Open Journal of Obstetrics and Gynecology
Vol. 2 No. 4 (2012) , Article ID: 24480 , 4 pages DOI:10.4236/ojog.2012.24079
Bartholin’s Gland Carcinomas: A 20 plus-year experience from Queensland*
![]()
1Queensland Centre for Gynaecological Cancer, University of Queensland, Brisbane, Australia
2Women’s and Newborn Services, Royal Brisbane and Women’s Hospital, Herston, Australia
Email: #Ian_Jones@health.qld.gov.au
Received 25 June 2012; revised 24 July 2012; accepted 3 August 2012
Keywords: Bartholin’s Gland Carcinomas; Cancer; Diagnosis; Management; Outcome; Follow Up
ABSTRACT
Objective: To review the records of cases of Bartholin’s Gland Carcinoma referred to the Queensland Centre for Gynaecological Cancer (QCGC) between mid 1993 and mid 2012. Methods: Bartholin’s Gland Carcinoma case data from QCGC were reviewed and analysed using the computer software Statistical Package for the Social Sciences 11.0. Results: Of the 12 cases four died of their disease, seven are still alive and disease free and one is alive with recurrent disease. The mean age at diagnosis was 52.8 years. Time from onset of symptoms to diagnosis averaged 5.8 months. All diagnoses were confirmed histologically. Presenting symptoms included a lump and pain. The most common presenting complaint was a lump. Treatment included surgical excision, occasional biopsy followed by radiotherapy with or without chemotherapy. In some cases radiation and chemotherapy was followed by vulvectomy of various extent. Conclusions: Bartholin’s Gland Carcinoma is a rare condition with outcome dependent on duration of symptoms, including delay in diagnosis, cell-type, cellular differentiation and the International Federation of Gynecology and Obstetrics (FIGO) classification. A Bartholin’s gland mass in a woman aged 40 years or more should be considered malignant until a biopsy proves otherwise.The incidence of Bartholin’s Gland Carcinoma in Queensland is less than that reported elsewhere but a higher proportion of squamous cell carcinomas was found in this small series.
1. INTRODUCTION
Bartholin’s gland in the human was first described by the Danish anatomist Caspar Bartholin in 1675 [1] and the first description of a Bartholin’s Gland Carcinoma (BGC) was in 1864 [2]. The incidence of Bartholin’s Gland Carcinoma has been variously reported as between two to seven % of all vulvar carcinomas [2]. These tumours can be of long standing because they are misdiagnosed as Bartholin’s gland cysts. When such “cysts” do not respond to conservative treatment biopsy reveals the true nature of the condition. Bartholin’s Gland Carcinomas have a wide range of histological features because they may arise from mucinous acini or ductal transitional cell epithelium [3]. Squamous cell carcinomas and adenocarcinomas each make up between 40% - 45% of Bartholin’s Gland Carcinomas [4].
The aim of the study was to review the clinical features, diagnosis, management and outcomes from the patient records of cases of Bartholin’s Gland Carcinoma held in the Queensland Centre for Gynaecological Cancer (QCGC) data base and determine if the type of management altered patient outcome.
2. MATERIALS AND METHODS
Case records, including pathology reports from the state wide QCGC data base for patients referred for registration and management of Bartholin’s Gland Carcinoma (BGC) between July 1989 and July 2012 were reviewed and abstracted relative to demographics, past surgical, medical and family history for cancer plus presenting symptoms, duration of symptoms prior to the commencement of treatment, clinical findings, International Federation of Gynecology and Obstetrics (FIGO) classification, treatment, histological findings, long term follow-up. In addition information was sought for the changes of human papilloma virus, vulvar in-situ carcinoma, cervical carcinoma in-situ, vulvar dystrophy, past history of cancer and a family history of cancer for each case. This information was analysed using the computer software Statistical Package for the Social Sciences 11.0. Where data was found to be incomplete attempts were made to collect this information by contacting referring doctors, reviewing patient case notes and pathology department records.
Ethics approval for the review of case records was obtained from the Clinical Research Ethics Committee of the Royal Brisbane and Women’s Hospital.
3. RESULTS
The incidence of Bartholin’s Gland Carcinoma (BGC) was 1.9% of all vulvar carcinomas recorded in the Queensland Centre for Gynaecological Cancer (QCGC) register between mid 1993 and mid 2012. Twelve cases of BGC were registered with QCGC during the study period. The frequency of cases registered each year ranged from zero to two. There were no cases of bilateral BGC and no cases of metastatic disease from other primary cancers. Of the 12 patients four have died of their disease, seven are still alive and disease free and one is alive with recurrent disease. The mean age at diagnosis was 52.8 years (range 38 - 70). Time from onset of symptoms to diagnosis for the 12 cases averaged 5.8 months. Nine cases had Squamous Cell Carcinomas with a symptom to diagnosis averaged 2.9 months, one had an Adenoid Cystic Carcinoma with a symptom to diagnosis time 24 months, one had a Small Cell Carcinoma with a symptom to diagnosis time five months and one had a Transitional Cell cancer, symptom to diagnosis 12 months. Age at presentation versus outcome data is presented in Table 1.
Presenting symptoms included lumps, pain and vaginal discharge. The most common presenting complaints were a painless lump (in 50% of cases), painful vulvar lump (in 25% of cases) and a groin lump (in 17% of cases). The size of the lesions ranged from 0.3 to 8 cm (mean 4.3 cm). Initial management was dictated by the extent of each patient’s condition. Four cases underwent biopsies of the lesions and eight underwent excision of Bartholin’s gland allowing the diagnoses to be confirmed. Of those cases who underwent biopsy three were FIGO 3 (3A, 3A, 3C) and one FIGO 4A. Following diagnosis by incomplete excision, the case with the small cell carcinoma FIGO grade 2 decided to go overseas for alternative
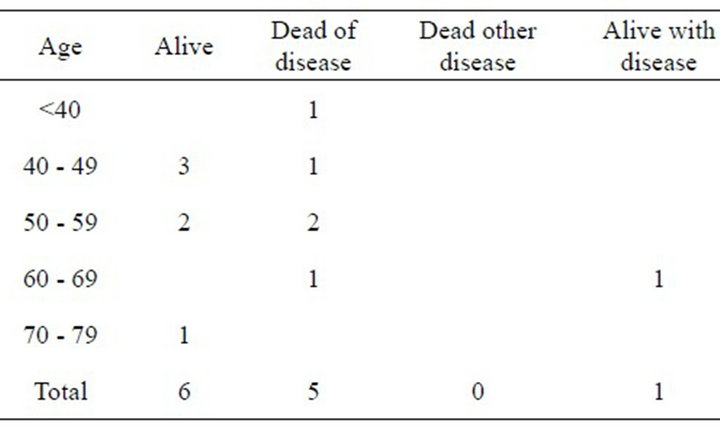
Table 1. Age versus patient status.
therapy. She died 34 months after diagnosis.
Subsequent treatment regimes were again determined by the extent of the disease. One case underwent chemoradiation therapy (Cisplatin and 5 Flurouracil), one received radiation therapy alone and another elected to seek treatment from an alternative practitioner overseas. The remaining eight underwent surgical procedures which included wide local excision hemi vulvectomy with or without groin node dissection, or in one case excision of groin nodes followed by radiation therapy (Table 2). The dose of radiation given to those receiving this treatment ranged from 43 to 60 Gy.
Patient outcome is shown in Table 3 and classified by FIGO grade and in Table 4 by histological cellular differ-
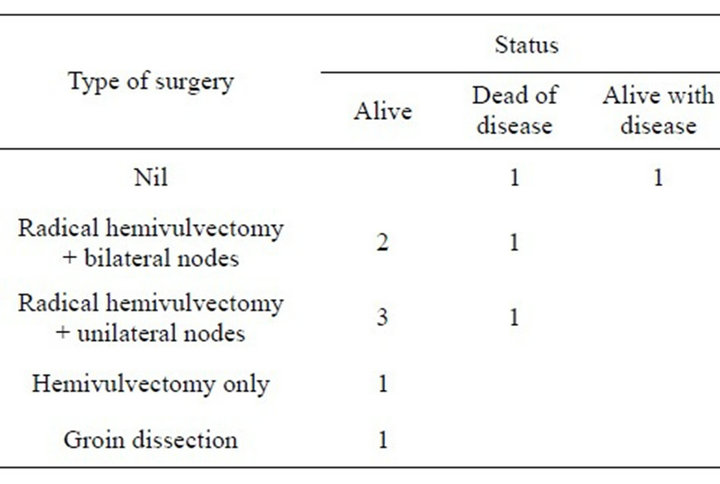
Table 2. Type of surgery versus patient status.
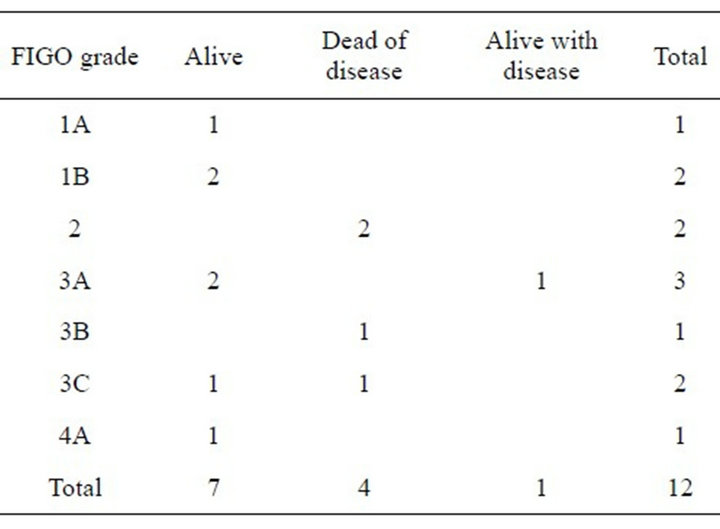
Table 3. FIGO grade versus patient status.
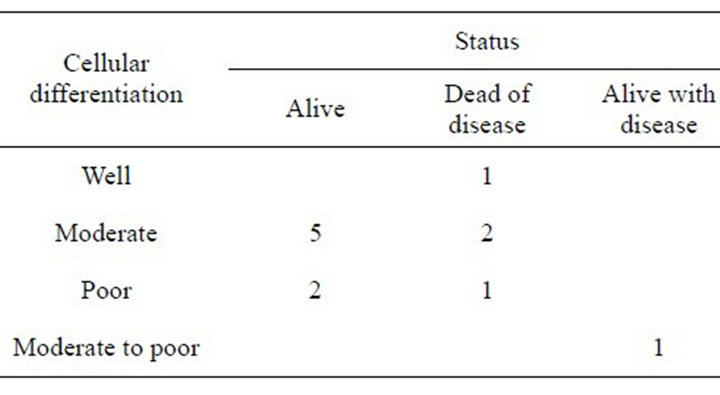
Table 4. Cell differentiation versus patient status.
entiation. Only two cases were found to have high grade VIN and the case of adenoid cystic carcinoma had lichen sclerosus and Carcinoma in-situ of the cervix (CIN 3). There were no cases of a family history of vulvar cancer but three cases had family histories of large bowel and breast cancer.
Of the nine cases of squamous cell carcinoma two had recurrences at 10 and 127 months post treatment and seven cases are alive at 3, 48, 60, 72, 120, 132 and 213 months without recurrence of disease. The case of adenoid cystic carcinoma recurred at 36 months and the transitional cancer recurred at 36 months. The remaining case of small cell cancer went overseas for treatment and we are unable to determine if she ever responded to treatment but she died 34 months after diagnosis.
4. DISCUSSION
Squamous cell carcinomas and adenocarcinomas make up 90% of Bartholin’s gland cancers (BGC) with the remaining types including adenoid cystic carcinomas, melanomas, sarcomas and undifferentiated cancers [5]. Since this report other types of BGC have been described including Merkel cell carcinoma [6] and epithelialmyoepithelial carcinomas [7,8]. In the current study seven of the 12 cases have been reported previously [9] which gave us the opportunity to review their histories and up date their progress. Three of the seven have died and one of the subsequent five cases has died. Of the 12 cases nine (75%), had SCC and there were single cases of adenoid cystic, transitional and small cell carcinomas. A case of leiomyosarcoma was excluded because it was not a carcinoma and so did not meet the criterion for inclusion in this paper. This incidence of SCC cases is higher than other reports mentioned above.
The human papilloma virus (HPV) has been shown to be associated with BGC of the squamous and adenocarcinoma types and may play a role in the genesis of this malignancy [10]. Two of our cases of SCC type had VIN changes on their histopathology and the case of adenoid cystic carcinoma had CIN 2 and lichen sclerosus. We did not DNA type the tissue from these three cases and so are unable to speculate as to whether HPV played any part in the genesis of these diseases. We also wondered if the presence of other cancers (lung, breast and endometrial) increased the risk for developing BGC but our numbers are too small to make any such prediction.
Adenoid cystic carcinoma of Bartholin’s gland is extremely rare with less than a hundred having been reported world wide. It is slow growing but locally aggressive carcinoma with a high risk of recurrence [11]. Our case, aged 43 years had a two-year delay in diagnosis, being told that her problem was due to a Bartholin’s gland cyst. She had recurrences four and six years after her initial surgical wide excision and radiotherapy. She died of her BGC with lung secondaries 12 years after her initial treatment began.
In the case of the small cell carcinoma she was aged 38 years and presented to a number of doctors while overseas with a vulvar mass being told that she had a Bartholin’s gland cyst. This resulted in a five month delay in diagnosis. Unfortunately she decided to seek alternative care overseas and died 34 months after leaving Queensland.
The patient with the transitional cell carcinoma was aged 58 years and her condition was complicated by breast cancer (treated six years earlier by surgery, chemotherapy and radiotherapy) that was thought to have metastasised and cause bilateral groin masses which were biopsied. She was then unsuccessfully treated with hormones for 12 months. Then a Bartholin’s gland mass was discovered, biopsied and treated with radiotherapy. Twenty-two months later she died of disease.
Factors which influence outcome for Bartholin’s Gland carcinoma include delay in diagnosis, FIGO staging where the four dead of disease cases had a grading of two or more (Table 2), histological type (these four cases had Adenoid cystic, Small cell and two cases of Squamous cell carcinoma [both with FIGO 3]) and cellular differentiation where three cases who are dead of disease had moderate or poor cellular differentiation (Table 3). We agree with Finan and Barre’s recommenddations [1] that a mass in the area of Bartholin’s gland in a post menopausal woman should be considered malignant until proven otherwise by biopsy but recommend that any woman aged 40 years or more should be managed this way. Even then we would not have diagnosed the 38 year old woman with the small cell carcinoma.
5. ACKNOWLEDGEMENTS
We gratefully acknowledge all those doctors who referred cases to the QCGC and the gynaecological oncologists who have updated QCGC records.
REFERENCES
- Finan, M.A. and Barre, G. (2003) Bartholin’s gland carcinoma, malignant melanoma and other rare tumours of the vulva. Best Practice & Research Clinical Obstetrics & Gynaecology, 17, 609-633. doi:10.1016/S1521-6934(03)00039-7
- Ridley, C.M. (2009) The vulva. In: Neill, S. and Lewis, F., Eds., 3rd Edition, Wiley-Blackwell, Oxford, 190.
- Ridley, C.M. (2009) The vulva. In: Neill, S. and Lewis, F., Eds., 3rd Edition, Wiley-Blackwell, Oxford, 191.
- Liebeskind, A., Machnicki, S. and Blackmun, D. (2004) Case of the month. Applied Radiology, 33, 49-53.
- Lelle, R.J., Davis, K.P. and Roberts, J.A. (1994) Adenoid cystic carcinoma of Bartholin’s gland: The University of Michigan experience. International Journal of Gynecological Cancer, 4, 145-149. doi:10.1046/j.1525-1438.1994.04030145.x
- Khoury-Collado, F., Elliot, K.S., Lee, Y., Chen, P.C. and Abulafia, O. (2005) Merkel cell carcinoma of Bartholin’s gland. Gynecologic Oncology, 97, 928-931. doi:10.1016/j.ygyno.2004.12.064
- McCluggage, W.G., Aydin, N.E., Wong, N.A. and Cooper, K. (2009) Low-grade epithelial-myoepithelial carcinoma of bartholin’s gland: Report of 2 cases of a distinct neoplasm arising in the vulvovaginal region. International Journal of Gynecological Pathology, 28, 286-291. doi:10.1097/PGP.0b013e31818e1040
- Kyriazi, M.A., Carvounis, E.E., Kitsou, M., Arkadopoulos, N., Nicolaidou, E., Fotiou, S. and Smymiotis, V. (2010) Myoepithelial carcinoma of the vulva mimicking bartholin’s gland abscess in a pregnant woman: Case report and review of literature. International Journal of Gynecological Pathology, 29, 501-504. doi:10.1097/PGP.0b013e3181d81cdb
- Obermair, A., Roller, S., Crandon, A.J., Perrin, L. and Nicklin, J.L. (2001) Primary bartholin gland carcinoma: A report of seven cases. ANZJOG, 41, 78-81.
- Felix, J.C., Cote, R.J., Kramer, E.W., Saigo, P. and Goldman, G.H. (1993) Carcinomas of bartholin’s gland. American Journal of Pathology, 142, 925-933.
- Woida, F.M. and Ribeiro-Silva, A. (2007) Adenoid cystic carcinoma of the Bartholin Gland. Archives of Pathology & Laboratory Medicine, 131, 796-798.
NOTES
*Conflict of Interest: The authors declare that there are no conflicts of interest.
#Corresponding author.

