International Journal of Organic Chemistry
Vol. 2 No. 3 (2012) , Article ID: 23143 , 9 pages DOI:10.4236/ijoc.2012.23036
Synthesis and Antibacterial Activity of Urea and Thiourea Derivatives of Anacardic Acid Mixture Isolated from A Natural Product Cashew Nut Shell Liquid (CNSL)
1Medicinal Chemistry Laboratory, GVK Biosciences Pvt. Ltd., Hyderabad, India
2Centre for Environment, Institute of Science and Technology, JNT University, Hyderabad, India
3 Dr. MACS Bio-Pharma Pvt. Ltd., Westren Hills, Hyderabad, India
Email: *machari100@yahoo.com
Received May 10, 2012; revised June 25, 2012; accepted July 4, 2012
Keywords: Synthesis; Urea and Thiourea Derivatives; Anacardic Acid; Anti-Bacterial Activity
ABSTRACT
Synthesis and antibacterial activity of some novel urea and thiourea derivatives (8a - 8k, 9a - 9f) of anacardic acid prepared from commercially available anacardic acid which is obtained from natural product Cashew Nut Shell Liquid (CNSL). Compounds (8a - 8k, 9a - 9f) were tested for Gram positive and Gram negative bacterial cultures. Most of the compounds were showed active compared with standard drug ampicilline.
1. Introduction
Anacardic acid mixture (1a-d) isolated from a natural product Cashew Nut Shell Liquid (CNSL) which is a by-product of cashew nut industry and these are salicylic acid derivatives with a nonisoprenoid alk(en)yl side chain [1]. Kubo et al. reported the separation of anacardic acid into monoene (15:1), diene (15:2) and triene (15:3) by preparative HPLC and tested against cancer cells, and found to show moderate cytotoxic activity on BT-20 breast and HeLa epithelioid cervix carcinoma cells [2]. The emergence of drug resistant strains in clinical applications [3-5] especially to Gram positive bacteria [6,7] has created a problem of global proportions [8,9]. This phenomenon has led in creating novel antibacterial agents distinct from existing classes of compounds. Anacardic acid (pentadecyl salicylic acid) is a phenolic constituent present in Cashew Nut Shell Liquid (CNSL); (Anacardium occidentale L.) and exhibits antimicrobial properties [10,11] which have led to the preparation of various analogues [12,13]. Anacardic acid and its derivatives exhibits biological activities like antimicrobial activity [10,11] and soybean lipoxygenase-1 inhibitory activity[14,15]. G. C. Reddy et al. reported the synthesis of benzamide derivatives of anacardic acid [16], sildenafil analogues [17], dihydropyridine analogues [18] as calcium channel blockers, isonicotinoylhydra zones for antimycobacterial activity [19] starting from anacardic acid. Recently, a few anacardic acid derivatives exhibited various activities like affect the structure of the enzyme [20], anacardic acid is a specific activator of kinase activity of Aurora Kinase A [21], suppresses expression of nuclear factor-kB regulated gene products leading to potentiation of apoptosis [22] inhibitor of the HAT activity of recombinant Plasmodium falciparum GCN5 [23] and as modulators of histone acetyltransferases [24].
In the present work we wish to report to synthesize novel cell permeable urea and thiourea compounds from cheaply available anacardic acid which was a major constituent of Cashew Nut Shell Liquid (CNSL) natural source to evaluate their biological activity by various antibacterial strains. This report describes the synthesis, spectroscopic identification and antibacterial activity of some novel urea and thiourea anacardic acid derivatives against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pyogenes bacterial strains.
2. Results and Discussion
Here we described the synthesis of various biologically active novel urea and thiourea derivatives using anacardic acid mixture as starting material and various reagents in the given below conditions (Scheme 1).
The anacardic acid mixture (1a-d) was isolated from commercially available CNSL by a reported method [25].
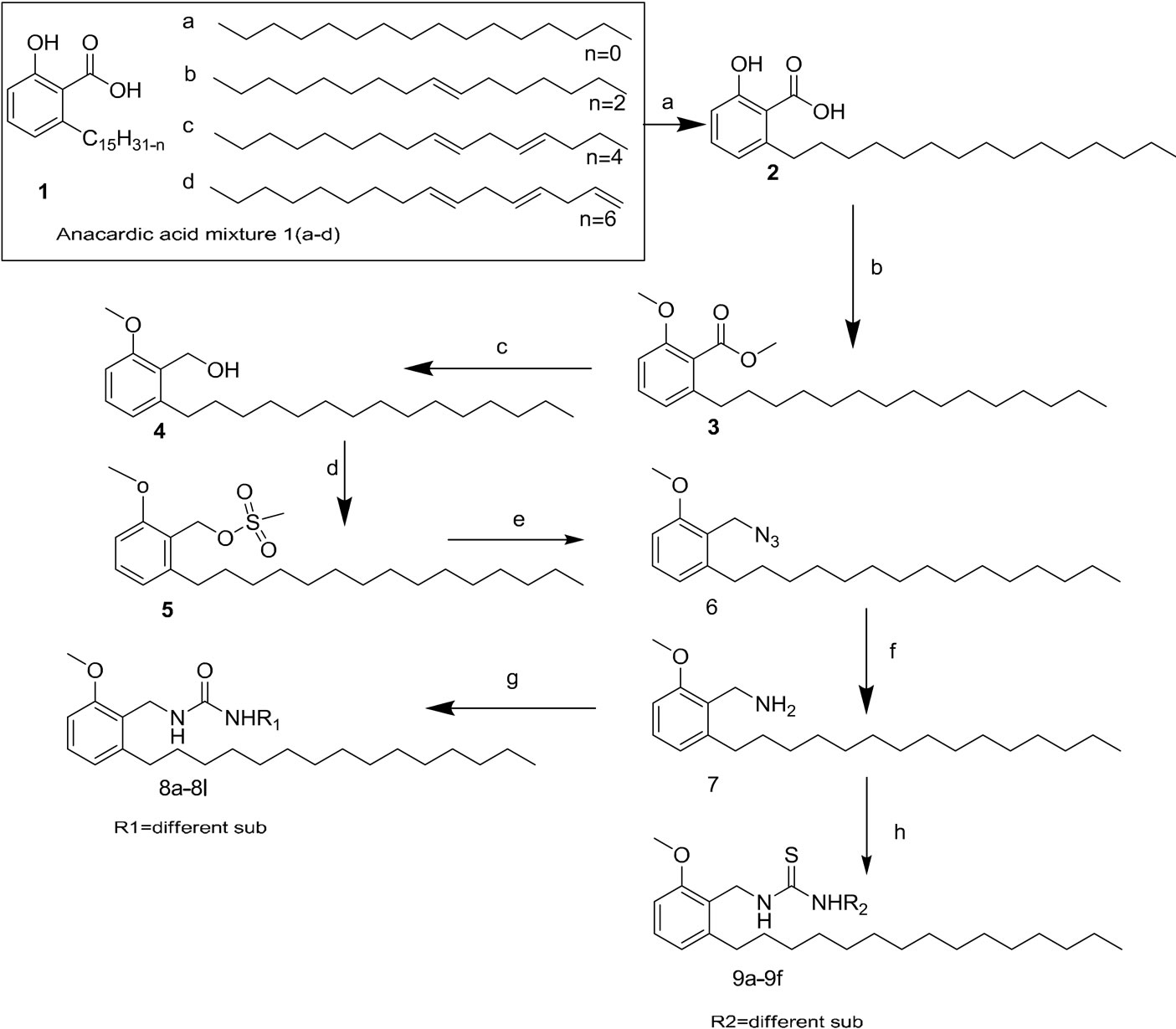
Scheme 1: Synthesis of various biologically active urea and thiourea derivatives from anacardic acid mixture.
Reagents: a) 10% Pd/C, EtOH, 50psi, RT, 2 hrs; b) Dimethyl sulfate, K2CO3, Acetonitrile, 90˚C, 24 hrs; c) LAH, THF, 0˚C-rt, 18 hrs; d) Methane sulphonyl chloride, Et3N, DCM, 0˚C-rt, 3 hrs; e) NaN3, DMF; f) 10% Pd/C, 50 psi, 2 hrs; g) different isocynate, CHCl3, h) different isothiocynate, CHCl3.
Accordingly CNSL was treated with calcium hydroxide, during which anacardic acid present in CNSL becomes calcium anacardate, which was isolated and hydrolyzed with dil. hydrochloric acid to generate anacardic acid ene mixture, which was a mixture of monoene, diene and triene located at (8’), (8’, 11’) and (8’, 11’, 14’) of the C15 alkyl chain respectively. Saturated anacardic acid was obtained by hydrogenation of the ene mixture of anacardic acid [17] and was further converted to dialkylated compound by reacting with dimethylsulfate in acetonitrile. Dialkylated anacardic acid was reduced to alcohol by treatment with lithium aluminium hydride in tetrahydrofuran and then protected with methane sulphonyl chloride in dichloromethane. Resultant mesylated compound was reacted with sodium azide followed by reduction with Pd/C under H2 pressure to obtained amine coupled with various isocynate or iso thiocynate in chloroform to obtain compounds (8a - 8k, 9a - 9f, Scheme 1) of urea and thiourea derivatives were purified by column chromatography to yield title compounds. The structure of urea and thiourea derivatives (8a - 8k, 9a - 9f) was determined by using different spectroscopic techniques 1H NMR, IR, Mass. The resulting compounds are screened for their antibacterial activity.
Biological Activity: The urea and thiourea derivatives (8a - 8k,9a - 9f) were screened for their antibacterial activity[26] against some of the pathogenic bacteria viz. E.coli (MTCC443), P. aeruginosa (MTCC424), S. aureus, (MTCC96) and S. pyogenes (MTCC443) using agar well diffusion method according to the literature protocol [26] The anti-bacterial activity of the analogues was compared with standard drug ampicilline and the results of investigation have been presented in Table 1 and observed that some of the compounds are showed high biological activity. Based on the test results it is evident that several of synthesized anacardic acid analogues

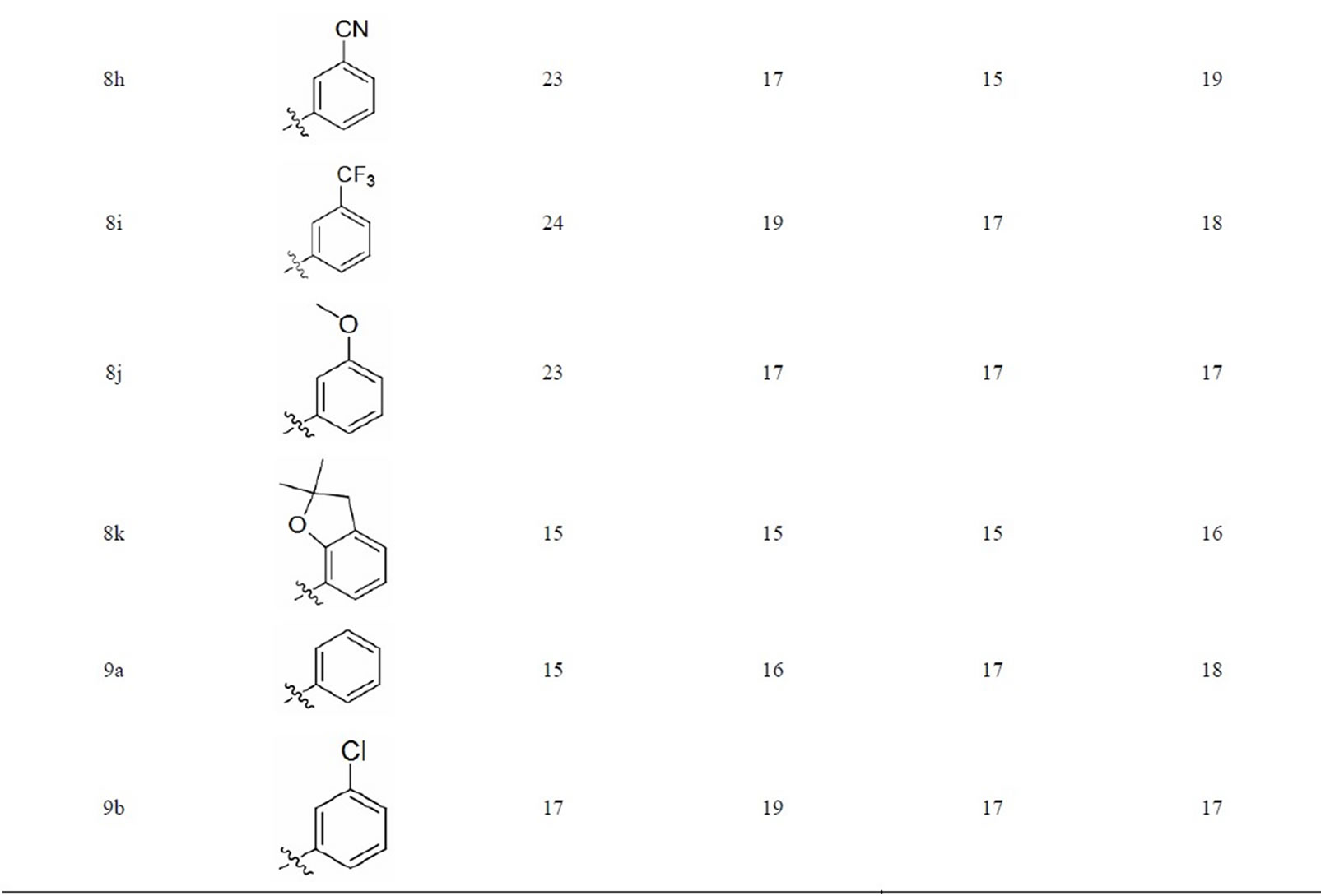
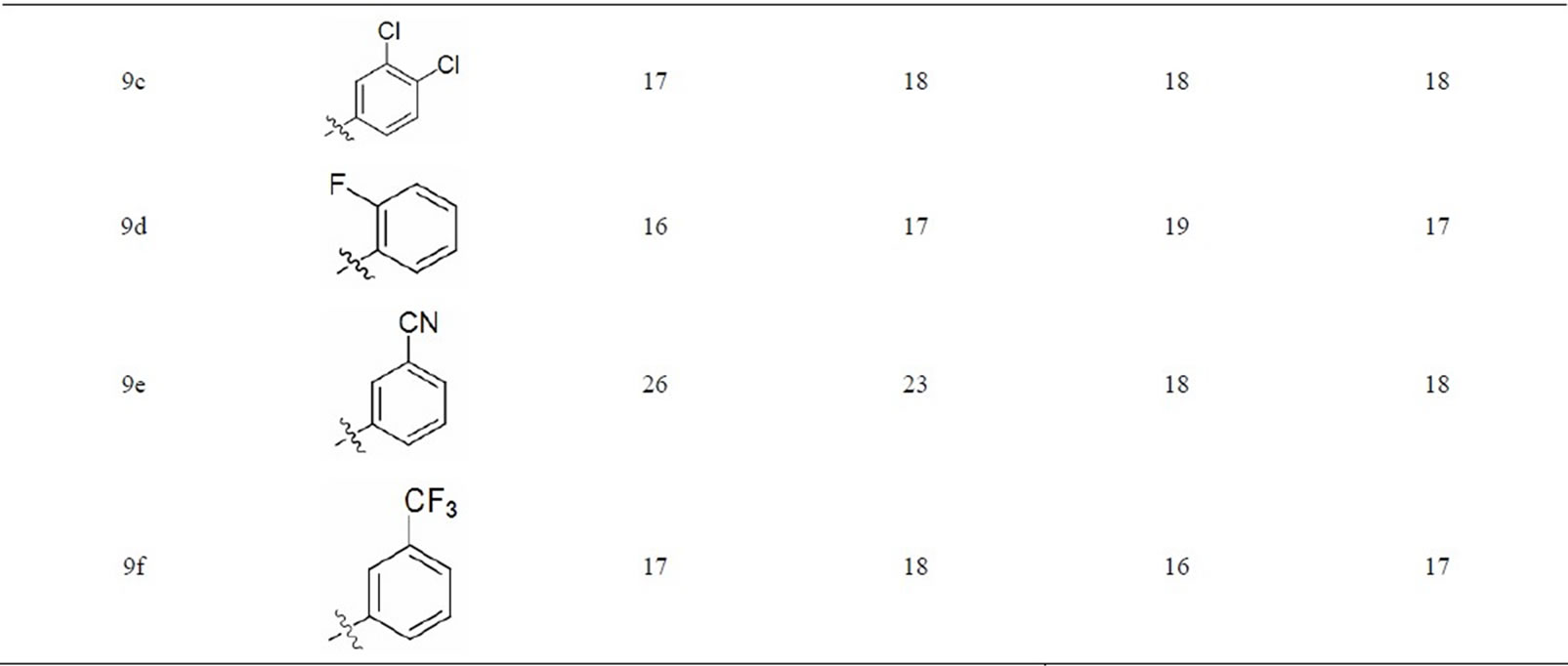
Table 1. Antibacterial activity of Urea and Thiourea derivatives of anacardic acid.
possess moderate to good activity against the Gram + ve and Gram – ve bacteria. Of all the compounds prepared entities 8h, 8i, 8j, 9e showed good activity against E. coli, 8b, 8c, 8e and 8f of E. coli MTCC443, and of P. aeruginosa MTCC424, display good to excellent activity while the remaining compounds showed moderate activity. The most active antibacterial agent against Escherichia coli found to be compound 8h, 8i, 8j, and 9e having -CN, -CF3 and -OCH3 groups and other compounds in the series exhibited moderate to good activity. The compound 9b, 9c, 9d and 9e showed good activity against almost all the strains and 9f also showed good activity against S. aureus MTCC96 and S. pyogenes MTCC442. This indicates chloro, dichloro, cyano and methoxy substituted compounds showed better activity when compared to other substituted groups. Here seems to be, thiourea substituted novel compounds are exhibiting better activity than urea substituted compounds. The activity depends to some extent on the R substituent, however all the compounds showed antibacterial activity. So other functionalities in the molecule will contribute to the activity as well. It may be suggested that the anacardic acid derivative with a suitable R may lead to a good antibacterial agent against all the Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pyogenes bacterial strains.
3. Conclusion
In summary, the present study describes a convenient and efficient protocol for the synthesis of sulfonamide derivatives by using anacardic acid mixture using various reagents and different conditions. We believe that this procedure is convenient, economic and a userfriendly process for the synthesis of these various novel urea and thiourea compounds from anacardic acid mixture. All compounds structures are supported by physico chemical and IR, NMR, Mass spectral data. Urea and thiourea derivatives were screened for their antibacterial activity against few bacterial strains and observed that some of the compounds are showed more biological activity than standards used.
4. Experimental Section
4.1. General Reagents and Equipment
All chemicals and solvents were obtained from Aldrich and Spectrochem., India and used without further purification. Column chromatographic separations were carried out on silica gel 60 - 120 mesh size. Melting points were determined in open glass capillaries on a Mel-temp apparatus and are uncorrected. The IR spectra were recorded on a Thermo Nicolet IR 200 FT-IR spectrometer as KBr pellets and the wave numbers were given in cm–1. The 1H and 13C NMR spectra of samples were recorded on a Varian EM-360, NMR spectrometer using TMS as an internal standard in CDCl3. The mass spectra were recorded on Jeol JMS-D 300 and Finnigan Mat b at 70 eV with an emission current of 100 µA.
Synthesis of 2-hydroxy-6-pentadecyl-benzoic acid (2):
A solution of compound 1 (100 g, 287.35 mmol) in ethanol (500 ml) was taken into a 1L Parr-hydrogenation vessel and added a suspension of 10% Pd/C (10 g, 10%) in 70 ml of ethanol under argon atmosphere and applied H2-pressure (50 psi) for 2h. Reaction mixture was filtered through celite bed and concentrated the filtrate under reduced pressure to obtained compound recrystallized in pet ether to get 2-hydroxy-6-pentadecyl-benzoic acid (2) as white color solid (70 g); (Yield: 70 g, 68.8%; white solid); M.p: 85˚C - 86˚C; IR (KBr): νmax 3010, 3002, 2918, 2851, 1655, 1450, 1246, 1214 cm–1; H1-NMR (400 MHz, CDCl3): δ 0.89 (t, 3H, J = 6.8 Hz), 1.27 (brs, 24H), 1.57 - 1.63 (m, 2H), 2.98 (t, 2H, J = 8 Hz), 6.78 (d, 1H, J = 7.6 Hz), 6.88 (d, 1H, J = 8.4 Hz), 7.37 (t, 1H, J = 8 Hz), 11.02 (bs, 1H); EI MS: m/z (rel.abund.%) 349 (M+, 100).
Synthesis of 2-methoxy-6-pentadecyl-benzoic acid methyl ester (3):
A solution of compound 2 (20 g, 57.471 mmol) in ACN (200 ml) was added K2CO3 (40.8 g, 287.35 mmol) and DMS (21.76 ml, 229.88 mmol). The content was heated refluxed for 24 hrs. Reaction mixture was filtered and distilled off filtrate, obtain residue was redissolved in ethyl acetate and washed with water (2 × 200 ml), brine solution (175 ml), dried over anhydrous Na2SO4, filtered and evaporated under vacuum to obtain 2-Methoxy-6-pentadecyl-benzoic acid methyl ester (3) as pale yellow solid 18.5 g; Yield: 85.5%; M.p: 36˚C - 37˚C; IR (KBr): νmax 3004, 2921, 2852, 1732, 1589, 1460, 1266, 1105, 1067, 954, 747 cm–1; H1-NMR (400 MHz, CDCl3) : δ 0.88 (t, 3H, J = 6.8 Hz), 1.25 (brs, 24H), 1.53 - 1.60 (m, 2H), 2.53 (t, 2H, J = 8 Hz), 3.81 (s, 3H), 3.90 (s, 3H), 6.75 (d, 1H, J = 8.4 Hz), 6.82 (d, 1H, J = 8 Hz), 7.25 (t, 1H, J = 8 Hz); 13C NMR (100 MHz, CDCl3): δ 14.07, 22.66, 29.32, 29.38, 29.48, 29.51, 29.61, 29.65, 31.12, 31.88, 33.45, 52.04, 55.80, 108.30, 121.45, 123.44, 130.17, 141.35, 156.20, 168.88; EI MS: m/z (rel.abund.%) 377 (M+, 100).
Synthesis of 2-Methoxy-6-pentadecyl-phenyl)-methanol (4):
A suspension of LiAlH4 (3.03 g, 79.787 mmol) in Dry THF (150 ml) was added compound 3 (20 g, 53.19 mmol) in THF (100 ml) drop wise over a period of 40 min at 0˚C. The content was slowly bringing it to room temp., stirred at room temp., for 18h. Reaction mixture was quenched with saturated brine solution (40 ml) at 0˚C diluted with ethyl acetate filtered (200 ml) and washed with ethyl acetate (100 ml), filtrate was washed with brine solution (200 ml) dried over anhydrous Na2SO4, filtered and evaporated under vacuum to obtain 2-Methoxy-6-pentadecyl-phenyl)-methanol (4) (15 gm) as off white solid; Yield: 81%; M.p. 60˚C - 62˚C; IR (KBr): νmax 3386, 3073, 2917, 2847, 1589, 1465, 1318, 1263, 1197, 1093, 1001, 739 cm–1; H1-NMR (400 MHz, CDCl3): δ 0.89 (t, 3H, J = 7.2 Hz), 1.27 (brs, 24H), 1.53 - 1.58 (m, 2H), 2.37 (t, 1H, J = 6.4 Hz), 2.68 (t, 2H, J = 6.4 Hz), 3.87 (s, 3H), 4.75 (d, 2H, J = 6.4 Hz) 6.77 (d, 1H, J = 8 Hz), 6.82 (d, 1H, J = 7.6 Hz), 7.20 (t, 1H, J = 8 Hz); 13C NMR (100 MHz, CDCl3): δ 14.07, 22.66, 29.32, 29.46, 29.57, 29.61, 29.65, 31.88, 32.10, 33.19, 55.37, 57.30, 108.01, 122.28, 126.83, 128.45, 142.62, 158.22; EI MS: m/z (rel.abund.%) 349 (M+, 100).
Synthesis of methanesulfonic acid 2-methoxy-6-pentadecyl-benzyl ester (5):
To a solution of compound 4 (15 g, 45.181 mmol) in DCM was added TEA (13.92 ml, 99.398 mmol) followed by mesyl chloride (4.2 ml, 54.21 mmol) at 0˚C over a period of 15 mins. The content was stirred at room temp., for 3 hrs. Reaction mixture was washed with water (150 ml), brine solution (200 ml) dried over anhydrous Na2SO4, filtered and evaporated under vacuum to obtain methanesulfonic acid 2-methoxy -6-pentadecyl-benzyl ester (5) (17 g, 92.59%) as offwhite color solid.
Synthesis of 2-(azidomethyl)-1-methoxy-3-penta-decyl-benzene (6):
To a solution of compound 5 (7.5 g, 17.605 mmol) in DMF (30 ml) was added sodium azide (1.49 g, 22.887 mmol), the content was heated at 100˚C for 2 hrs. Reaction mixture was cooled to room temp., and poured into cool water (150 ml) solid compound was precipitated filtered and dried to get Azide (6) (6.2 g, 94.4%) as white color solid; IR (KBr): νmax 3087, 3013, 2924, 2854, 2096, 1589, 1463, 1316, 1262, 1089, 782, 751 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.25 (brs, 24H), 1.52 - 1.56 (m, 2H), 2.63 (t, 1H, J = 7.6 Hz), 3.85 (s, 3H), 4.42 (s, 2H) 6.78 (d, 1H, J = 8 Hz), 6.83 (d, 1H, J = 8 Hz), 7.24 - 7.26 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 14.09, 22.67, 29.35, 29.48, 29.56, 29.66, 31.56, 31.91, 32.98, 45.14, 55.49, 108.11, 121.55, 121.97, 129.27, 143.70, 158.28; ESIMS(m/z): 331 (M-N3) (M + H)+.
Synthesis of 2-methoxy-6-pentadecyl-benzyl amine (7):
A solution of compound 6 (1.5 g, 4.021 mmol) in ethanol (30 ml) was taken into a 500 ml Parr-hydrogenation vessel and added a suspension of 10% Pd/C (250 mg, 10%) in 20 ml of ethanol under argon atmosphere and applied H2-pressure (60 psi) for 2 hrs. Reaction mixture was filtered through celite bed and concentrated the filtrate under reduced pressure to obtain 2-methoxy-6-pentadecyl-benzyl amine (7) (1.1 g, 78.8%) as a off-white solid. M.p.: 55-56˚C, IR (neat): νmax 3400, 3066, 2924, 2853, 1583, 1464, 1439, 1374, 1259, 1149, 1121, 1079, 879, 787, 742 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.89 (t, 3H, J = 7.2 Hz), 1.26 (brs, 24H), 1.51 - 1.57 (m, 2H), 1.76 (bs, 2H), 2.65(t, 2H, J = 7.6 Hz), 3.84 (s, 5H), 6.74 (d, 1H, J =8 Hz), 6.79 (d, 1H, J = 7.6 Hz), 7.15 (t, 1H, J = 7.6 Hz); 13C NMR (100 MHz, CDCl3): δ 14.06, 22.64, 29.31, 29.49, 29.57, 29.61, 31.88, 32.14, 33.14, 37.54, 55.24, 107.96, 122.02, 127.33, 129.76, 141.62, 157.87; MS (m/z): 348 (M + H)+.
Synthesis of urea and thiourea compounds:
A solution of amine (1.0 eq) in dry CHCl3 was taken in seal tube was added isocynate or iso thiocynate and stirred at room temperature for 3 hrs to 8 hrs reaction went to completed distilled off solvent crude compound was purified by column chromatography.
Synthesis of 1-ethyl-3-(2-methoxy-6-pentadecylbenzyl) urea (8a):
Using 7 and ethyl isocyanate as starting materials, the title compound 8a was obtained as a white solid (Yield = 63.1%); m.p.114˚C - 115˚C; IR (KBr): νmax 3335, 3050, 2967, 2921, 2849, 1622, 1576, 1467, 1260, 1095, 785, 732 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.12 (t, 3H, J = 7.2 Hz), 1.25 (brs, 24H), 1.48 - 1.58 (m, 2H), 2.73 (t, 2H, J = 7.8 Hz), 3.17-3.20 (m, 2H), 3.84 (s, 3H), 4.3 (brs, 1H), 4.40 (d, 2H, J = 6 Hz), 4.65 (bs, 1H), 6.73 (d, 1H, J = 8.4 Hz), 6.82 (d, 1H, J = 8 Hz) 7.18 (t, 1H, J = 7.6 Hz; ESIMS (m/z): 419 (M + H)+.
Synthesis of 1-cyclopentyl-3-(2-methoxy-6-pentdecylbenzyl) urea (8b):
Using 7 and cyclopentyl isocyanate as starting materials, the title compound 8b was obtained as a white solid (Yield = 37.8%); M.p.: 128˚C - 129˚C; IR (DCM film): νmax 3328, 2921, 2851, 1621, 1573, 1466, 1260, 1092, 782 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.24 - 1.37 (m, 26H), 1.50 - 1.66 (m, 6H), 1.88 - 1.96 (m, 2H), 2.73 (t, 2H, J = 7.6 Hz), 3.84 (s, 3H), 3.86 - 3.95 (m, 1H), 4.32 (bs, 1H), 4.40 (d, 2H, J = 5.2 Hz), 4.65 (s, 1H), 6.73 (d, 1H, J = 8.4 Hz), 6.82 (d, 1H, J = 7.6 Hz), 7.18 (t, 1H, J = 7.6Hz); 13C NMR (100 MHz, CDCl3): δ 14.09, 22.66, 23.56, 29.33, 29.63, 29.66, 31.89, 33.11, 33.58, 35.87, 52.15, 55.43, 107.89, 122.30, 125.19, 128.11, 143.25, 157.71, 157.97; ESIMS (m/z): 459 (M + H)+.
Synthesis of 1-(2-methoxy-6-pentadecylbenzyl)-3-phenylurea (8c):
Using 7 and phenyl isocyanate as starting materials, the title compound 8c was obtained as a white solid (Yield = 58.3%); M.p.105˚C - 106˚C; IR (KBr): νmax 3324, 3041, 2923, 2851, 1639, 1559, 1465, 1306, 1238, 1088, 912, 782, 729 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.24 (brs, 24H), 1.54 - 1.56 (m, 2H), 2.76 (t, 2H, J = 7.6 Hz), 3.82 (s, 3H), 4.49 (d, 2H, J = 5.6 Hz), 5.2 (brs, 1H), 6.25 (bs, 1H), 6.73 (d, 1H, J = 8.4 Hz), 6.82 (d, 1H, J = 7.6 Hz), 7.05 (m, 1H) 7.18 (t, 1H, J = 7.6 Hz), 7.24 - 7.28 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 13.90, 22.05, 28.67, 28.87, 29.00, 31.26, 31.56, 32.38, 34.15, 55.54, 108.34, 117.30, 120.80, 121.67, 124.97, 128.11, 128.54, 140.50, 142.29, 154.71, 157.97; ESIMS (m/z): 467 (M + H)+.
Synthesis of 1-(3-chlorophenyl)-3-(2-methoxy-6-pentadecylbenzyl)urea (8d):
Using 7 and 3-chlorophenyl isocyanate as starting materials, the title compound 8d was obtained as a off white solid (Yield = 80.8%); M.p.101˚C - 102˚C; IR (DCM film): νmax 3318, 2999, 2922, 2850, 1632, 1582, 1557, 1470, 1260, 1235, 1089, 770 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.87 (t, 3H, J = 7.2 Hz), 1.24 - 1.36 (brs, 24H), 1.48 - 1.6 (m, 2H), 2.75 (t, 2H, J = 8 Hz), 3.86 (s, 3H), 4.50 (d, 2H, J = 6 Hz), 5.16 (brs, 1H), 6.3 (brs, 1H), 6.75 (d, 1H, J = 7.6 Hz), 6.83 (d, 1H, J = 8 Hz), 6.99 (d, 1H, J = 8 Hz), 7.125 - 7.22 (m, 3H), 7.38 (s, 1H); ESIMS (m/z): 501 (M + H)+.
Synthesis of 1-(3,4-dichlorophenyl)-3-(2-methoxy-6- pentadecylbenzyl)urea (8e):
Using 7 and 3,4-dichlorophenyl isocyanate as starting materials, the title compound 8e was obtained as a white solid (Yield = 68.1%); M.p.118˚C - 119˚C; IR (DCM film): νmax 3355, 3288, 2955, 2920, 2848, 1634, 1582, 1549, 1466, 1381, 1264, 1120, 1088, 776 cm–1; 1H NMR (CDCl3, 400 MHz): δ 1.02 (t, 3H, J = 7.2 Hz), 1.38-1.5 (m, 24H), 1.62-1.67 (m, 2H), 2.88 (t, 2H, J = 7.6 Hz), 4.01 (s, 3H), 4.63 (d, 2H, J = 5.6 Hz), 5.25 (s, 1H), 6.5 (s, 1H), 6.91 (d, 1H, J = 8 Hz), 6.98 (d, 1H, J = 8 Hz), 7.29 - 7.45 (m, 3H), 7.67 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ 13.88, 22.07, 28.69, 28.92, 29.03, 31.26, 31.62, 32.41, 34.16, 55.54, 108.32, 117.30, 118.32, 121.69, 122.02, 124.62, 128.20, 130.30, 130.93, 140.68, 142.32, 154.28, 157.96; ESIMS (m/z): 535 (M + H)+.537 (chloro).
Synthesis of 1-(2-fluorophenyl)-3-(2-methoxy-6- pentadecylbenzyl)urea (8f):
Using 7 and 2-fluorohenyl isocyanate as starting materials, the title compound 8f was obtained as a white solid (Yield = 40.5%); M.p.: 110˚C - 111˚C; IR (DCM film): νmax 3321, 3066, 3002, 2923, 2851, 1639, 1601, 1560, 1462, 1310, 1258, 1092, 750 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.87 (t, 3H, J = 6.8 Hz), 1.24 - 1.36 (m, 24H), 1.51-1.56 (m, 2H), 2.76 (t, 2H, J = 8 Hz), 3.86 (s, 3H), 4.52 (d, 2H, J = 5.6 Hz), 5.16 (s, 1H), 6.4 (s, 1H), 6.75 (d, 1H, J = 8.4 Hz), 6.83 (d, 1H, J = 7.2 Hz), 6.85 - 7.08 (m, 3H), 7.20 (t, 1H, J = 8 Hz), 8.01-8.10 (m, 1H); ESIMS (m/z): 485 (M+H)+.
Synthesis of 1-(2-cyanophenyl)-3-(2-methoxy-6-pentadecylbenzyl)urea (8g):
Using 7 and 2-cyanophenyl isocyanate as starting materials, the title compound 8g was obtained as a white solid (Yield = 45.8%); M.p.: 140˚C - 141˚C; IR (DCM film): νmax 3325, 3037, 2918, 2847, 2225, 1702, 1641, 1550, 1468, 1298, 1240, 1087, 756 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.87 (t, 3H, J = 6.8 Hz), 1.24 - 1.4 (m, 24H), 1.49-1.57 (m, 2H), 2.78 (t, 2H, J = 8 Hz), 3.89 (s, 3H), 4.53 (d, 2H, J = 5.2 Hz), 5.39 (s, 1H), 6.76-6.83 (m, 3H), 7.02 (t, 1H), 7.19 - 7.21 (m, 1H), 7.47 - 7.51 (m, 2H), 8.31 (d, 1H, J = 8.4 Hz); ESIMS (m/z): 492 (M + H)+.
Synthesis of 1-(3-cyanophenyl)-3-(2-methoxy-6-pentadecylbenzyl)urea (8h):
Using 7 and 3-cyanophenyl isocyanate as starting materials, the title compound 8 hrs was obtained as a cream color solid (Yield = 70.6%); M.p.: 100˚C - 101˚C; IR (DCM film): νmax 3324, 3196, 3078, 2921, 2850, 2230, 1636, 1559, 1467, 1239, 1091, 882, 786, 731 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.24 (brs, 24H), 1.45 - 1.56 (m, 2H), 2.75 (t, 2H, J = 8 Hz), 3.89 (s, 3H), 4.50 (d, 2H, J = 5.2 Hz), 5.14 (brs, 1H), 6.5 (bs, 1H), 6.77 (d, 1H, J = 8 Hz), 6.84 (d, 1H, J = 8 Hz), 7.19 - 7.28 (m,2H), 7.35 (t, 1H, J = 7.6 Hz), 7.55 (d, 1H, J = 7.2 Hz), 7.69 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 14.08, 22.65, 29.32, 29.54, 29.59, 29.66, 31.88, 33.10, 35.69, 55.50, 108.03, 112.49, 118.80, 121.89, 122.39, 123.30, 124.34, 125.81, 128.43, 129.72, 140.26, 143.02, 154.86, 157.93; ESIMS (m/z): 492 (M + H)+.
Synthesis of 1-(2-methoxy-6-pentadecylbenzyl)-3-(3- (tri-fluoromethyl) phenyl) urea (8i):
Using 7 and 3-(trifluoromethyl)phenyl isocyanate as starting materials, the title compound 8i was obtained as a off white solid (Yield = 90.9%); M.p.: 88˚C - 89˚C; IR (DCM film): νmax 3323, 3088, 2922, 2851, 1638, 1560, 1454, 1332, 1253, 1169, 1122, 1076, 888, 755 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.87 (t, 3H, J = 6.8 Hz), 1.23 (brs, 24H), 1.48 - 1.57 (m, 2H), 2.74 (t, 2H, J = 7.2 Hz), 3.85 (s, 3H), 4.50 (d, 2H, J = 5.6 Hz), 5.19 (brs, 1H), 6.6 (bs, 1H), 6.75 (d, 1H, J = 8.4 Hz), 6.83 (d, 1H, J = 7.2 Hz), 7.19 (t, 1H, J =8 Hz), 7.24 - 7.26 (m, 1H), 7.36 (t, 1H, J = 8 Hz), 7.52 - 7.56 (m, 2H); ESIMS(m/z): 535 (M + H)+.
Synthesis of 1-(2-methoxy-6-pentadecylbenzyl)-3-(3- methoxyphenyl) urea (8j):
Using 7 and 3-methoxyphenyl isocyanate as starting materials, the title compound 8j was obtained as a off white solid (Yield = 69.9%); M.p.: 77˚C - 78˚C; IR (DCM film): νmax 3318, 2997, 2923, 2851, 1629, 1604, 1562, 1467, 1264, 1212, 1165, 1091, 844,779 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.24 (brs, 24H), 1.51 - 1.57 (m, 2H), 2.75 (t, 2H, J = 8 Hz), 3.76 (s, 3H), 3.82 (s, 3H), 4.49 (d, 2H, J = 5.2 Hz), 5.27 (t, 1H, J = 5.6 Hz), 6.31 (s, 1H), 6.59 - 6.62 (m, 1H), 6.72 - 6.82 (m, 3H), 6.94 (s, 1H), 7.14 - 7.20 (m, 2H); ESIMS(m/z): 497 (M+H)+.
Synthesis of 1-(2,2-dimethyl-2,3-dihydrobenzofuran-7yl)-3-(2-methoxy-6-pentadecylben zyl) urea (8k):
Using 7 and 2,2-dimethyl-2,3-dihydrobenzofuran-7yl isocyanate as starting materials, the title compound 8k was obtained as a light yellow solid (Yield = 66.8%); M.p.: 112˚C - 113˚C; IR (DCM film): νmax 3356, 3299, 3045, 2923, 2854, 1692, 1635, 1556, 1448, 1323, 1304, 1263, 1228, 1132, 1099, 1056, 879,759 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.87 (t, 3H, J = 7.2 Hz), 1.23 - 1.36 (m, 24H), 1.42 (s, 6H), 1.49 - 1.55 (m, 2H), 2.75 (t, 2H, J = 8 Hz), 3.00 (s, 2H), 3.83 (s, 3H), 4.50 (d, 2H, J = 5.2 Hz), 5.16 (bs, 1H), 6.13 (brs, 1H), 6.72 - 6.82 (m, 4H), 7.17 (t, 1H, J = 8.4 Hz), 7.60 (d, 1H, J =7.6 Hz); ESIMS (m/z): 537 (M + H)+.
Synthesis of 1-(2-methoxy-6-pentadecylbenzyl)-3-phenylthiourea (9a):
Using 7 and phenyl thioisocyanate as starting materials, the title compound 9a was obtained as a off white solid (Yield = 86.3%); M.p.: 71˚C - 72˚C; IR (DCM film): νmax 3387, 3192, 3005, 2924, 2853, 1590, 1529, 1461, 1309, 1253, 1171, 1081, 750, 695 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.25 - 1.38 (m, 24H), 1.53 - 1.57 (m, 2H), 2.83 (t, 2H, J = 8 Hz), 3.65 (s, 3H), 4.92 (d, 2H, J = 3.6 Hz), 6.67 (d, 1H, J = 8 Hz), 6.82 (d,1H, J = 7.6 Hz), 6.9 (bs, 1H), 7.13 - 7.19 (m, 3H), 7.23 - 7.29 (m, 1H), 7.36 - 7.40 (m, 2H), 7.52 (s, 1H); ESIMS (m/z): 483 (M + H)+.
Synthesis of 1-(3-chlorophenyl)-3-(2-methoxy-6- pentadecylbenzyl)thiourea (9b):
Using 7 and 3-chloro phenyl thioisocyanate as starting materials, the title compound 9b was obtained as a off white solid (Yield = 76.6%); M.p.: 81˚C - 82˚C; IR (DCM film): νmax 3332, 3089, 3029, 2921, 2849, 1634, 1583, 1558, 1469, 1259, 1088, 894, 860, 779 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.24 - 1.40 (m, 24H), 1.50 - 1.56 (m, 2H), 2.75 (t, 2H, J = 8 Hz), 3.85 (s, 3H), 4.49 (d, 2H, J = 6 Hz), 5.2 (s, 1H), 6.40 (s, 1H), 6.75 (d, 1H, J = 8 Hz), 6.82 (d, 1H, J = 7.2 Hz), 6.98 - 7.02 (m, 1H), 7.12 - 7.22 (m, 3H), 7.36-7.39 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 14.11, 22.68, 29.35, 29.62, 29.69, 31.91, 33.15, 35.81, 55.50, 108.00, 117.91, 119.89, 122.41, 122.99, 124.52, 128.37, 129.95, 134.59, 140.28, 143.15, 154.98, 157.96; ESIMS (m/z): 517 (M + H)+.
Synthesis of 1-(3,4-dichlorophenyl)-3-(2-methoxy-6- pentadecylbenzyl) thiourea (9c):
Using 7 and 3,4-dichloro phenyl thioisocyanate as starting materials, the title compound 9c was obtained as a off white solid (Yield = 73.5%); M.p.:92˚C - 93˚C; IR (DCM film): νmax 3262, 3052, 3003, 2924, 2853, 1587, 1529, 1469, 1313, 1255, 1128, 1084, 1031, 775 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.25 - 1.40 (m, 24H), 1.52 - 1.60 (m, 2H), 2.8 (brs, 2H), 3.77 (s, 3H), 4.90 (s, 2H), 6.72 - 7.01 (m, 4H), 7.18 - 7.3 (m, 2H), 7.41 - 7.51 (m, 2H); ESIMS (m/z): 551 (M + H)+. 553 (chloro).
Synthesis of 1-(2-fluorophenyl)-3-(2-methoxy-6-pentadecylbenzyl)thiourea (9d):
Using 7 and 2-fluoro-phenyl thioisocyanate as starting materials, the title compound 9d was obtained as a off white solid (Yield = 87.9%); M.p.: 80˚C - 81˚C; IR (DCM film): νmax 3276, 3224, 3000, 2923, 2852, 1585, 1531, 1462, 1318, 1257, 1085, 860, 752 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.25 - 1.40 (m, 24H), 1.51 - 1.60 (m, 2H), 2.8 (bs, 2H), 3.69 (s, 3H), 4.91 (s, 2H), 6.63 - 6.66 (m, 1H), 6.82 - 6.90(m, 2H), 7.14 - 7.3 (m, 5H); ESIMS (m/z): 501 (M + H)+.
Synthesis of 1-(2-methoxy-6-pentadecylbenzyl)-3- cyano phenylthiourea (9e):
Using 7 and 3-cyano phenyl thioisocyanate as starting materials, the title compound 9e was obtained as a yellow solid (Yield = 82.1%); M.p.: 81˚C - 82˚C; IR (DCM film): νmax 3349, 2929, 2853, 2228, 1587, 1529, 1463, 1258, 1085, 757 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.25 - 1.38 (m, 24H), 2.50 - 2.60 (m, 2H), 2.79 (bs, 2H), 3.78 (s, 3H), 4.92 (s, 2H), 6.74 (d, 1H, J =7.6 Hz), 6.85 (d, 1H, J = 7.2 Hz), 7.20 - 7.29 (m, 3H), 7.55 - 7.66 (m, 3H); ESIMS (m/z): 508 (M + H)+.
Synthesis of 1-(2-methoxy-6-pentadecylbenzyl)-3-(3- (trifluoromethyl) phenyl) thiourea (9f):
Using 7 and 3- (trifluoromethyl)phenyl thioisocyanate as starting materials, the title compound 9f was obtained as a off white solid (Yield = 84.2%); IR (DCM film): νmax 3374, 3204, 2922, 2852, 1586, 1530, 1459, 1328, 1256, 1126, 1075, 894, 780 cm–1; 1H NMR (CDCl3, 400 MHz): δ 0.88 (t, 3H, J = 6.8 Hz), 1.25 - 1.38 (m, 24H), 1.54 - 1.56 (m, 2H), 2.82 (brs, 2H), 3.69 (s, 3H), 4.92 (s, 2H), 6.65-6.84 (m, 3H), 7.18 - 7.21 (m, 1H), 7.28 - 7.6 (m, 4H); ESIMS (m/z): 551 (M+H)+.
Antibacterial Bioassay [26]:
Urea and thiourea derivatives of Anacardic acid (8a - 8k, 9a - 9f) were dissolved in dimethyl sulphoxide at 250 μg/ml concentration. The composition of nutrient agar medium was Bactotryptone (10 g), yeast extract (5 g), NaCl (10 g), final pH 7.4. After 18 h the exponentially growing cultures of the six bacteria in nutrient broth at 37˚C were diluted in sterile broth. From each of these diluted cultures, 1mL was added to 100 mL sterilized and cooled nutrient agar media to give a final bacterial count of 1 × 106 cell/ml. The plates were set at room temperature and later dried at 37˚C for 20 hrs. Paper discs (6 mm, punched from Whatmann no. 41 paper) were ultraviolet sterilized and used for the assays. Discs were soaked in different concentration of the test solution and placed on the inoculated agar media at regular intervals of 6 - 7 cm, care was taken to ensure that excess solution was not on the discs. All the samples were taken in triplicates. The plates were incubated at 37˚C in an inverted fashion. Activity was determined by zones showing complete inhibition (mm). Growth inhibition was calculated with reference to positive control.
5. Acknowledgements
We thank GVK Biosciences Private Limited for the financial support and encouragement. We also thankful to the analytical department for their analytical data and to Dr. Balaram Patro for his helpful suggestions.
REFERENCES
- J. H. P. Tyman, “Non-Isoprenoid Long Chain Phenols,” Chemical Society Reviews, Vol. 8, No. 4, 1979, pp. 499- 537. doi:10.1039/cs9790800499
- I. Kubo, M. Ochi, P. C. Viera and S. Komatsu, “Antitumor Agents from the Cashew (Anacardium occidentale) apple juice,” Journal of Agricultural and Food Chemistry, Vol. 41, No. 6, 1993, pp. 1012-1015. doi:10.1021/jf00030a035
- M. J. Rybak and R. L. Akins, “Emergence of Methicillin-Resistant Staphylococcus Aureus with Intermediate Glycopeptide Resistance: Clinical Significance and Treatment Options,” Drugs, Vol. 61, No. 1, 2001, pp. 1-7. doi:10.2165/00003495-200161010-00001
- M. Lipsitch, “The Rise and Fall of Antimicrobial Resistance,” Trends Microbiology, Vol. 9, No. 9, 2001, pp. 438-444. doi:10.1016/S0966-842X(01)02130-8
- B. A. Cunha, Drugs Today, Vol. 34, 1998, pp. 691-698.
- A. Marchese, G. C. Schito and E. A. Debbia, “Evolution of Antibiotic Resistance in Gram-Positive Pathogens,” Journal of Chemotherapy, Vol. 12, No. 6, 2000, pp. 459- 462.
- Y. Cetinkaya, P. Falk and C. G. Mayhall, “Vancomycin-Resistant Enterococci,” Clinical Microbiology Reviews, Vol. 13, No. 4, 2000, pp. 686-707. doi:10.1128/CMR.13.4.686-707.2000
- G. H. Cassell and J. Mekalanos, “Development of Antimicrobial Agents in the Era of New and Reemerging Infectious Diseases and Increasing Antibiotic Resistance,” Journal of the American Medical Association, Vol 285, No. 5, 2001, pp. 601-605. doi:10.1001/jama.285.5.601
- D. T. W. Chu, J. J. Plattner and L. Katz, “New Directions in Antibacterial Research,” Journal of Medicinal Chemistry, Vol. 39, No. 20, 1996, pp. 3853-3874. doi:10.1021/jm960294s
- I. Kubo, H. Muroi and M. Himejima, “Structure-Anti Bacterial Activity Relationships of Anacardic Acids,” Journal of Agricultural and Food Chemistry, Vol. 41, No. 6, 1993, pp. 1016-1019. doi:10.1021/jf00030a036
- J. L. Gillerman, N. J. Walsh, N. K. Werner and H. Schlenk, “Antimicrobial Effects of Anacardic Acids,” Canadian Journal of Microbiology, Vol. 15, No. 10, 1969, pp. 1219- 1223. doi:10.1139/m69-220
- A. S. Gulati and B. C. Subba Rao, Indian Journal of Chemistry, Vol. 2, 1964, pp.337.
- M. A. EIShol, P. D. Adawadkar, D. A. Benign, E. S. Watson and T. L. Little Jr., “6-(Alkylamino)-9-benzyl- 9H-purines. A New Class of Anticonvulsant Agents,” Journal of Medicinal Chemistry, Vol. 29, 1986, pp. 606- 612.
- S. V. Shoba, C. S. Ramadoss and B. Ravindranath, “Inhibition of Soybene Lipoxygenase-1 by Anacardic Acids, Cardol, and Cardanols,” Journal of Natural Products, Vol. 57, No. 12, 1994, pp. 1755-1757. doi:10.1021/np50114a025
- T. J. Ha and I. Kubo, “Lipoxygenase Inhibitory Activity of Anacardic Acids,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 11, 2005, pp.4350-4354. doi:10.1021/jf048184e
- V. Chandregowda, A. Kush and G. C. Reddy, “Synthesis of Benzamide Derivatives of Anacardic Acid and Their Cytotoxic Activity,” European Journal of Medicinal Chemistry, Vol. 44, No. 6, 2009, pp. 2711-2719. doi:10.1016/j.ejmech.2009.01.033
- R. Paramashivappa, P. Phanikumar, P. V. Subba Rao and A. Srinivasa Rao, “Synthesis of Sildenafil Analogues from Anacardic Acid and Their Phosphodiesterase-5 Inhibition,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 26, 2002, pp. 7709-7713. doi:10.1021/jf0258050
- P. Phanikumar, S. C. Stotz, R. Paramashivappa, M. Beedle, G. W. Zamponi and A. Srinivasa Rao, “Synthesis and Evaluation of a New Class of Nifedipine Analogs with T-Type Calcium Channel Blocking Activity,” Molecular Pharmacology, Vol. 61, No. 3, 2002, pp. 649-658. doi:10.1124/mol.61.3
- B. Narayana Swamy, T. K. Suma, G. Venkateswara Rao and G. Chandrasekara Reddy, European Journal of Medicinal Chemistry, Vol. 42, 2007, pp. 422-424.
- K. Mantelingu, A. H. Kishore, K. Balasubramanyam, G. V. Kumar, M. Altaf, S. N. Swamy, R. Selvi, C. Das, C. Narayana, K. S. Rangappa and T. K. Kundu, “Activation of p300 Histone Acetyltransferase by Small Molecules Altering Enzyme Structure: Probed by Surface-Enhanced Raman Spectroscopy,” Journal of Physical Chemistry B, Vol. 111, No. 17, 2007, pp. 4527-4534. doi:10.1021/jp067655s
- A. Hari Kishore, B. M. Vedamurth, K. Mantelingu, S. Agrawal, B. A. Ashok Redd, S. Roy, K. S. Rangappa and T. K. Kundu, “Specific Small-Molecule Activator of Aurora Kinase A Induces Autophosphorylation in a CellFree System,” Journal of Medicinal Chemistry, Vol. 51, No. 4, 2008, pp. 792-797. doi:10.1021/jm700954w
- B. Sung, M. K. Pande, K. S. Ahn, T. Yi, M. M. Chaturved, M. Liu and B. B. Aggarwal, “Anacardic Acid (6-Nonadecyl Salicylic Acid), an Inhibitor of Histone Acetyltransferase, Suppresses Expression of Nuclear Factor-κB-Regulated Gene Products Involved in Cell Survival, Proliferation, Invasion, and Inflammation through Inhibition of the Inhibitory Subunit of Nuclear Factor- κBα Kinase, Leading to Potentiation of Apoptosis,” Blood, Vol. 111, No. 10, 2008, pp. 4880-4891. doi:10.1182/blood-2007-10-117994
- L. Cui, J. Miao, T. Furuya, Q. Fan, X. Li, P. K. Rathod, X. Z. Su and L. Cui, “Histone Acetyltransferase Inhibitor Anacardic Acid Causes Changes in Global Gene Expression during in Vitro Plasmodium Falciparum Development,” Eukaryotic Cell, Vol.7, No. 7, 2008, pp. 1200- 1210. doi:10.1128/EC.00063-08
- G. Sbardella, S. Castellano, C. Vicidomini, D. Rotili, A. Nebbioso, M. Miceli, L. Altucci and A. Mai, “Identification of Long Chain Alkylidenemalonates as Novel Small Molecule Modulators of Histone Acetyl-Transferases,” Bioorganic & Medicinal Chemistry Letters, Vol. 18, No. 9, 2008, pp. 2788-2792. doi:10.1016/j.bmcl.2008.04.017
- R. Paramashivappa, P. Phanikumar, P. J. Vithayathil and A. Srinivasa Rao, “Novel Method for Isolation of Major Phenolic Constituents from Cashew (Anacardium occidentale L.) Nut Shell Liquid,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 5, 2001, pp. 2548-2551. doi:10.1021/jf001222j
- A. Rahman, M. L. Choudhary and W. J. Thomson, “Bioassay Techniques for Drug Development,” Harwood Academic Publishers, The Netherlands, 2001. doi:10.4324/9780203304532
NOTES
*Corresponding author.

