World Journal of Nuclear Science and Technology
Vol.05 No.03(2015), Article ID:58482,10 pages
10.4236/wjnst.2015.53024
Elemental Analysis of Some Nigerian Food Legumes by ko-ENAA and INAA
F. T. Sheyin1*, M. O. A. Oladipo1, S. A. Jonah1, U. Sadiq2
1Centre for Energy Research and Training, Ahmadu Bello University, Zaria, Nigeria
2Department of Physics, Ahmadu Bello University, Zaria, Nigeria
Email: *fsheyin@abu.edu.ng
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 May 2015; accepted 28 July 2015; published 31 July 2015
ABSTRACT
Samples of the food legumes, cowpeas (Vigna unguiculata), soya beans (Glycine max) and groundnuts (Arachis hypogaea) collected from rural farmers in four rural villages around Zaria, Nigeria were analysed for the concentrations of the major, minor and trace elements by the ko-ENAA and INAA methods. Irradiation and γ-ray counting were performed using the NIRR-1 and associated facilities. The NIST 1515 Apple leaves were used as the quality control material. The concentrations of the elements Se, Br and Rb were determined by ko-ENAA while those of Na, K, Zn, La, Br, Sm and Sc were determined in INAA in the food legumes. The concentrations of Zn found in all the legumes are within the limit specified in international guidelines. The concentrations of Se found in the food legumes are above the limit specified in international guidelines. The food legumes are good sources of the elements Na, K, Zn which can meet adequate dietary requirements provided the legumes are not refined. Bromine is not at toxic levels in the food legumes.
Keywords:
ko-ENAA of Food Legumes, INAA of Food Legumes

1. Introduction
The 30 kW Nigeria Research Reactor-1 (NIRR-1) is one of the commercial Miniature Neutron Source Reactors (MNSRs) installed outside China. It was commissioned in 2004 and has been standardized for instrumental neutron activation analysis (INAA) [1] . In order to extend its utilization for epithermal neutron activation analysis (ENAA) protocol, the cadmium (Cd) lined irradiation channel was installed in one of the large outer irradiation channels [2] . Prior to now, the NIRR-1 has been used for elemental analysis via the ko-standardization method based on the Ko-IAEA software [3] .
The use of ENAA greatly reduces the interferences caused by the major and minor elements with low resonance integral to thermal neutron cross section ratios (Qo) that are easily activated by thermal neutrons such as Al, Mn, Na, Cr and Sc in geological samples and Na, K, Zn, Mn, Fe and Cl in biological samples and enhance the measurement of the activities of the trace elements with high Qo values such as I, Br, As, Sb, U, Th, Se and Mo, which strongly absorb neutrons of specific resonance energies in the epithermal neutron region (0.5 eV - 1 MeV) [4] [5] . Epithermal neutron activation analysis has been used by many laboratories for the analysis of different types of samples of materials [4] -[9] .
The trace elements generally recognized to be essential for human health are Cr, Co, Cu, Fe, I, Mn, Zn, F, Se and Mo [10] -[13] . Some other trace elements such as Pb, As, Hg, Cd, Sb are recognized to be toxic as they have adverse effects on human health [11] . In general, all chemical elements are potentially toxic when injested in large amounts.
Plant foodstuffs are major sources of the total daily intake of the essential and non essential elements by the people in Nigeria. The food legumes such as cow peas (Vigna unguiculata), soya beans (Glycine max) and groundnuts (Arachis hypogaea) form about 30% - 40% of the daily dietary intake in Nigeria. It is therefore important to determine the concentrations of the mineral element nutrients and the toxic elements in these food legumes to increase our understanding of their distribution in the food legumes geographically, for the calculation of the dietary intake of the essential elements for human health and for the evaluation of human exposure to the toxic elements.
In this work, the single comparator method of epithermal neutron activation analysis (ko-ENAA) which uses Al-0.1%Au thin foil as the single comparator and the instrumental neutron activation analysis (INAA) method were used for elemental determination in some Nigerian food legumes such as cowpeas (Vigna unguiculata), soya beans (Glycine max) and groundnuts (Arachis hypogaea).The aim was to assess the nutritional quality of the food legumes based on the concentrations of the elements with high resonance integral to thermal neutron cross section ratios (Qo) determined by epithermal activation in the Cd lined irradiation channel and also the concentrations of the elements with low Qo values determined by thermal neutron activation in the thermal neutron irradiation sites.
2. Materials and Methods
Twenty legumes: Cowpeas (Vigna unguiculata), soya beans (Glycine max) and groundnuts (Arachis hypogaea) were collected from rural farmers at four rural villages: Tudun Sarki, Unguwa Rafin Magaji, Kufena and Unguwa Goruba around Zaria in Kaduna state, Nigeria. All the legume samples were put in different strong polyethylene bags and labeled appropriately.
All the samples of legumes in the polyethylene bags were homogenized using the quartering technique. The selected sub-samples of legumes were ground whole using the Thomas Wiley Laboratotry Mill Model 4 made of stainless steel so that the ground whole samples of legumes passed through a sieve with 1 mm diameter openings inserted in the Thomas Wiley laboratory mill. The ground whole legume samples were dried in the Memmert air Oven dryer. The samples of ground whole cow peas (Vigna unguiculata), and soya beans (Glycine max) were dried at a temperature 101˚C ± 5˚C for 7 hours until constant weight was achieved. The samples of ground whole groundnuts (Arachis hypogaea) were dried at a temperature of 101˚C ± 5˚C for 5 hours until constant weight was achieved. Small quantities of the dried ground whole samples of legumes were taken at random using pre-cleaned small plastic vials for analysis.
Twenty samples of ground whole legumes weighing 0.2529 - 0.2970 g were packed in pre-cleaned polyethylene films and heat sealed for analysis by the ko-ENAA method. They were divided into three sets. The first set consisted of five samples of legumes, the standard reference materials NIST 1573a Tomato leaves and NIST 1574a typical diet.The second set consisted of twelve samples of legumes, NIST 1515Apple leaves and NIST 1573a Tomato leaves. The third set consisted of three samples of legumes, NIST 1515 Apple leaves, NIST 1547 Peach leaves, NIST 1573a Tomato leaves and Al-0.1%Au thin foil. All the sets of legume samples were put into different pre-cleaned 25 cm3 polyethylene vials, covered with cotton wool, the tops sealed with a celotape.
Twenty other samples of ground whole legumes weighing 0.2539 - 0.2728 g were packed in pre-cleaned polyethylene films and heat sealed for analysis by the INAA method. They were divided into three sets. The first set consisted of six samples of legumes and the standard reference material NIST 1515 Apple leaves. The second set consisted of six samples of legumes and the NIST 1573a Tomato leaves. The third set consisted of eight samples of legumes and the NIST 1515 Apple leaves. All the sets of samples were put into different pre-cleaned 7 cm3 polyethylene vials, covered with cotton wool and the tops sealed with a celotape.
All the sets of samples of legumes for ko-ENAA were irradiated separately with epithermal neutrons in the Cd lined irradiation channel of the NIRR-1 at a preset thermal neutron flux of 5 × 1011 n∙cm−2∙s−1 for 6 hours. The value of the epithermal neutron shape factor (α) in the Cd lined irradiation channel determined by the cadmium covered multimonitor method using the monitor set Al-0.1%Au, Zr and Zn thin foils and Mo thin wire is −0.137 ± 0.018. A detailed description of the method used for the determination of the epithermal neutron shape factor (α) is given elsewhere [2] [14] .
After irradiation, all the polyethylene vials containing the irradiated sets samples of legumes with the standards were put inside a small lead shield, kept in the fumed chamber and allowed to cool before counting.
The induced activities on the elements of interest in all the samples of legumes and standard reference materials (SRMs) were measured under the same counting conditions using the full energy peak efficiency calibrated P-type GEM 30195 HPGe coaxial detector system at the distance of 2 cm from the detector. The energy resolution of the detector system is 1.95 keV for the 1332 keV peak of 60Co and the relative detector efficiency is 30%. The full energy peak efficiency of the P-type GEM 30195 HPGe coaxial detector was measured at the distances 2 cm and 15 cm from the detector over the energy range 59.54 -1408 keV using the set IAEA standard sources 241Am, 152Eu 226Ra, 137Cs, 60Co and 22Na. A detailed description of the measured full energy peak efficiency curves and the theoretical fitting function to the experimental efficiency curves is given elsewhere [14] . The photopeak areas of the radionuclides found in the spectra were obtained from the gamma-ray acquisition system of the P-type GEM 30195 HPGe coaxial detector system that consists of the Maestro Multicannel Annalyser (MCA) emulation software card coupled to the detector via electronic nuclear instrumentation modules (NIM) manufactured by Ortec. The analysis scheme employed for the elements measured in the samples of legumes and NIST 1515 Apple leaves in the Cd lined irradiation channel for analysis by ko-ENAA is shown in Table 1.
All the other sets of samples of legumes together with the standard reference materials for INAA were irradiated separately with thermal neutrons in the inner irradiation sites B2 and B3 of the NIRR-1 at a preset thermal neutron flux of 5 × 1011 n∙cm−2∙s−1 for 6 hours. After irradiation, all the polyethylene vials containing the irradiated sets samples of legumes with the standard reference materials were put inside a small lead shield, kept in the fumed chamber and allowed to cool before counting. The induced activities on the elements of interest in all the samples of legumes and standard reference materials (SRMs) were measured under the same counting conditions using the same set up as above. The analysis scheme employed for the elements measured in the irradiated samples of legumes and the standard reference material NIST 1515 Apple leaves for INAA is shown in Table 2.
Table 3 shows the nuclear data for the product nuclides measured in the samples of legumes and NIST 1515 Apple leaves for ko-ENAA. The nuclear data ko, Qo,and
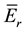 and FCd for 198Au and the nuclides of the elements of interest measured in the samples of legumes and the NIST 1515 Apple leaves were obtained from the literature [15] -[20] . The corrected Qo (α) values for 198Au and the elements of interest measured in the samples of legumes
and FCd for 198Au and the nuclides of the elements of interest measured in the samples of legumes and the NIST 1515 Apple leaves were obtained from the literature [15] -[20] . The corrected Qo (α) values for 198Au and the elements of interest measured in the samples of legumes
Table 1. Analysis scheme for elements measured in samples of legumes and NIST 1515 Apple leaves for ko-ENAA.
Table 2. Analysis scheme for elements measured in legume samples and NIST 1515 Apple leaves for INAA.
and NIST 1515 Apple leaves were calculated with the MS Excel from the equation
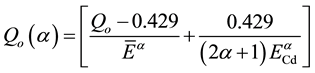 (1)
(1)
where
 effective resonance energy,
effective resonance energy,
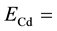 cadmium cut-off energy (0.55 eV),
cadmium cut-off energy (0.55 eV),
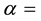 value of the epithermal neutron shape factor in the cadmium lined irradiation channel,
value of the epithermal neutron shape factor in the cadmium lined irradiation channel,
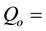 resonance integral to thermal neutron cross section ratios of nuclides in the
resonance integral to thermal neutron cross section ratios of nuclides in the
 epithermal neutron spectrum,
epithermal neutron spectrum,
 corrected resonance integral to thermal neutron cross section ratios of nuclides in the cadmium lined irradiation channel.
corrected resonance integral to thermal neutron cross section ratios of nuclides in the cadmium lined irradiation channel.
Using the nuclear data in Table 3, the unknown concentrations of elements measured in the samples of legumes and NIST 1515 Apple leaves were calculated by the single comparator method of ENAA using the activity of 198Au in Al-0.1% Au thin foil as the single comparator with the MS Excel from the expression [21]
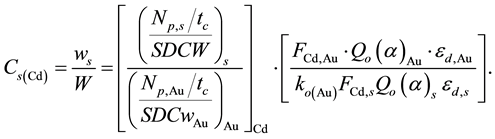 (2)
(2)
The
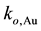 factor is a compound nuclear constant of the nuclide in the sample of material with respect to Au which is independent of the irradiation and counting conditions and is defined theoretically in the ko-ENAA method as [22]
factor is a compound nuclear constant of the nuclide in the sample of material with respect to Au which is independent of the irradiation and counting conditions and is defined theoretically in the ko-ENAA method as [22]
 (3)
(3)
In Equation (2) and Equation (3)
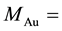 atomic mass of gold,
atomic mass of gold,
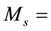 atomic mass of the element of interest in the sample matrix,
atomic mass of the element of interest in the sample matrix,
 isotopic abundance of the element of interest in the sample matrix,
isotopic abundance of the element of interest in the sample matrix,
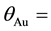

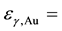
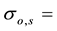

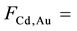

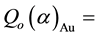
Table 3. Nuclear data for nuclides measured in samples of legumes and NIST 1515 Apple leaves for ko-ENAA.
lined irradiation channel,
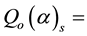
in the legume samples and NIST 1515 Apple leaves in the cadmium lined irradiation channel,
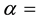
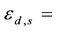



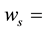

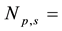


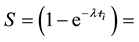
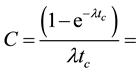


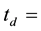
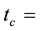
The self-shielding correction factors for epithermal neutrons (Gepi) were assumed to be negligible since the masses of the samples of legumes and Al-0.1%Au thin foil were small.
The precision in the concentrations of elements determined in the legume samples and NIST 1515 Apple leaves by the ko-ENAA method is due mainly to random errors. Random errors are mainly due to counting statistics of the activities of the product nuclides in the irradiated legume samples and 198Au used as the single comparator. From Equation (2), the precision in the concentrations of the elements of interest in the samples of legumes and NIST 1515 Apple leaves was calculated using the MS Excel spreadsheet from the error propagation equation
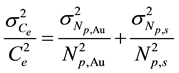
where
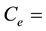

ko-ENAA,
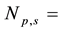
leaves,
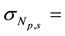
1515 Apple leaves,
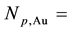
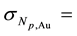
The accuracy of the INAA method was assessed by the analysis of the SRM NIST 1515 Apple leaves irradiated along with the legume samples using the software WINSPAN 2004 [23] . The concentrations of the elements measured in the SRM NIST 1515 Apple leaves using the same set up as the one used to measure the legume samples were compared with the certified values.
The unknown concentrations of only seven elements Na, K, Zn, Br, La, Sm and Sc were calculated by the instrumental neutron activation analysis method using the nuclear data of the elements determined in the NIST 1515 Apple leaves as the comparator. The photopeak analysis and the neutron activation analysis calculations of the radionuclides found in the spectra were carried out using the software WINSPAN 2004 [23] . The results are shown in Table 7.
3. Results and Discussions
From Table 4, the concentrations of the elements Sm and Br with high Qo values are better determined in the NIST 1515 Apple leaves by the ko-ENAA method because they strongly absorb neutrons of specific resonance energies in the epithermal neutron region. The elements Sm and Br have high resonance activation integrals compared to their thermal neutron cross sections in the epithermal neutron region. The concentrations of Sm and Br in the NIST 1515 Apple leaves are in good agreement with the certified values. The ko-ENAA method in the cadmium lined irradiation channel of the NIRR-1 is a reliable method of neutron activation analysis (NAA) in the NIRR-1.
Table 5 shows the results of the concentrations of the elements determined in the legume samples by the
Table 4. Results of concentrations of elements determined in NIST 1515 Apple leaves by ko-ENAA in mg∙kg−1.
Table 5. Results of concentrations of elements determined in samples of legumes by ko-ENAA in mg∙kg−1.
BDL―Below detection limit.
ko-ENAA method. The elements Se, Br and Rb are better determined in the samples of legumes by the ko-ENAA method because of their high Qo values.
From Table 6, it can be observed that the concentrations of only nine elements Na, K, Br, La, Sm, Zn, Sc, Rb and Yb determined in the standard reference material NIST 1515 Apple leaves are in good agreement with the certified values. This indicates the reliability of the INAA method for analysis of the food legumes in this work.
Table 7 shows the elements Na, K, Zn, Br, La,Sm and Sc determined in the samples of legumes by the INAA method. A comparison of the results of the analysis of the legume samples by the ko-ENAA in Table 5 and by INAA in Table 7 shows that the ko-ENAA method in the cadmium lined irradiation channel is a favourable me-
Table 6. Results of the concentrations of elements determined in NIST 1515 Apple leaves by INAA in mg∙kg−1.
Table 7. Results of concentrations of elements determined in samples of legumes by INAA in mg∙kg−1.
BDL―Below detection limit.
thod for the determination of the elements Se, Br and Rb with high Qo values.This is because these elements strongly absorb neutrons of specific resonance energies in the epithermal neutron region. The elements Se, Br and Rb have high resonance activation integrals compared to their thermal neutron cross sections in the epithermal neutron region. The INAA method is a favourable method for the determination of the elements Na, K, Zn, La and Sc with low Qo values because they have relatively large thermal neutron cross sections compared to their resonance activation integrals in the reactor neutron spectrum. The elements Sm and Br have high Qo values but can be determined by the INAA method because they have relatively large thermal neutron cross sections.
3.1. Sodium
Sodium is an essential major element for human health. It is a primary extracellular cation and is fundamental in regulating osmolarity and body fluid volume. It also acts as a buffer in maintaining the body’s acid-base balance [10] . Table salt (sodium chloride) is the major dietary source of sodium. The high intake of table salt in diets is correlated with elevated blood pressure (hypertension) [10] [12] . The source of sodium in the Nigerian food legumes used in this work is the soil. Sodium was found in all the legume samples. The range of the concentrations of Na were found to be higher in cow peas than in groundnuts and soya beans.
3.2. Potassium
Potassium is an essential major element for human health. It is a primary cation in intracellular fluids of the human body. The high intake of K lowers blood pressure in hypertensive patients [10] [12] . Potassium is not generally added to human diets in Nigeria. However, K is used medicinally by some communities in Nigeria to control stomach constipation or disorder and in some types of diets for women that have newly given birth to children and even healthy people. The sources of uptake of K by legumes grown in Nigeria are the soil and the use of K containing chemical fertilizers during their growing period. Potassium was found in all the legume samples. The range of the concentrations of K were found to be relatively higher in soya beans than in cow pea and groundnuts.
3.3. Zinc
Zinc is an essential trace element for human health. Zinc is a constituent of insulin and many enzymes for the metabolic processes of the human body. It supports the immune system, the synthesis of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), the healthy growth and development on the body during childhood, adolescence and pregnancy, rapid wound healing and fertility [10] [12] . The deficiency symptoms of Zn in the human body include retarded growth, anaemia, decreased wound healing, diminished taste, diarrhea, infertility and delayed sexual maturation [10] [12] . The source of Zn in the samples of legumes in this work is the soil. Zinc was found in all the legume samples. The range of the concentrations of Zn in soya beans are relatively higher than that in cow pea and groundnuts. The concentrations of Zn found in all the legume samples in this study are lower than the maximum permissible concentrations (MPCs) in foodstuffs for human consumption that has been recommended by Reilly [24] which is 40 - 50 ppm.
3.4. Selenium
Selenium is an essential trace element to human health. Selenium is part of an antioxidant sytem. The best understood enzymatic function of selenium occurs as part of two enzymes, each named glutathione peroxidase (one is inside cells, the other is outside cells including in the serum portion of the blood) [10] [12] . The symptoms of the deficiency of selenium in humans include liver and muscle damage, cancers, tumors and cardiomyopathy. The excessive intake of selenium is toxic and can cause vomiting, nausea, weakness, liver damage and hair loss [10] [12] .
Selenium was found in only four samples of cow peas, one sample of soya beans and two samples of groundnuts. The concentrations of selenium in all the legume samples are higher than the maximum permissible concentrations (MPCs) in foodstuffs for human consumption that has been recommended by Reilly [24] which is 2 ppm. The high concentrations of Se in the samples legumes in this study could be due to the uptake of this element from the soil.
3.5. Bromine
Bromine is one of the most abundant and very common of the recognized trace elements in the biosphere [11] . It is not an essential element for human health. The excessive intake of Br can result in bromism, a toxic condition with symptoms that include mental disturbance, impaired memory, drowsiness and damage to heart tissues [25] . No Br containing chemicals such as methyl bromide were used as pesticides during the growing period of the legume samples or for the control of pests during storage. This suggests that the main source of Br found in the legume samples in this study is from the naturally occurring Br in the soils they were grown. Bromine was found in the legume samples by ko-ENAA and INAA. The range of the concentrations of Br by INAA were found to be relatively lower in groundnuts than those for cow peas and soya beans. The ranges of the concentrations of Br in soya beans, cow pea and groundnuts by INAA are higher than those by ko-ENAA in the soya beans, cow pea and groundnuts.
3.6. Rubidium
Rubidium is not an essential trace element for human health. Rubidium can interchange with potassium in biological systems. There is little evidence of the toxicity of Rb in biological systems. Rubidium was found in only three samples of cow peas, one sample of soya beans and one sample of groundnuts.
3.7. Lanthanum
Lanthanum is not an essential trace element for human health. The low concentrations of La found in almost all the legume samples of cow peas, soya beans and groundnuts indicates that it occurs widely in small amounts in soils but is poorly absorbed by plants.
3.8. Samarium
Samarium is not an essential trace element for human health. Samarium was found in only four samples of cow peas and one sample of groundnuts. The low concentrations of Sm found in the few legume samples indicates that Sm is not absorbed to a measurable concentration by plants.
3.9. Scandium
Scandium is not an essential trace element for human health. Scandium was found in low concentrations in only four samples of cow peas, one sample of soya beans and one sample of groundnuts. The low concentrations of Sc in only a few legume samples indicates that Sc occurs in soils in very small amounts and is poorly absorbed by plants.
4. Conclusions
The ko-ENAA and INAA methods were successfully employed to analyse some samples of the Nigerian food legumes cow peas (Vigna unguiculata), soya beans (Glycine max) and groundnuts (Arachis hypogaea). The standard reference material NIST 1515 Apple leaves was used as the quality control material in this work. The concentrations of the elements Se, Br and Rb with high Qo values were determined in the samples of legumes by the ko-ENAA method in the Cd lined irradiation channel. The elements Na, K, Zn, La and Sc with low Qo values were determined in the samples of legumes by the INAA method. The elements Br and Sm have high Qo values but were determined by the INAA method because they have relatively large thermal neutron cross sections.
The concentrations of Zn are within the limit specified in international guidelines while that of Se is above the limit specified in international guidelines. It can be considered that the legume samples used in this work are good sources of the elements Na, K and Zn which can meet dietary requirement provided the legumes are not refined [26] . The concentrations of Br found in the legume samples in this study are from the naturally occurring Br in the soil and are not at toxic levels in the food legumes. The results of the analysis of the legume samples by the ko-ENAA and INAA methods in this work will be useful in the provision of necessary data from the Zaria environment needed as base line data in studies on the elemental composition of foodstuffs for human consumption in Nigeria.
The ko-ENAA in the Cd lined irradiation channel using MS Excel and INAA are reliable methods for optimization of the neutron activation analysis (NAA) scheme in the NIRR-1. It will be necessary in future to use moveable boron vials with cut-off energy of range up to 280 eV depending on thickness for epithermal activation of samples of materials in the Cd lined irradiation channel to improve the accuracy of the ko-ENAA method in the NIRR-1.
Acknowledgements
The authors are thanking the management of the Centre for Energy Research and Training, Ahmadu Bello University, Zaria, Nigeria and members of staff of the Nuclear Science and Technology Section, Centre for Energy Research and Training, Ahmadu Bello University, Zaria, Nigeria for their assistance in the experimental work of this study.
Cite this paper
F. T.Sheyin,M. O. A.Oladipo,S. A.Jonah,U.Sadiq, (2015) Elemental Analysis of Some Nigerian Food Legumes by ko-ENAA and INAA. World Journal of Nuclear Science and Technology,05,241-251. doi: 10.4236/wjnst.2015.53024
References
- 1. Jonah, S.A., Umar, I.M., Oladipo, M.O.A., Balogun, G.I. and Adeyemo, D.J. (2006) Standardization of NIRR-1 Irradiation and Counting Facilities for Instrumental Neutron Activation Analysis. Applied Radiation and Isotopes, 64, 818-822. http://dx.doi.org/10.1016/j.apradiso.2006.01.012
- 2. Sheyin, F.T., Oladipo, M.O.A., Jonah, S.A. and Sadiq, U. (2015) Determination of α in the Cadmium Lined Irradiation Channel of the NIRR-1 for Use in ko-ENAA. Unpublished.
- 3. Jonah, S.A., Sadiq, U., Okunade, I.O. and Funtua, I.I. (2009) The Use of the ko-IAEA Program in NIRR-1 NAA Laboratory. Journal of Radioanalytical and Nuclear Chemistry, 279, 1-7.
- 4. Bem, H. and Ryan, D.E. (1981) Choice of Boron Shield in Epithermal Neutron Activation Analysis Determinations. Analytica Chimica Acta, 124, 373-380.
http://dx.doi.org/10.1016/S0003-2670(01)93585-8 - 5. Hou, X., Wang, K. and Chai, C. (1996) Epithermal Neutron Activation and Its Application in the Miniature Neutron Source Reactor. Journal of Radioanalytical and Nuclear Chemistry, 210, 137-148.
http://dx.doi.org/10.1007/BF02055413 - 6. Bereznal, T., Bodizs, D. and Keomley, G. (1977) Investigation on the Comparator Technique used in Epithermal Neutron Activation Analysis. Journal of Radioanalytical and Nuclear Chemistry, 36, 509-524.
http://dx.doi.org/10.1007/BF02517018 - 7. Parry, S.J. (1982) Epithermal Neutron Activation Analysis of Short-Lived Nuclides in Geological Material. Journal of Radioanalytical and Nuclear Chemistry, 72, 195-207. http://dx.doi.org/10.1007/BF02516784
- 8. Zaghloul, R. (1986) Multielement Epithermal Neurton Activation Analysis Using an Internal Single Comparator. Journal of Radioanalytical and Nuclear Chemistry, 100, 215-218.
- 9. Zaghloul, R., Gantner, E., Mostafa, M. and Ache, N.J. (1987) Epithermal Neutron Actvation Analysis Using the Monostandard Method. Journal of Radioanalytical and Nuclear Chemistry, 109, 291-307.
http://dx.doi.org/10.1007/BF02037873 - 10. Garrison, R.G. and Somer, E. (1995) The Nutrition Desk Reference, 3rd Edition, Keats Publishing Inc., New Cannan.
- 11. Underwood, E.J. (1977) Trace Elements in Human and Animal Nutrition. 4th Edition, Academic Press, New York.
- 12. Wardlaw, G.M., Hampl, J.S. and Di Sivelstro, R.A. (2004) Perspectives in Nutrition. 6th Edition, McGraw Hill Companies, Inc., New York.
- 13. WHO (1996) Trace Elements in Human Nutrition and Health. World Health Organization, Geneva.
- 14. Sheyin, F.T. (2015) Characterization of the Cadmium Lined Irradiation Channel of the Nigeria Research Reactor-1 (NIRR-1) and Its Application for Elemental Determination in Some Legumes. Ph.D. Thesis, Ahmadu Bello University, Zaria.
- 15. De Corte, F. and Simonits, A. (2003) Recommended Nuclear Data for Use in the ko Standardization of Neutron Activation Analysis. Atomic Data and Nuclear Data Tables, 85, 47-67.
http://dx.doi.org/10.1016/S0092-640X(03)00036-6 - 16. Moens, L., De Corte, F., De Wispelaere, A., Hoste, J., Simonits, A., Elek, A. and Szabo, E. (1984) Part II: ko-Measurements and Related Nuclear Data Compilation for (n, γ) Reactor Neutron Activation Analysis. Journal of Radioanalytical and Nuclear Chemistry, 82, 385-452.
- 17. Simonits, A., Moens, L., De Corte, F., De Wispelaere, A., Elek, A. and Hoste, J. (1990) ko-Measurements and Related Nuclear Data Compilation For (n, γ) Reactor Neutron Activation Analysis, Part I. Journal of Radioanalytical and Nuclear Chemistry, 60, 461-516. http://dx.doi.org/10.1007/BF02518906
- 18. De Corte, F., Simonits, A., De Wispelaere, A. and Elek, A. (1989) ko-Measurements and Related Nuclear Data Compilation for (n, γ) Reactor Neutron Activation Analysis, IIIa: Experimental. Journal of Radioanalytical and Nuclear Chemistry, 133, 3-41.
- 19. De Corte, F. and Simonits, A. (1989) ko-Measurements and Related Nuclear Data Compilation For (n, γ) Reactor Neutron Activation Analysis, IIIb: Tabulation. Journal of Radioanalytical and Nuclear Chemistry, 133, 43-130.
- 20. El Nimr, T., De Corte, F., Moens, L. and Hoste, J. (1981) Epicadmium Neutron Activation Analysis (ENAA) Based on the ko-Comparator Method. Journal of Radioanalytical and Nuclear Chemistry, 67, 421-430.
http://dx.doi.org/10.1007/BF02516355 - 21. De Corte, F., Dejaeger, M., Hossain, S.M., Vandenberghe, D., De Wespelaere, A. and Van den Haute, P. (2005) A Performance Comparison of ko-Based ENAA and NAA in the (K, Th, U) Radiation Dose Rate Assessment for the Luminescence Dating of Sediments. Journal of Radioanalytical and Nuclear Chemistry, 263, 659-665.
- 22. Simonits, A., De Corte, F. and Hoste, J. (1975) Single Comparator Methods in Reactor Neutron Activation Analysis. Journal of Radioanalytical and Nuclear Chemistry, 24, 31-46.
http://dx.doi.org/10.1007/BF02514380 - 23. Liyu, W. (2004) CIAE/WINSPAN, Multi-Purpose Gamma-Ray Spectrum Analysis Software. China Institute of Atomic Energy, Beijing.
- 24. Reilly, C. (1980) Metal Contamination of Food. Applied Science Publishers, London, 46-63.
- 25. Waheed, S., Zaidi, J.H. and Ahmad, S. (2003) Instrumental Neutron Activation Analysis of 23 Individual Food Articles from a High Altitude Region. Journal of Radioanalytical and Nuclear Chemistry, 258, 73-81.
http://dx.doi.org/10.1023/A:1026253924758 - 26. Schroeder, H.A. (1976) The Trace Elements in Nutrition. Faber and Faber, London, 36-46.
NOTES
*Corresponding author.



