Technology Guwahati, Guwahati, India
2Bioengineering Research Laboratory, Department of Biotechnology, Indian Institute of Technology Guwahati, Guwahati, India
3Department of Civil Engineering, Indian Institute of Technology Guwahati, Guwahati, India
Email: #ubora@iitg.ernet.in, #ubora@rediffmail.com
Received September 22, 2012; revised October 28, 2012; accepted November 8, 2012
Keywords: Gold Nanoparticles; Leaf Extract; Anti-Oxidant; Amaranthus spinosus; Crystal Growth
ABSTRACT
We report here the application of a medicinally important plant Amaranthus spinosus for the synthesis of gold nanoparticles (AuNPs). Different concentrations of ethanolic leaf extract of the plant were reacted with aqueous solution of HAuCl4·4H2O under mild reaction conditions. Synthesis of AuNPs was confirmed from the UV-Vis study of surface plasmon resonance property of the colloidal solution. Transmission electron microscopy (TEM) revealed particles as spherical and triangular in shape. X-ray diffraction (XRD) confirmed the crystalline nature of AuNPs with average size of 10.74 nm as determined by Debye-Scherrer’s Equation. Fourier transform infra-red (FT-IR) analysis of leaf extract and lyophilized AuNPs showed the presence of various functional groups present in diverse phytochemicals. Energy dispersive X-ray (EDX) of purified AuNPs confirmed the formation of AuNPs and surface adsorption of biomolecules. We further investigated the toxicity of the synthesized AuNPs and found non toxic to the cancer cell lines and could be used for biomedical applications.
1. Introduction
In recent years, gold nanoparticles (AuNPs) have become prominent for its diverse applications [1-5]. The initial step in the synthesis of AuNPs involves reducetion of gold ions (Au1+ or Au3+) to neutral atoms (Au0) with a strong reducing agent. Synthetic reducing agents are not favoured for synthesizing AuNPs for biomedical applications as traces of such chemicals left unreacted in the process can be harmful. Thus, alternative methodologies to replace synthetic chemical reducing agents for AuNPs preparation have recently been explored. Many plants and microbes have been found to be excellent sources of natural reducing agents [6-13]. Phytochemicals like phenolic compounds, terpenoids, alkaloids etc. have been found to be suitable reducing agents with high efficiency [14-15]. In the present study, we selected the plant Amaranthus spinosus Linn. (Family: Amaranthaceae) known as “Khutura” or “Kanatabhajii” in the Indian traditional system of medicine [16].
The species is well known for its many remedial properties [17-27]. Reports on the strong antioxidant nature of the ethanolic leaf extract highly encouraged us to carry out the present research work and evaluate the plant for metallic nanoparticle synthesis [28].
2. Experimental
2.1. Materials
Healthy and fresh leaves of A. spinosus were collected from a local farm. Cell culture-related plasticware were obtained from Sigma-Aldrich (Bangalore, India). Chlorauric acid (HAuCl4) was purchased from Sigma (Bangalore, India). 3,4,5-Dimethylthiazol-2-yl-2-5-diphenyltetrazoliumbro-mide (MTT) was purchased from Hi Media (Bangalore, India). Cell lines were obtained from the National Centre for Cell Sciences (Pune, India). Other chemicals used were of analytical grade and obtained from Merck (Mumbai, India).
2.2. Synthesis of AuNPs
The collected leaves were washed with double distilled water and shadow dried before being grinded to fine powder and sieved to remove coarse particles. One gram leaf powder was mixed with 100 ml of ethanol and the mixture was left in a shaking incubator operating at 200 rpm, 25˚C for 24 h. The extract was then filtered and the filtrate was used for AuNPs synthesis. Various concentrations (1% - 5%, v/v) of the ethanolic leaf extract of A. spinosus were mixed with aqueous solution of HAuCl4 (1 mM) and the reaction volume was made upto 2 ml with distilled water. The mixture solution was left on constant magnetic stirring at room temperature (25˚C) and observed for change in colour.
2.3. Characterization of AuNPs
UV-visible spectroscopic measurements were carried out on a Cary 100 (BIO UV-Vis spectrophotometer, Varian, CA, USA). Few drops of AuNPs solution were placed over a copper grid and allowed to dry in hot air oven at 50˚C for 4 hrs. Morphological details of the AuNPs were analyzed by TEM (JEOL 2100 UHR-TEM) operating at an accelerating voltage of 200 kV. 10 ml of AuNPs solution was freeze dried in a lyophiliser (Christ Gefriertrocknungsanlagen GmbH Model 1-4) for 16 hours and obtained powder was used for FT-IR analysis. The AuNPs solution was placed on microscope glass slide and allowed to dry in hot air oven at 50˚C and the process was repeated until it forms a layer on the glass slide. The dried sample was analysed with the help of an XRD instrument (Bruker Advance D8 XRD machine).
2.4. Cytotoxicity Studies
To examine the cytotoxicity effect of AuNPs on cancer cells, monocultures of the HeLa and MCF-7 cell lines were incubated with increasing concentrations of filter (0.2 micron) sterilized AuNPs for 24 hrs and the cell viability was estimated by MTT dye conversion assay. Cells were seeded and maintained (1 × 104) in a 96-well plate (Cell Bind, Corning) using the minimal essential medium with serum. After 24 h of growth, the medium was replaced with the serum free medium that contained varied concentrations of AuNPs (10 - 100 µM). The medium was removed after 24 hrs of treatment and cells were washed with phosphate-buffered saline (PBS, 0.01 M, pH-7.2). This was followed by addition of 100 μl of MTT (0.5 mg/ml) prepared in serum free medium to each well and incubated for 4 h at 37˚C. After incubation the medium was removed and 100 μl of dimethyl sulphoxide (DMSO) was added to each well to solubilise the formazan crystals and the concentration of formazan was determined by measuring its absorbance at 70 nm using a multiwell plate reader (Tecon micro plate reader, model 680, CA, USA). The cell viability was calculated with the following equation:
Viability (%) = Nt/Nc × 100 where Nt, Nc are mean absorbance of AuNPs treated and control cells respectively, (n = 5; where n is the no. of independent experiments).
3. Results and Discussion
3.1. Effect of Amaranthus spinosus Leaf Extract on AuNPs synthesis
We observed that the yellow colour of the reaction mixture (HAuCl4 and ethanolic leaf extract) kept at room temperature (25˚C) under constant stirring gradually turned into ruby-red after 4 h. Scanning of the coloured solution in the Ultraviolet-Visible spectroscopy (400 - 800 nm) range showed absorption bands with sharp peaks (535 - 565 nm) (Figure 1).
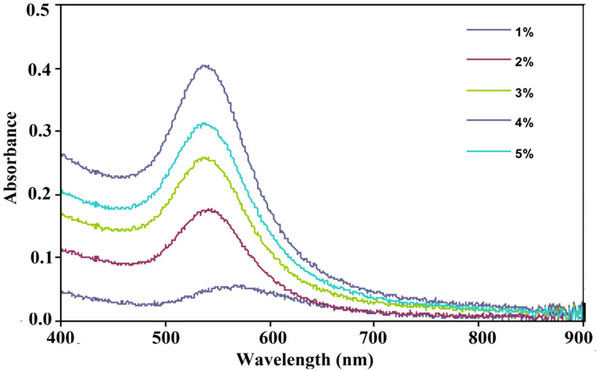
Figure 1. UV–Vis absorption spectra of AuNPs synthesized by treating 1 mM HAuCl4·4H2O solution with different concentrations (1% - 5%, v/v) of the ethanolic leaf extract of A. spinosus at 25˚C under magnetic stirring.
The absorption bands originated from the surface optical property of gold exhibited only at nano dimension, known as surface plasmon resonance (SPR). Appearance of SPR bands confirmed the formation of AuNPs. At 1% plant extract concentration, SPR peak was centered at around 565 nm. At higher concentrations (2% - 5%), a blue shift in the peaks was observed and peak maxima were located at around 535 ± 3 nm. As position of SPR peaks is correlated with particles size, it is evident that for 1 mM of HAuCl4·4H2O solution, more than 1% of plant extract is required to synthesize AuNPs of suitable size (<50 nm) for bio-medical applications. With higher plant extract concentrations the absorbance intensities of SPR band gradually increased indicating the formation of more AuNPs.
3.2. Monographs of TEM Confirmed the Formation of AuNPs
AuNPs synthesized with 5% (v/v) A. spinosus leaf extract against 1 mM aqueous solution of HAuCl4, mostly appeared to be spherical in shape with few triangular morphologies (Figure 2(A)). Selected area electron diffraction pattern (SAED) of a single spherical particle confirmed the crystalline nature of AuNPs. We observed rings corresponding to (111), (200) and (220) planes of fcc crystalline lattices of gold (Figure 2(B)). The ultra high resolution TEM (UHRTEM) images displayed clear lattice fringes on the particle surfaces (Figure 2(C)).
3.3. XRD Analysis Revealed the Crystalline Nature of AuNPs
XRD analysis of the AuNPs exhibited Bragg’s reflections, which was indexed on the basis of the facecentered cubic (fcc) gold structure.The diffraction peaks (111), (200) and (220) corresponding to 38.1˚, 44.5˚ and 64.8˚ 2θ angles, respectively, confirmed that the synthesized AuNPs were of crystalline nature.
A strong diffraction peak located at 38.15˚ was ascribed to the (111) facets of face-centered cubic metal gold structures, while diffraction peaks of other facets were much weaker (Figure 3). The broadening of Bragg’s peaks provided additional indication of the formation of AuNPs. The width of the (111) Bragg reflection, was determined for calculating the mean size of AuNPs by using Debye-Scherer’s equation which was found to be around 10.74 nm.
3.4. Functional Groups Being Involved in AuNPs Synthesis
FTIR spectrum of ethanolic leaf extract of A. spinosus showed characteristic bands for several functional groups. IR peaks for hydroxyl (-OH), aromatic amines
(-C6H5NH2), aliphatic amines (R-NH2), carbonyl (>C=O), C-H and C=C (benzene) functional groups were observed at around 3378, 1118, 1380, 1040, 1694, 2848 and 1604 cm−1 respectively (Figure 4(A)). These findings were supported by some previous reports that evaluated the profile of compounds (like amaranthine type betacyanin, amaranthine, isoamaranthine, phenols, flavanoids, lysine, etc.) present in alcoholic leaf extract of many species under the genus Amaranthus [28]. Chemical structures of these compounds justified the IR bands obtained. To determine whether during AuNPs synthesis some biomolecules particularly those with free carboxylic(-COOH) or amino (-NH2) groups present in the leaf extract have bound to gold surface, purified and lyophilized AuNPs samples were subjected to FTIR studies. IR band of AuNPs showed peaks for hydroxyl (3388 cm−1), aromatic and aliphatic amines (1391 & 1018 cm−1 respectively), carbonyl (1685 cm−1), C=C of benzene (1607 cm−1) and C-H (2842 cm−1) functional groups (Figure 4(B)). As reported earlier, alcoholic leaf extract of A. spinosus is very rich in anti-oxidant property due to the presence of many compounds with functional groups (hydroxyl or imino)
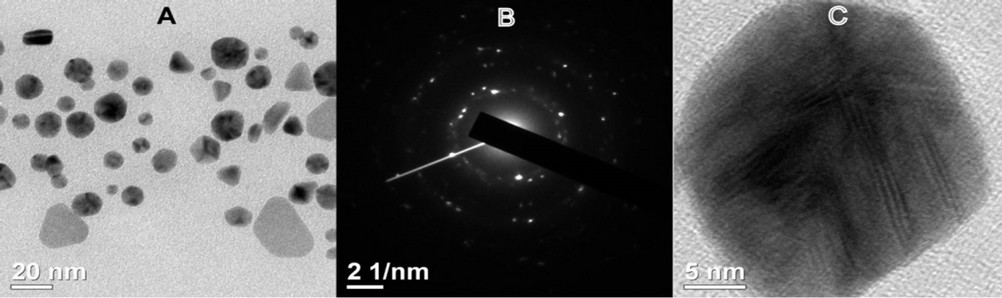
Figure 2. (A) TEM image of AuNPs synthesized by reacting 5% (v/v) ethanolic leaf extract of A. spinosus against 1 mM HAuCl4·4H2O aqueous solution at 25°C under magnetic stirring; (B) SAED pattern of a AuNPs; (C) URHTEM image of a spherical GNP showing lattice fringes.
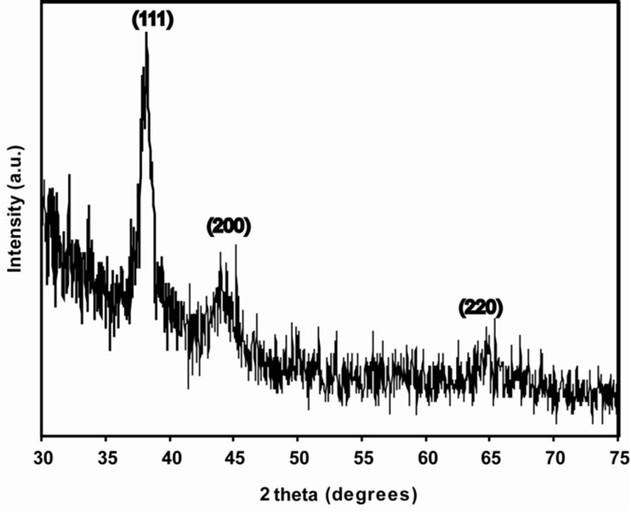
Figure 3. XRD spectrum of AuNPs synthesized with A. spinosus ethanolic leaf extract. The principal Bragg reflections are identified.
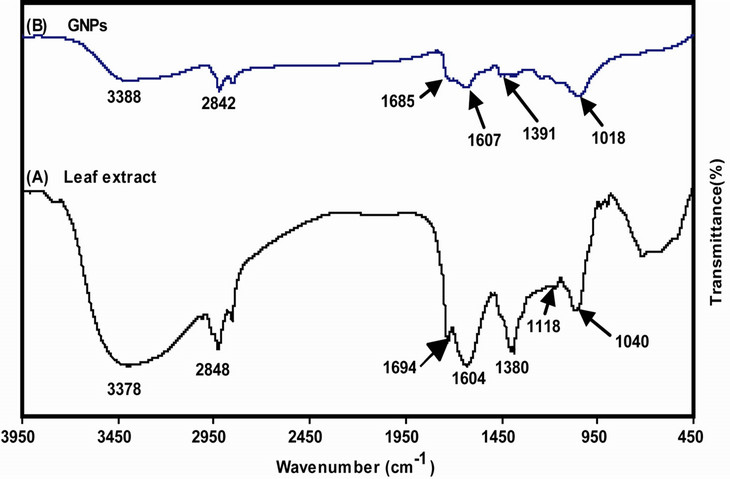
Figure 4. FT-IR spectra of (A) A. spinosus leaf extract; and (B) AuNPs synthesized from A. spinosus ethanolic leaf extract.
that can donate hydrogen atoms and many free amino or carboxylic moieties capable of binding to free gold surface. This suggested that during the reduction process of Au3+ ions to Au0, such molecules tightly bound to the gold surface as detected in the FTIR spectrum of AuNPs.
The spot profile EDAX of AuNPs showed a strong signal for gold along with weak oxygen, carbon, and potassium peaks, further suggested them to be originated from the biomolecules bound to the surface of the AuNPs
(Figure 5). The strong signal for gold confirmed the successful formation of AuNPs.
3.5. Amaranthus spinosus Leaf Extract Provides the Non Toxic Coating on AuNPs
The cytotoxicity of AuNPs under in vitro conditions in HeLa and MCF-7 cells was examined in terms of effect of AuNPs on cell toxicity by MTT assay for 24 h. The cellular morphologies of two cell lines were unaltered
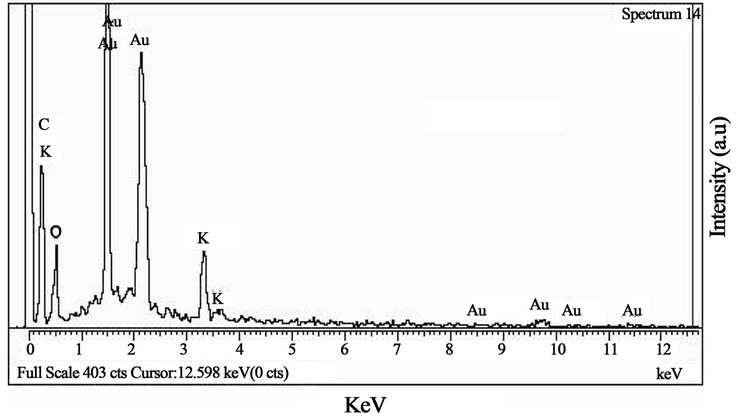
Figure 5. EDAX profile of AuNPs synthesized from A. spinosus ethanolic leaf extract.
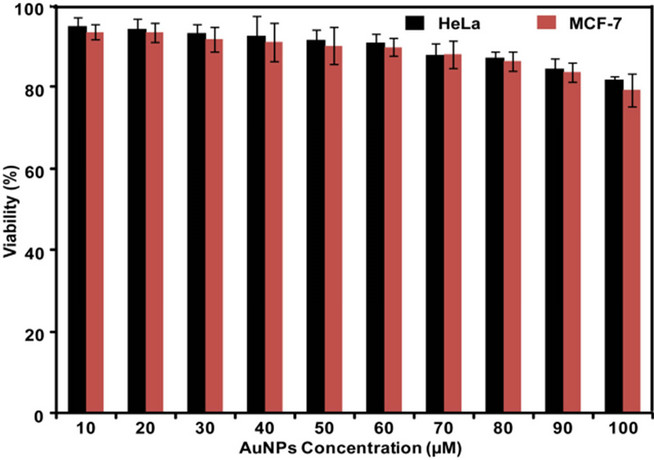
Figure 6. Cytotoxicity assay: cell viability of HeLa and MCF-7 cells exposed to different concentrations of AuNPs (10 - 100 μM) over a period of 24 h treatment.
after treatment with AuNPs suggesting that treatment with AuNPs did not induce any cytotoxic effect causing significant damage or death of the treated cells. The two cancer cell lines (HeLa and MCF-7) after 24 h of post treatment showed excellent viability up to as high as 100 μmol/L of AuNPs (Figure 6). This indicated that the SFE provided a nontoxic coating on the surface of AuNPs. This revealed that the synthesized AuNPs were of insignificant toxicity on cancer line provides an opportunity for application in drug delivery and molecular imaging such as magnetic resonance imaging (MRI), positron emission tomography (PET) and single photon emission computed tomography (SPECT).
4. Conclusion
We report here the application of the ethanolic leaf extract of Amaranthus spinosus for the synthesis of AuNPs from HAuCl4·4H2O. Characterization of AuNPs was done with UV-Vis study which exhibited the typical surface plasmon resonance property of the colloidal solution. TEM analysis showed the particle shape dominantly as spherical with few having triangular geometries. The AuNPs were crystalline as revealed from XRD studies. The average size of the AuNPs was 10.74 nm as determined with Debye-Scherrer’s equation utilizing XRD data. FTIR and EDX analyses of leaf extract showed the presence of various functional groups present on the surface AuNPs. Cytotoxicity studies revealed the non toxic nature of synthesized AuNPs and thus provided us an opportunity to use in biomedical applications.
5. Acknowledgements
We thank the Department of Biotechnology, Govt. of India, for funding this work (Sanction No: BT/04/NE/ 2009.
REFERENCES
- J. F. Hainfeld, D. N. Slatkin, T. M. Focella and H. M. Smilowitz, “Gold Nanoparticles: A New X-Ray Contrast Agent,” British Journal of Radiology, Vol. 79, No. 939, 2006, pp. 248-253. doi:10.1259/bjr/13169882
- C. Alric, J. Taleb, G. L. Duc, C. Mandon, C. Billotey, A. L. Meur-Herland, T. Brochard, F. Vocanson, M. Janier, P. Perriat, S. Roux and O. Tillement, “Gadolinium Chelate Coated Gold Nanoparticles as Contrast Agents for Both X-Ray Computed Tomography and Magnetic Resonance Imaging,” Journal of the American Chemical Society, Vol. 133, No. 18, 2008, pp. 5908-5915. doi:10.1021/ja078176p
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, “Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods,” Journal of the American Chemical Society, Vol. 128, No. 6, 2006. pp. 2115-2120.
- W. B. Cai, T. Gao, H. Hong and J. T. Sun, “Applications of Gold Nanoparticles in Cancer Nanotechnology,” Journal of Nanotechnology, Science and Applications, Vol. 1, No. 1, 2008, pp. 17-32.
- S. Eustis and M. A. El-Sayed, “Why Gold Nanoparticles Are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and Its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes,” Chemical Society Reviews, Vol. 35, No. 3, 2006, pp. 209-217. doi:10.1039/b514191e
- K. B. Narayanan and N. Sakthivel, “Coriander Leaf Mediated Biosynthesis of Gold Nanoparticles,” Materials Letter, Vol. 62, No. 30, 2008, pp. 4588-4590. doi:10.1016/j.matlet.2008.08.044
- S. L. Smitha. D. Philip and K. G. Gopchandran, “Green Synthesis of Gold Nanoparticles Using Cinnamomum zeylanicum Leaf Broth,” Spectrochimica Acta Part A, Vol. 74, No. 3, 2009, pp. 735-739. doi:10.1016/j.saa.2009.08.007
- M. Agnihotri, S. Joshi, R. K. Ameeta, S. Zinjarde and S. Kulkarni, “Biosynthesis of Gold Nanoparticles by the Tropical Marine Yeast Yarrowia lipolytica NCIM 3589,” Materials Letter, Vol. 63, No. 15, 2009, pp. 1231-1234. doi:10.1016/j.matlet.2009.02.042
- M. Gericke and A. Pinches, “Biological Synthesis of Metal Nanoparticles,” Hydrometallurgy, Vol. 83, No. 1-4, 2006, pp. 132-140.
- S. P. Chandran, M. Chaudhary, R. Pasricha and A. Ahmad, “Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloevera Plant Extract,” Biotechnology Progress, Vol. 22, No. 2, 2006, pp. 577-583. doi:10.1021/bp0501423
- P. Mohanouria, N. K. Rana and S. K. Yadav, “Biosynthesis of Nanoparticles by Microorganisms and Their Applications,” Journal of Nanoparticle Research, Vol. 10, No. 3, 2008, pp. 507-517.
- S. S. Shankar, A. Rai, A. Ahmad and M. Sastry, “Rapid Synthesis of Au, Ag, and Bimetallic Au Core-Ag Shell Nanoparticles Using Neem (Azadirachta indica) Leaf Broth,” Journal of Colloid and Interface Science, Vol. 275, No. 2, 2004, pp. 496-502. doi:10.1016/j.jcis.2004.03.003
- K. N. Satish, C. Nripen, S. Ravi, K. Kavita, R. K. Rajesh, T. Subramanian, M. Swapna, K. Raghuraman and V. K. Kattesh, “Green Nanotechnology from Tea: Phytochemicals in Tea as Building Blocks for Production of Biocompatible Gold Nanoparticles,” Journal of Materials Chemistry, Vol. 19, No. 19, 2009, pp. 2912-2920. doi:10.1039/b822015h
- J. Y. Song, H. K. Jang and B. S. Kim, “Biological Synthesis of Gold Nanoparticles Using Magnolia kobus and Diopyros kaki Leaf Extracts,” Process Biochemistry, Vol. 44, No. 10, 2009, pp. 1133-1138. doi:10.1016/j.procbio.2009.06.005
- J. Y. Song, E. Y. Kwon and B. S. Kim, “Biological Synthesis of Platinum Nanoparticles Using Diopyros kaki Leaf Extract,” Bioprocess and Biosystems Engineering, Vol. 33, No. 1, 2010, pp. 159-164. doi:10.1007/s00449-009-0373-2
- K. R. Kirtikar and B. D. Basu, “Indian Medicinal Plants,” Vol. 9, 2nd Edition, Oriental Enterprises, Rajpur, 2001, pp. 2832-2836.
- H. Zeashan, G. Amresh, S. Singh and C. V. Rao, “Hepatoprotective and Antioxidant Activity of Amaranthus spinosus against CCl4 Induced Toxicity,” Journal of Ethnopharmacology, Vol. 125, No. 2, 2009, pp. 364-366. doi:10.1016/j.jep.2009.05.010
- E. S. Hema, M. Sivadasan and K. N. Anil, “Studies on Edible Species of Amaranthaceae and Araceae Used by Kuruma and Paniya Tribes in Wayanad District, Kerala, India,” Ethnobotany, Vol. 18, No. 1. 2006, pp. 122-126.
- B. Odhav, S. Beekrum, U. Akula and H. Baijnath, “Preliminary Assessment of Nutritional Value of Traditional Leafy Vegetables in KwaZulu-Natal, South Africa,” Journal of Food Composition and Analysis, Vol. 20, No. 5, 2007, pp. 430-435. doi:10.1016/j.jfca.2006.04.015
- G. Cao, E. Sofic and R. L. Prior, “Antioxidant Capacity of Tea and Common Vegetables,” Journal of Agriculture and Food Chemistry, Vol. 44, No. 11, 1996, pp. 3426- 3431. doi:10.1021/jf9602535
- M. I. Gil, F. Ferreres and F. A. Thomas-Barberan, “Effect of Postharvest Storage and Processing on the Antioxidant Constituents (Flavonoids and Vitamin C) of Fresh-Cut Spinach,” Journal of Agriculture and Food Chemistry, Vol. 47, No. 6, 1999, pp. 2213-2217. doi:10.1021/jf981200l
- J. A. Vinson, Y. Hap, X. Su and L. Zubik, “Phenol Antioxidant Quantity and Quality in Foods: Vegetables,” Journal of Agriculture and Food Chemistry, Vol. 46, No. 9, 1998, pp. 3630-3634. doi:10.1021/jf980295o
- K. Srinivasan, C. L. Kaul and P. Ramarao, “Partial Protective Effect of Rutin on Multiple Low Dose Streptozotocin-Induced Diabetes in Mice,” Indian Journal of Pharmacology, Vol. 37, No. 5, 2005, pp. 327-328. doi:10.4103/0253-7613.16859
- J. C. Ibewuike, A. O. Ogundaini, L. Bohlin and F. O. Ogungbamila, “Anti Inflammatory Activity of Selected Nigerian Medicinal Plants,” Nigerian Journal of Natural Products and Medicine, Vol. 1, No. 1, 1997, pp. 10-14.
- R. P. Rastogi and B. N. Mehrotra, “Compendium of Indian Medicinal Plants,” CDRI and NISCAIR, Lucknow, 1999.
- F. C. Stintzing, D. Kammerer, A. Schieber, A. Hilou, O. Nacoulma and R. Carle, “Betacyanins and Phenolic Compounds from Amaranthus spinosus L. and Boerhavia erecta L.,” Zeitschrift fur Naturforschung, Vol. 59, No. 1, 2004, pp. 1-8.
- A. Hilou, O. G. Nacoulma and T. R. Guiguemde, “In Vivo Antimalarial Activities of Extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in Mice,” Journal of Ethnopharmacology, Vol. 103, No. 2, 2006, pp. 236- 240.
- Y. Cai, M. Sun and H. Corke, “Antioxidant Activity of Betalains from Plants of the Amaranthaceae,” Journal of Agriculture and Food Chemistry, Vol. 51, No. 8, 2003, pp. 2288-2294. doi:10.1021/jf030045u
NOTES
*Authors contributed equally.
#Corresponding author.

