 Vol.1, No.3, 166-175 (2009) doi:10.4236/ns.2009.13021 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ Natural Science Ion exchange recovery of palladium (II) from nitrate weak acidic solutions Olga N. Kononova1*, Nataliya G. Goryaeva1, Olga V. Dychko1 1Department of Chemistry, Siberian Federal University, Krasnoyarsk, Russian Federation; cm2@bk.ru Received 12 August 2009; revised 2 September 2009; accepted 4 September 2009. ABSTRACT Sorption recovery of palladium (II) from nitrate weak acidic model solutions and solutions of spent catalysts on some ion exchangers with different physical and chemical structure has been investigated. The palladium concentration in contacting solutions was 5.0 · 10-5 – 1.0 · 10-3 mol/L at nitric acid and potassium nitrate con- centrations 0.01 and 1.0 mol/L, respectively. It was shown that anion exchangers AV-17-8 as well as Purolite S 985 and A 500 possess the best sorption and kinetic properties. These sorbents can be recommended for selective recovery of palladium from solutions of spent catalysts. Keywords: Palladium; Ion Exchange; Anion Exchangers; Nitrate Solutions 1. INTRODUCTION As natural deposits of precious metals are being depleted, the technologies for precious metals recovery from dif- ferent secondary raw materials are becoming more im- portant. The hydrometallurgical methods are success- fully used for these purposes [1-3]. One of the most promising methods for recovery of platinum group met- als (in particular, of palladium) is sorption, characterized by high efficiency and selectivity [1,3-5]. However, the majority of investigations devoted to the sorption recov- ery of palladium, deal with chloride or sulfate solutions [6-14], and the studies of sorption of noble metals from nitric acidic media are rather limited [1,15-20]. At the same time, the recovery of palladium from some kinds of secondary sources (e.g. electronic scrap and ex- hausted nuclear fuel) requires its isolation from nitric acidic and nitrate solutions [1,21]. Our previous investigations were focused on sorption recovery of palladium from chloride model solutions and solutions of spent catalysts by various ion ex- changers and carbon adsorbents [9,22,23]. Apart from this, we started the research of sorption concentration of palladium on carbon adsorbents during its recovery from model nitric acidic solutions [24]. We have revealed high sorption abilities of some carbon adsorbents to palla- dium (II) ions depending on initial concentrations of nitric acid andPd . )(II The present paper is focused on sorption recovery of palladium from nitrate weak acidic solutions (model and of spent catalysts) by some ion exchangers with different physical and chemical structure. 2. MATERIALS AND METHODS 2.1. Characteristics of Ion Echangers Some ion exchangers from various manufacturers were taken for investigation. These sorbents possess different physical and chemical structure. Their physical-chemical characteristics are summarized in Table 1. It should be noted that ion exchangers produced by Purolite Com- pany for the first time were used for recovery of plati- num group metals. However, these ion exchangers were successfully applied in our previous investigations on sorption recovery of gold and silver [25]. Before sorption all the ion exchangers were prepared according to the standard procedures and then loaded with 1 M solution, in order to convert them to chloride form (anion exchangers) or to NaCl ,Na Cl -form (amphoteric resin). The acid-base properties of ion exchangers investi- gated were studied by a potentiometric titration with the glass electrode. Based on the experimental data obtained, we have calculated the average apparent ionization con- stants of functional groups of ion exchangers [5]. The calculation procedure is described below and the values of constants are presented in Table 1. 2.2. Preparation of Palladium Ntrate Solutions The initial model stock solution of palladium was pre- pared according to works [26,27]. The accurately 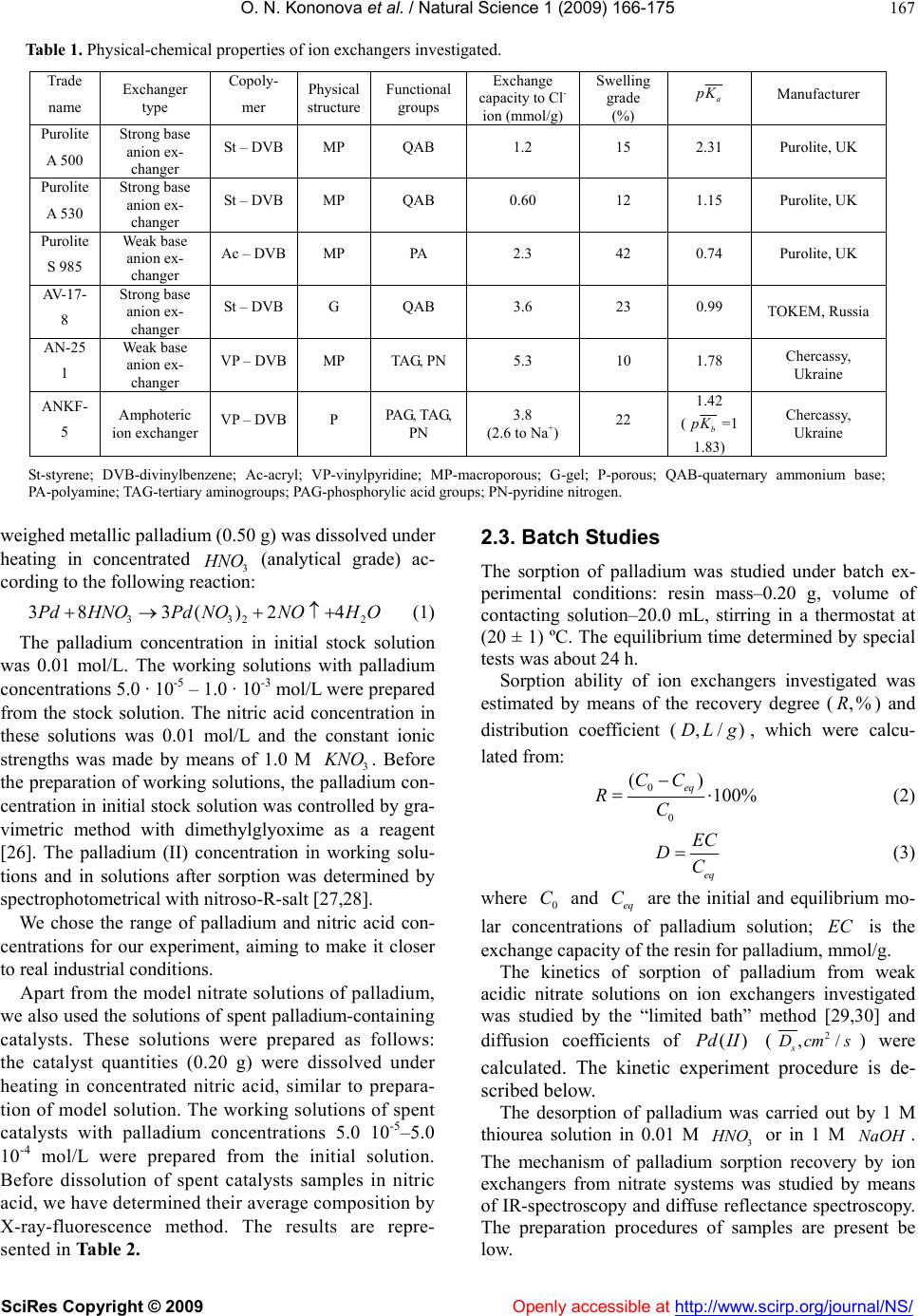 O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 167 Table 1. Physical-chemical properties of ion exchangers investigated. Trade name Exchanger type Copoly- mer Physical structure Functional groups Exchange capacity to Cl- ion (mmol/g) Swelling grade (%) a K Manufacturer Purolite A 500 Strong base anion ex- changer St – DVB MP QAB 1.2 15 2.31 Purolite, UK Purolite A 530 Strong base anion ex- changer St – DVB MP QAB 0.60 12 1.15 Purolite, UK Purolite S 985 Weak base anion ex- changer Ac – DVB MP PA 2.3 42 0.74 Purolite, UK AV-17- 8 Strong base anion ex- changer St – DVB G QAB 3.6 23 0.99 TOKEM, Russia AN-25 1 Weak base anion ex- changer VP – DVB MP TAG, PN 5.3 10 1.78 Chercassy, Ukraine ANKF- 5 Amphoteric ion exchanger VP – DVB P PAG, TAG, PN 3.8 (2.6 to Na+) 22 1.42 (b K=1 1.83) Chercassy, Ukraine St-styrene; DVB-divinylbenzene; Ac-acryl; VP-vinylpyridine; MP-macroporous; G-gel; P-porous; QAB-quaternary ammonium base; PA-polyamine; TAG-tertiary aminogroups; PAG-phosphorylic acid groups; PN-pyridine nitrogen. weighed metallic palladium (0.50 g) was dissolved under heating in concentrated 3 (analytical grade) ac- cording to the following reaction: HNO 332 383()2 4PdHNOPdNONOH O 2 (1) The palladium concentration in initial stock solution was 0.01 mol/L. The working solutions with palladium concentrations 5.0 · 10-5 – 1.0 · 10-3 mol/L were prepared from the stock solution. The nitric acid concentration in these solutions was 0.01 mol/L and the constant ionic strengths was made by means of 1.0 M 3 NO . Before the preparation of working solutions, the palladium con- centration in initial stock solution was controlled by gra- vimetric method with dimethylglyoxime as a reagent [26]. The palladium (II) concentration in working solu- tions and in solutions after sorption was determined by spectrophotometrical with nitroso-R-salt [27,28]. We chose the range of palladium and nitric acid con- centrations for our experiment, aiming to make it closer to real industrial conditions. Apart from the model nitrate solutions of palladium, we also used the solutions of spent palladium-containing catalysts. These solutions were prepared as follows: the catalyst quantities (0.20 g) were dissolved under heating in concentrated nitric acid, similar to prepara- tion of model solution. The working solutions of spent catalysts with palladium concentrations 5.0 10-5–5.0 10-4 mol/L were prepared from the initial solution. Before dissolution of spent catalysts samples in nitric acid, we have determined their average composition by X-ray-fluorescence method. The results are repre- sented in Table 2. 2.3. Batch Studies The sorption of palladium was studied under batch ex- perimental conditions: resin mass–0.20 g, volume of contacting solution–20.0 mL, stirring in a thermostat at (20 ± 1) ºС. The equilibrium time determined by special tests was about 24 h. Sorption ability of ion exchangers investigated was estimated by means of the recovery degree () and distribution coefficient (, which were calcu- lated from: ,%R ,/)DL g 0 0 () 100% eq CC RC (2) eq EC DC (3) where and are the initial and equilibrium mo- lar concentrations of palladium solution; is the exchange capacity of the resin for palladium, mmol/g. 0 Ceq C EC The kinetics of sorption of palladium from weak acidic nitrate solutions on ion exchangers investigated was studied by the “limited bath” method [29,30] and diffusion coefficients of ( ()Pd II2 ,/ s cms ) were calculated. The kinetic experiment procedure is de- scribed below. The desorption of palladium was carried out by 1 M thiourea solution in 0.01 M or in 1 M . The mechanism of palladium sorption recovery by ion exchangers from nitrate systems was studied by means of IR-spectroscopy and diffuse reflectance spectroscopy. The preparation procedures of samples are present be 3 HNO NaOH low.  O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 168 Table 2. Average composition of initial sample of palla- dium-containing spent catalyst. All the results were statistically processed by standard methods [31,32]. The average experimental error for 3-4 parallel runs was below 6 %. 2.4. Calculation of Apparent Constants of Acid-Base Ionization of Ion Exchangers The constants values were calculated using potentiomet- ric titration data. For each point of the titration curve, the functional groups content was determined and the ap- parent ionization degree ( ) of the resin was calculated: 0 [] C (4) where [] is the equilibrium concentration of ions in the ion exchanger phase, mmol/mL; is the initial concentration of the titrant solution (0.1 M 0 C Cl ). Then a curve was plotted on the coordinates (log ) 1 pH f and at 0.5 , the apparent acid-base ionization constants of functional groups of the ion exchangers (a pK ) were calculated from Hen- derson’s equation: log( ) 1 a pKpH m (5) where is the slope angle tangent of the curve. m 2.5. “Limited Bath” Method for Sorption Kinetics of Palladium (Ii) The quantities of preswollen resin (0.10 g) were stirred with 25.0 mL of palladium nitrate solutions at (20 ± 1)ºС over a period of 30 s to 24 h. The suspensions were in- tensively stirred (more than 800 rev/min). After a certain time period, the resins and solutions were quickly sepa- rated and the concentration of was determined in the solutions. Then the exchange degree ( ()Pd II ) was calculated from t Q FQ (6) where and t Q Q are the amounts (in mmol) of the palladium sorbed to the time (s) and to the equilib- rium time. t According to the Boyd’s method [29,30,33], the ki- netic coefficient was calculated from B 22 (1.08) Bt (7) The data obtained were plotted as a function()Btf t . If the process is controlled by gel diffusion [29,30,33], this function should be linear. After that, the diffusion coefficients ( D) were calculated according to the equa- tion: 2 2 s Br D (8) where is the radius of the resin grain (cm). The half-exchange time of the kinetic process () was calculated as follows: 1/ 2 t 2 1/2 2 4 r tD (9) 2.6. Preparation of Sample for FT-IR-Spectroscopy IR–spectra of ion exchangers investigated were recorded by means of FT-IR- spectrometer Vector 22 (Bruker). Before that, ion exchanger samples were dried during 4 h at 40ºС in convection drier. Then the samples were held in a vacuum-desiccator over freshly calcinated cal- cium chloride. The specimens were ground in a me- chanical mill without air access and after that were pressed with spectrally pure Br to discs. The quanti- ties of resin samples and potassium bromide were con- stant (200 mg each of resin and Br ). 2.7. Preparation of Samples for Diffuse Reflectance Spectroscopy The diffuse reflectance spectra were recorded by means of spectrometer PULSAR (Russia). The resin quantities (0.20 g) were preliminary saturated with palladium (II) ions with concentration 5.0· 10-4 mol/L during 24 h. Af- ter that, the resins were filtered and wet samples were placed into cell. Then the diffuse reflectance spectra Component Content (%) Palladium 0.79 Sodium 0.41 Aluminum oxide ~ 84 Silicon 0.03 Sulfur 0.12 Chlorine 0.80 Iron 0.19 Nickel < 0.02 Zinc < 0.02 Gallium < 0.02 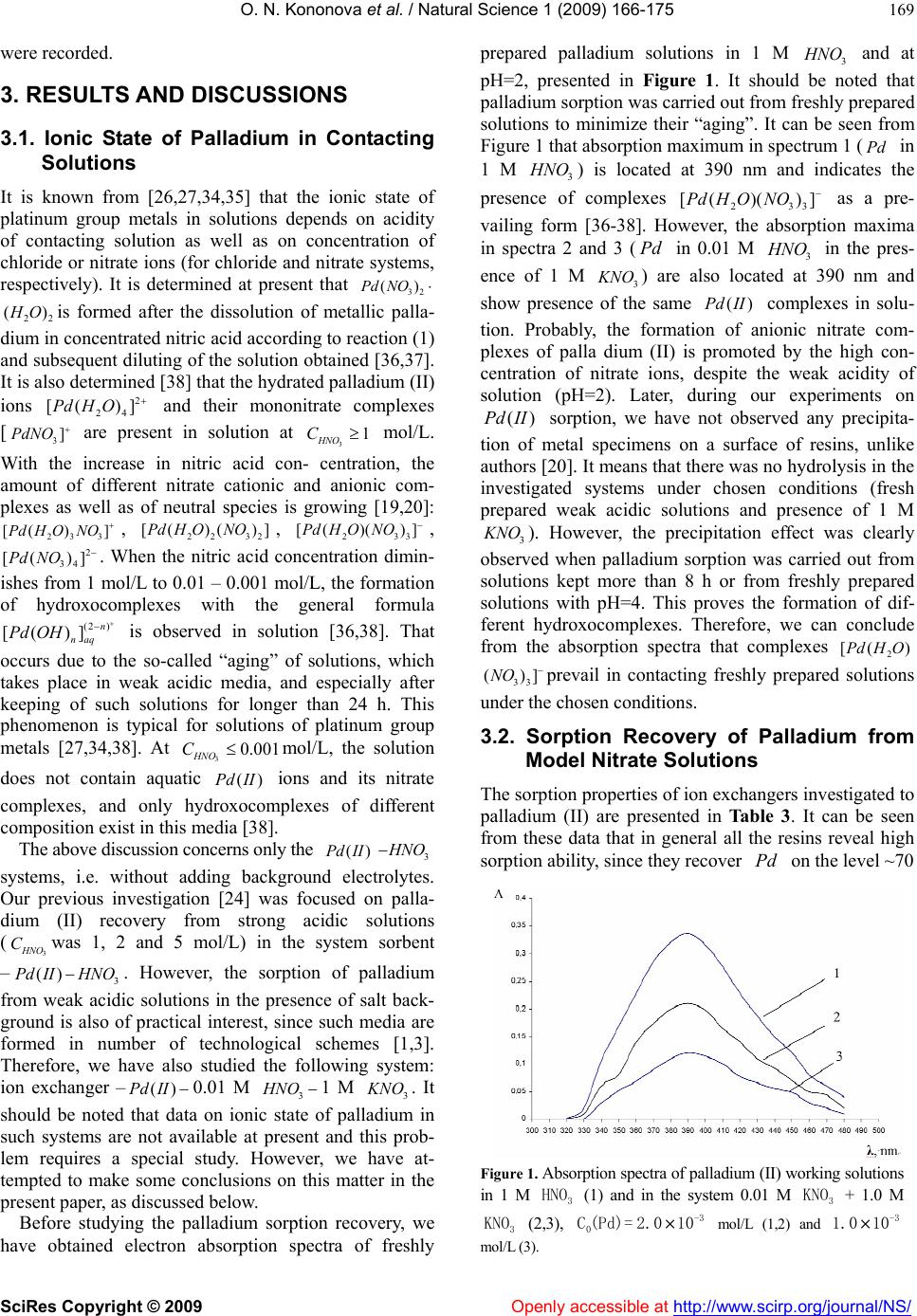 O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 169 were recorded. 3. RESULTS AND DISCUSSIONS 3.1. Ionic State of Palladium in Contacting Solutions It is known from [26,27,34,35] that the ionic state of platinum group metals in solutions depends on acidity of contacting solution as well as on concentration of chloride or nitrate ions (for chloride and nitrate systems, respectively). It is determined at present that 32 ()Pd NO is formed after the dissolution of metallic palla- dium in concentrated nitric acid according to reaction (1) and subsequent diluting of the solution obtained [36,37]. It is also determined [38] that the hydrated palladium (II) ions and their mononitrate complexes [ are present in solution at mol/L. With the increase in nitric acid con- centration, the amount of different nitrate cationic and anionic com- plexes as well as of neutral species is growing [19,20]: 22 ()HO 3]PdNO [( 2 24 [( )]PdH O 23 3 ) ] 3 HNO C1 dHONO , , 22 32 [()( )]PdHONO233 )()]O NO[(PdH , . When the nitric acid concentration dimin- ishes from 1 mol/L to 0.01 – 0.001 mol/L, the formation of hydroxocomplexes with the general formula is observed in solution [36,38]. That occurs due to the so-called “aging” of solutions, which takes place in weak acidic media, and especially after keeping of such solutions for longer than 24 h. This phenomenon is typical for solutions of platinum group metals [27,34,38]. At mol/L, the solution does not contain aquatic ions and its nitrate complexes, and only hydroxocomplexes of different composition exist in this media [38]. 2 34 )] (2 ) )] n naq [(Pd NO [(Pd OH 30.001 HNO C ()Pd II The above discussion concerns only the ()Pd II3 HNO systems, i.e. without adding background electrolytes. Our previous investigation [24] was focused on palla- dium (II) recovery from strong acidic solutions (3 NO C () was 1, 2 and 5 mol/L) in the system sorbent –3 dIIHNO. However, the sorption of palladium from weak acidic solutions in the presence of salt back- ground is also of practical interest, since such media are formed in number of technological schemes [1,3]. Therefore, we have also studied the following system: ion exchanger –() dII 0.01 M 1 M 3 HNO 3 NO . It should be noted that data on ionic state of palladium in such systems are not available at present and this prob- lem requires a special study. However, we have at- tempted to make some conclusions on this matter in the present paper, as discussed below. Before studying the palladium sorption recovery, we have obtained electron absorption spectra of freshly prepared palladium solutions in 1 M 3 NO and at pH=2, presented in Figure 1. It should be noted that palladium sorption was carried out from freshly prepared solutions to minimize their “aging”. It can be seen from Figure 1 that absorption maximum in spectrum 1 (Pd 1 M in 3 NO is located at 390 nm and indicates the presence of complexes [( as a pre- vailing form [36-38]. However, the absorption maxima in spectra 2 and 3 ( in 0.01 M in the pres- ence of 1 M ) 2 )(PdH ONO HN 33 )] 3 O Pd 3 NO ) are also located at 390 nm and show presence of the same () dII complexes in solu- tion. Probably, the formation of anionic nitrate com- plexes of palla dium (II) is promoted by the high con- centration of nitrate ions, despite the weak acidity of solution (pH=2). Later, during our experiments on sorption, we have not observed any precipita- tion of metal specimens on a surface of resins, unlike authors [20]. It means that there was no hydrolysis in the investigated systems under chosen conditions (fresh prepared weak acidic solutions and presence of 1 M (PdII) 3 NO (NO ). However, the precipitation effect was clearly observed when palladium sorption was carried out from solutions kept more than 8 h or from freshly prepared solutions with pH=4. This proves the formation of dif- ferent hydroxocomplexes. Therefore, we can conclude from the absorption spectra that complexes 2 [(H)OPd 33 )] prevail in contacting freshly prepared solutions under the chosen conditions. 3.2. Sorption Recovery of Palladium from Model Nitrate Solutions The sorption properties of ion exchangers investigated to palladium (II) are presented in Table 3. It can be seen from these data that in general all the resins reveal high sorption ability, since they recover on the level ~70 Pd Figure 1. Absorption spectra of palladium (II) working solutions in 1 M (1) and in the system 0.01 M + 1.0 M (2,3), mol/L (1,2) and mol/L (3). 3 HNO C 3 KNO 3 KNO -3 0 (Pd)= 2.0×10-3 1.0×10  O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 170 3 % (ANKF-5) and more than 90 % (Purolite S 985). The studied sorbents can be arranged by their affinity grade to palladium in the following order: Purolite S 985 > Purolite A 530 > Purolite A 500 ~ AV-17-8 > AN-251 > АNKF-5. It is interesting to note, that all ion exchangers except Purolite S 985 possess approximately equal affinity to , despite of their different physical and chemical structure. The nature of functional groups of the ion ex- changers investigated allows to form the following types of chemical bonds during their contact with noble metal ions [5,6,19,20]: Pd ionic, i.e. ion – ion interaction, which takes place on strong basic anion exchangers; coordination, forming as a result of conservation of electron pair between ligand (electron-pair donor), which is the nitrogen atom of resin functional groups, and the metal (electron-pair acceptor); this bond takes place on weak basic anion exchangers. The fact that amphoteric ion exchanger ANKF-5 re- covers ions from nitrate solutions on the same level (and even less) than anion exchanger AN-251 (both resins were synthesized on the basis of vinylpyridine), unambiguously points out to ionic state of in contacting solution. If cationic hydroxocomplexes or hydrated ions were present in this solu- tion, the degree of palladium (II) recovery would proba- bly be above 69% due to the activity of phosphorylic acid and pyridine nitrogen functional groups in weak acidic media. ()Pd II ()Pd II 2 24 [( )]PdHO Moreover, it is noteworthy that strong basic anion ex- changers recover to a greater extent (Table 3), although the additional complex formation of palladium ions with functional groups of quaternary ammonium base is impossible (unlike the weak basic anion ex- changers). Therefore, we consider highly probable that anionic palladium complexes ()Pd II 23 [()()]PdH ONO or exist in the systems investigated in the presence of 1 M 2 34 [( )]Pd NO 3 NO . Table 3. Sorption of palladium from nitrate model solutions on ion exchangers investigated Initial concentration is 5.0 · 10-4 mol/L, pH = 2. Pd(II) Trade name log D (%)R Trade name log D (%) R Purolite A 500 2.79 86 AV-17-8 2.77 85 Purolite A 530 2.87 88 AN-251 2.54 78 Purolite S 985 3.23 94 ANKF-5 2.34 69 As for sorption recovery of palladium (II) on anion exchanger Purolite S 985, which reaches 94%, there is little doubt that this recovery proceeds according not only to anion exchange, but also to complexation proc- ess, taking into account the presence of polyamine groups in the structure of this sorbent. 3.3. FT-IR Study of Palladium Sorption To study the palladium recovery from nitrate solutions in more detail, we have carried out IR-spectroscopic inves- tigation. We have obtained IR-spectra of anion exchang- ers Purolite S 985, AV-17-8 and AN-251, the fragments of which are shown in Figure 2. IR-spectra of initial samples of these resins in chloride form are presented for comparison. It can be seen from IR-spectra that appearance of in- tensive peaks in the range of 1400–1300 cm-1 (1384, 1352 and 1300 cm-1) is observed for all the ion exchang- ers, independently of their basicity and structure of polymeric matrix. These peaks are assigned to vibrations of N–O bonds of nitrate ion: peak at 1384 cm-1 corre- sponds with stretching vibrations of free 3 NO [20,39], whereas peaks at 1352 and 1300 cm-1 can be assigned to the N–O stretching vibrations in palladium complex [39]. It should be noted that the greatest intensity of these peaks is revealed in IR-spectrum of strong basic anion exchanger AV-17-8 (Figure 2, spectrum 5). It was men- tioned above that the functional groups of this resin (quaternary ammonium base) cannot react with palla- dium through additional coordination. Therefore, it can be concluded that the anion exchange takes place in this case: 233 233 [()( )][()( )]RClPdHONOR PdHONOCl (10) Since the similar but less intensive peaks are re- vealed in IR-spectra of weak basic anion exchangers Purolite S 985 and AN-251 (Figure 2, spectra 1 and 3), it can be concluded that the reaction (10) is to some ex- tent attributable to these resins too. However, in case of recovery on weak ba- sic anion exchanger AN-251, the redistribution of the intensities in the range of 1700 – 1400 cm-1, corre- sponding to symmetric and asymmetric stretching vi- brations of pyridine ring, is observed in IR-spectrum of this resin (Figure 2, spectrum 3). The absorption bands at 1600, 1558, 1494 and 1417 cm-1 are assigned to С = С and C = N stretching in pyridine ring [20,39,40]. The reduction of peak intensity at 1510 cm-1 and depression of that one at 1492 cm-1 points out to the probable complexation between palladium and pyridine nitrogen [5,40]. ()Pd II 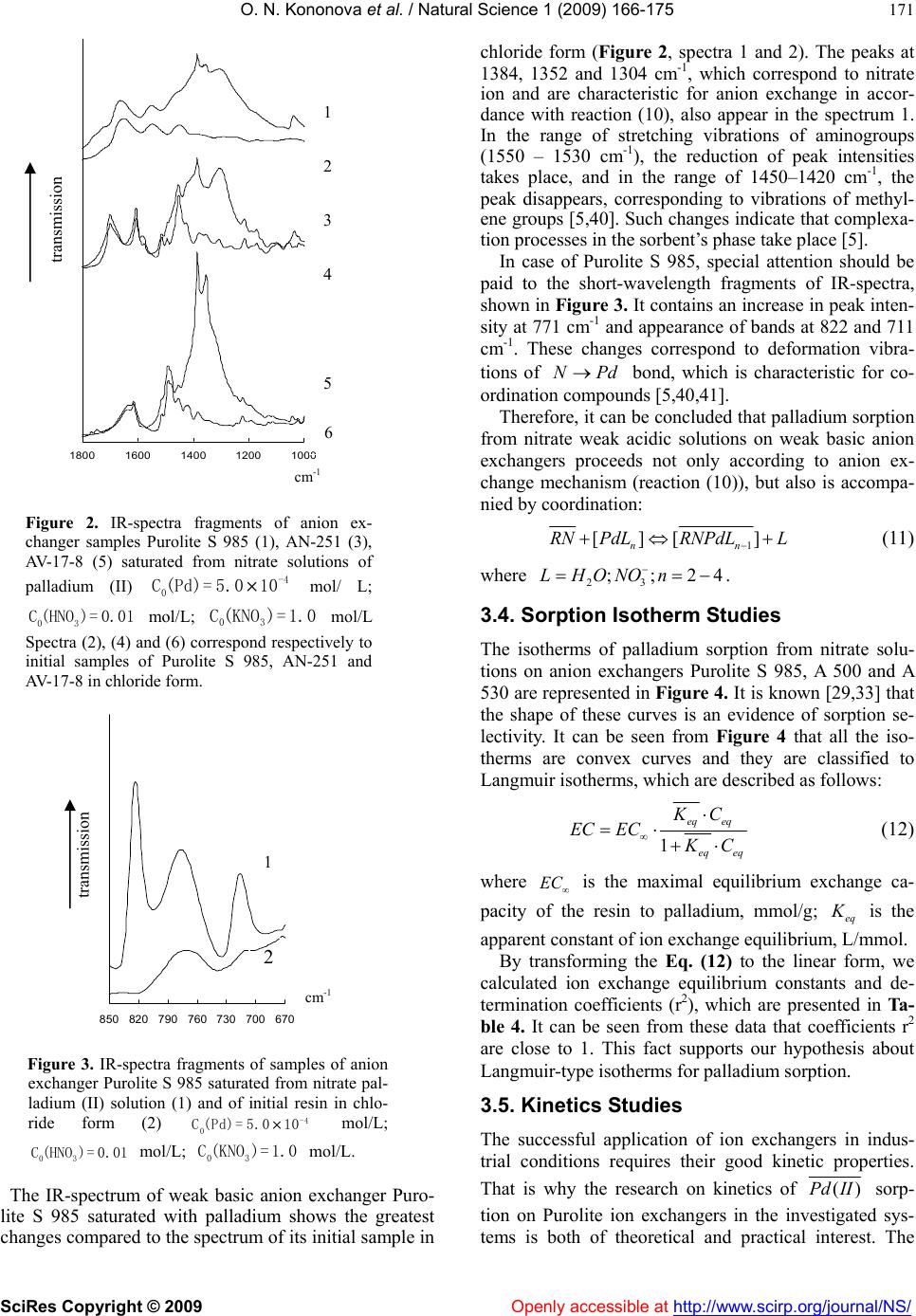 O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 171 Figure 2. IR-spectra fragments of anion ex- changer samples Purolite S 985 (1), AN-251 (3), AV-17-8 (5) saturated from nitrate solutions of palladium (II) mol/ L; mol/L; mol/L Spectra (2), (4) and (6) correspond respectively to initial samples of Purolite S 985, AN-251 and AV-17-8 in chloride form. -4 0 C(Pd)= 5.0×10 03 C(KNO)=1 03 C (HNO)= 0.01.0 Figure 3. IR-spectra fragments of samples of anion exchanger Purolite S 985 saturated from nitrate pal- ladium (II) solution (1) and of initial resin in chlo- ride form (2) mol/L; mol/L; mol/L. -4 0 C (Pd)= 5.0×10 03 C(KNO)= 1.0 03 C (HNO)= 0.01 The IR-spectrum of weak basic anion exchanger Puro- lite S 985 saturated with palladium shows the greatest changes compared to the spectrum of its initial sample in chloride form (Figure 2, spectra 1 and 2). The peaks at 1384, 1352 and 1304 cm-1, which correspond to nitrate ion and are characteristic for anion exchange in accor- dance with reaction (10), also appear in the spectrum 1. In the range of stretching vibrations of aminogroups (1550 – 1530 cm-1), the reduction of peak intensities takes place, and in the range of 1450–1420 cm-1, the peak disappears, corresponding to vibrations of methyl- ene groups [5,40]. Such changes indicate that complexa- tion processes in the sorbent’s phase take place [5]. In case of Purolite S 985, special attention should be paid to the short-wavelength fragments of IR-spectra, shown in Figure 3. It contains an increase in peak inten- sity at 771 cm-1 and appearance of bands at 822 and 711 cm-1. These changes correspond to deformation vibra- tions of bond, which is characteristic for co- ordination compounds [5,40,41]. NPd Therefore, it can be concluded that palladium sorption from nitrate weak acidic solutions on weak basic anion exchangers proceeds not only according to anion ex- change mechanism (reaction (10)), but also is accompa- nied by coordination: 1 [][ ] nn RN PdLRNPdLL 4 (11) where 23 ;;2LHONOn . 3.4. Sorption Isotherm Studies The isotherms of palladium sorption from nitrate solu- tions on anion exchangers Purolite S 985, A 500 and A 530 are represented in Figure 4. It is known [29,33] that the shape of these curves is an evidence of sorption se- lectivity. It can be seen from Figure 4 that all the iso- therms are convex curves and they are classified to Langmuir isotherms, which are described as follows: 1 eq eq eq eq KC EC EC C (12) where EC is the maximal equilibrium exchange ca- pacity of the resin to palladium, mmol/g; eq is the apparent constant of ion exchange equilibrium, L/mmol. By transforming the Eq. (12) to the linear form, we calculated ion exchange equilibrium constants and de- termination coefficients (r2), which are presented in Ta- ble 4. It can be seen from these data that coefficients r2 are close to 1. This fact supports our hypothesis about Langmuir-type isotherms for palladium sorption. 3.5. Kinetics Studies The successful application of ion exchangers in indus- trial conditions requires their good kinetic properties. That is why the research on kinetics of ()Pd II sorp- tion on Purolite ion exchangers in the investigated sys- tems is both of theoretical and practical interest. The 10001200140016001800 transmission c -1 670700730760790820850 transmission cm-1 1 2 3 4 5 6 1 2 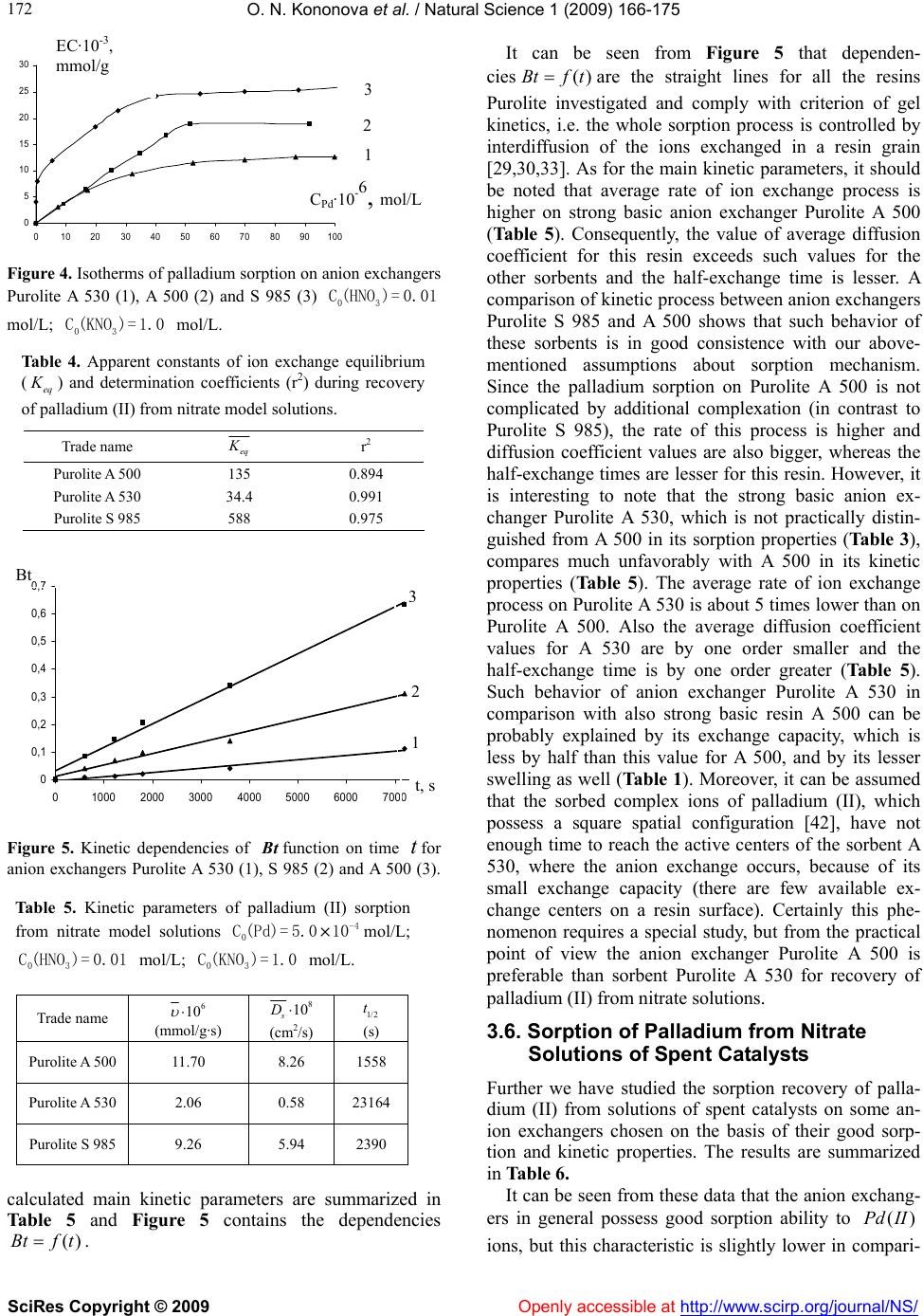 O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 172 Figure 4. Isotherms of palladium sorption on anion exchangers Purolite A 530 (1), A 500 (2) and S 985 (3) mol/L; mol/L. 03 C (HNO)= 0.01 03 C(KNO)= 1.0 Table 4. Apparent constants of ion exchange equilibrium (eq ) and determination coefficients (r2) during recovery of palladium (II) from nitrate model solutions. Trade name eq r2 Purolite A 500 135 0.894 Purolite A 530 34.4 0.991 Purolite S 985 588 0.975 Figure 5. Kinetic dependencies of function on time for anion exchangers Purolite A 530 (1), S 985 (2) and A 500 (3). Bt t Table 5. Kinetic parameters of palladium (II) sorption from nitrate model solutions mol/L; -4 0 C (Pd)= 5.0×10 03 C (HNO)= 0.01 mol/L; mol/L. 03 C(KNO)= 1.0 Trade name 6 10 (mmol/g·s) 8 10 s D (cm2/s) 1/ 2 t (s) Purolite A 500 11.70 8.26 1558 Purolite A 530 2.06 0.58 23164 Purolite S 985 9.26 5.94 2390 calculated main kinetic parameters are summarized in Table 5 and Figure 5 contains the dependencies . ()Btf t It can be seen from Figure 5 that dependen- cies ()Btf t are the straight lines for all the resins Purolite investigated and comply with criterion of gel kinetics, i.e. the whole sorption process is controlled by interdiffusion of the ions exchanged in a resin grain [29,30,33]. As for the main kinetic parameters, it should be noted that average rate of ion exchange process is higher on strong basic anion exchanger Purolite A 500 (Table 5). Consequently, the value of average diffusion coefficient for this resin exceeds such values for the other sorbents and the half-exchange time is lesser. A comparison of kinetic process between anion exchangers Purolite S 985 and A 500 shows that such behavior of these sorbents is in good consistence with our above- mentioned assumptions about sorption mechanism. Since the palladium sorption on Purolite A 500 is not complicated by additional complexation (in contrast to Purolite S 985), the rate of this process is higher and diffusion coefficient values are also bigger, whereas the half-exchange times are lesser for this resin. However, it is interesting to note that the strong basic anion ex- changer Purolite A 530, which is not practically distin- guished from A 500 in its sorption properties (Table 3), compares much unfavorably with A 500 in its kinetic properties (Table 5). The average rate of ion exchange process on Purolite A 530 is about 5 times lower than on Purolite A 500. Also the average diffusion coefficient values for A 530 are by one order smaller and the half-exchange time is by one order greater (Table 5). Such behavior of anion exchanger Purolite A 530 in comparison with also strong basic resin A 500 can be probably explained by its exchange capacity, which is less by half than this value for A 500, and by its lesser swelling as well (Table 1). Moreover, it can be assumed that the sorbed complex ions of palladium (II), which possess a square spatial configuration [42], have not enough time to reach the active centers of the sorbent A 530, where the anion exchange occurs, because of its small exchange capacity (there are few available ex- change centers on a resin surface). Certainly this phe- nomenon requires a special study, but from the practical point of view the anion exchanger Purolite A 500 is preferable than sorbent Purolite A 530 for recovery of palladium (II) from nitrate solutions. 3.6. Sorption of Palladium from Nitrate Solutions of Spent Catalysts Further we have studied the sorption recovery of palla- dium (II) from solutions of spent catalysts on some an- ion exchangers chosen on the basis of their good sorp- tion and kinetic properties. The results are summarized in Table 6. It can be seen from these data that the anion exchang- ers in general possess good sorption ability to ions, but this characteristic is slightly lower in compari- ()Pd II 0 5 10 15 20 25 30 0 102030405060708090100 EC·10-3, mmol/g CPd·10-6, mol/L 1 2 3 0 0,1 0,2 0,3 0,4 0,5 0,6 0,7 010002000 3000 4000 5000 60007000 t, s  O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 173 son with the data obtained for model solutions, exclud- ing anion exchanger AV-17-8 (Table 3). Partly the de- crease in recovery from real solutions can be explained by complex composition of initial solution of spent catalyst (Table 2), where a number of ions produce a competing effect on palladium sorption process. ()Pd II ()Pd II HNO In order to determine the form of palladium in con- tacting solution of spent catalyst as well as in the phase of strong basic anion exchanger, we have obtained the diffuse reflectance spectra presented in Figure 6. It can be seen from these data that the maximum in diffuse reflectance spectra is located at 410 nm. It corresponds with the maximum in absorption spectrum at 390 nm (Figure 1), since the bathochromic shift of maximum in diffuse reflectance spectra is observed during sorption concentration of noble metal ions in view of matrix ef- fect of solid phase [43,44]. Therefore, the prevailing form of existence in nitrate weak acidic solu- tions of spent catalysts is its complex 2 [( )PdH O 33 ()]NO It is known [2-5,15] that the desorption of noble metals from highly selective ion exchangers is hardly achievable process because of strong retention of ad- sorbed metal ions by functional groups of resins. Due to that, for the successful regeneration of these sor- bents it is necessary to use the elution agents which form more stable complexes with the recovered metal ions than the complexes of these metals existing in the resin phase. From this point of view, the acidic or ba- sic thiourea solutions are widely applied as eluting agent [1,15,19,22-24,27]. We have also used in the present work the thiourea solutions for palladium de- sorption after its sorption recovery. The results are shown in Table 7. It can be seen from the presented data that the palladium (II) elution degrees after its recovery from model solutions reach the level of 78-82% – the result which is considered quite satis- factory. However, the palladium desorption after its recovery from solutions of spent catalysts proceeds on the level of 22–25%. When carrying out this process, we have changed thiourea concentration and used its solutions in or , but the best result we could reach was ~ 25% by palladium elution with 1 M thiourea solution in 1 M 3NaOH aOH. It should be noted that the recovery of noble metals from industrial solu- tions after their sorption on highly selective ion ex- changers is often carried out by burning of such resins, since the value of noble metals exceeds the costs of ion exchange materials, even selective ones [2,4,24,45]. Therefore, it is necessary to continue the research for improving the palladium recovery after its sorption on selective ion exchangers from solutions of spent cata- -lysts. Figure 6. Diffuse reflectance spectra of anion exchangers’ samples AV-17-8 (1) and Purolite A 500 (2) saturated with palladium (II) from nitrate solutions of spent catalysts. mol/L; mol/L; mol/L F(R) – diffuse reflectance coefficient. -4 0 C(Pd)= 5.0×10 03 C(KNO)= 1.0 03 C(HNO)= 0.01 Table 6. Sorption recovery of palladium (II) from nitrate solutions of spent catalysts mol/L; mol/L; mol/L. -4 0 C(Pd)= 5.0×10 03 (KNO)= 1.0 03 C(HNO)= 0.01C Trade name log D(%)R Purolite A 500 2.31 65 Purolite S 985 2.86 88 AV-17-8 2.72 84 Table 7. Elution of palladium from anion exchangers in- vestigated by thiourea solution (1.0 mol/L) in 0.01 M after palladium sorption from nitrate weak acidic model solutions. 3 HNO Trade name Desorption degree (%) Purolite S 985 78 Purolite A 500 82 4. CONCLUSIONS Sorption recovery of palladium (II) from nitrate weak acidic model solutions and solutions of spent catalysts on some ion exchangers with different physical and chemical structure was investigated. Based on electron absorption spectra, it was determined that complexes 233 [()()]PdH ONO prevail in contacting solutions. It was shown that ion exchangers investigated possess good sorption and kinetic properties. The mechanism of palladium sorption recovery on strong and weak basic anion exchangers from nitrate weak acidic solution was outlined by means of FT-IR-spectroscopy. It was shown that strong basic an- ion exchangers sorb palladium according to anion ex- change, whereas weak basic resins recover ()Pd II 0 4 8 12 16 20 24 380 400 420440 460480 500 520 540 560 , nm F(R) 1 2  O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 Openly accessible at http://www.scirp.org/journal/NS/ 174 complexes not only by anion exchange, but also by means of additional coordination with nitrogen atoms of functional groups. The palladium (II) desorption after its recovery on se- lective ion exchangers from model solutions and solu- tions of spent catalysts was carried out using thiourea solutions. It was shown that the degree of palladium elution by 1 M thiourea solution in 0.01 M 3 NO after its sorption from model solutions is on the level of 78 – 82 %, whereas this value does not exceed 25% after sorption from solutions of spent catalysts. The improvement of this process should be a subject for fur- ther research. ()Pd II Based on results of present investigation, the anion exchangers AV-17-8 as well as Purolite S 985 and A 500 can be recommended for selective recovery of palladium (II) from nitrate weak acidic solutions. 5. ACKNOWLEDGEMENT The authors would like to express profound gratitude to the team of Moscow office of Purolite International Ltd, who kindly provided us with ion exchanger samples. REFERENCES [1] R. Pfrepper, (1988) Möglichkeiten Der Wiedergewin- nung Von Pd Aus Sekundären Rohstoffquellen. ZFI- Mitteilungen. Akademie-Verlag, Berlin, 3-292. [2] I. F. Khudyakov, A. P. Doroshkevich, S. V. Karelov, (1990) Metallurgy of the secondary non-ferrous heavy metals. Nauka, Moscow, 15-25. [3] M. A. Meretukov and A. M. Orlov, (1990) Metallurgy of noble metals. Metallurgiya, Moscow, 27-30. [4] A. G. Kholmogorov, (1986) Ion exchange in hydro- metallurgical processing of palladium-containing spent catalysts. Sibir, Krasnoyarsk, 150-151. [5] K. M. Saldadze and V. D. Kopylova-Valova, (1980) Com- plex-forming ion exchangers. Nauka, Moscow, 3- 336. [6] S. A. Simanova, A. S. Tevlina, and I. S. Bazhanova, (1994) Formation of palladium complexes during sorp- tion on high permeable sorbents with heterocyclic nitro- gen atoms. J. Inorg. Chem., Moscow, 39(1), 121-126. [7] I. A. Kovalev, G. I. Tsyzin, and A. A. Formanovskiy, (1995) Concentration of rhodium, palladium and plati- num on sorbent with ethylenetriamine groups. J. Inorg. Chem., Moscow, 40(5), 828-833. [8] I. A. Tarkovskaya, L. P. Tikhonova, and N. V. Kulik, (1997) Sorption of platinum group metals from aqueous solutions on inorganic and synthetic ion exchange mate- rials. Khimiya I Tekhnologiya Vody, 19(3), 227-239. [9] A. G. Kholmogorov, O. N. Kononova, S. V. Kachin, and S. V. Razvodovskaya, (1998) Sorption of palladium from chloride solutions on anion exchangers with macro-re- ticular and porous structure. J. Phys. Chem., Moscow, 72(2), 318-321. [10] O. P. Shvoeva, V. P. Dedkova, and S. B. Savvin, (2004) Effect of masking compounds on color reactions of di- thizone with Pd (II), Hg (II) and Ag (I) after sorption from chloride solutions on fibrous anion exchanger. J. Anal. Chem., Moscow, 59(7), 704-709. [11] V. P. Dedkova, O. P. Shvoeva, and S. B. Savvin, (2005) Test-method for determination of palladium in chloride solutions in solid phase of fibrous ion exchanger. J. Anal. Chem., Moscow, 60(1), 85-89. [12] Z. Hubicki and M. Leszczynska, (2005) Sorption of pal- ladium (II) chloride complexes on weakly, intermediate and strongly basic anion exchangers. Desalination, 175, 227-236. [13] Z. Hubicki and A. Wolowicz, (2009) Adsorption of pal- ladium (II) from chloride solutions on Amberlyst A 29 and Amberlyst A 21 resins. Hydrometallurgy, 96, 159- 165. [14] Z. Hubicki and A. Wolowicz, (2009) A comparative study of chelating and cationic ion exchange resins for removal of palladium (II) complexes from acidic chloride media. J. Hazard. Mater., 164, 1414-1419. [15] V. V. Milyutin, S. V. Peskishev, and V. M. Gelis, (1994) Sorption of palladium, ruthenium and rhodium from ni- tric acidic solutions on different sorbents. Radiokhimiya, 36(1), 25-28. [16] G. R. Anpilogova and E. V. Kondratieva, (1996) Study of palladium (II) sorption on polyethylene-monosulfide from nitric acidic solutions. J. Inorg. Chem., Moscow 41(3), 447-452. [17] V. I. Skorokhodov, O. Y. Goryaeva, B. K. Radionov, (2004) Choice of optimal structure of ion exchangers for palladium sorption from nitric acidic solutions. Tsven- naya Metallurgiya, 1, 31-33. [18] Z. Hubicki, M. Leszczynska, B. Lodyga, and and A. Lo- dyga, (2007) Recovery of palladium (II) from chloride and chloride-nitrate solutions using ion exchange resins with S-donor atoms. Desalination, 207, 80-86. [19] K. A. Venkatesan, B. R. Selvan, M. P. Antony, T. G. Srinivasan, and P. R. V. Rao, (2007) Extraction of palla- dium (II) from nitric acid medium by imidazolium nitrate immobilized resin. Hydrometallurgy, 86, 221-229. [20] R. Kumaresan, K. N. Sabharval, T. G. Srinivasan, P. R. V. Rao, and G. Dhekane, (2008) Studies on the sorption of palladium using cross-linked poly(4-vinylpyridine- divi- nylbenzene) resins in nitric acid medium. Solv. Extr. Ion Exchange, 26, 643-671. [21] A. V. Belyaev, (2003) Chemical-technological problems of platinum group metals during processing of exhausted nuclear fuel. Zhurnal Strukturnoy Khimii, 44(1), 39-47. [22] O. N. Kononova, A. G. Kholmogorov, and E. V. Mikhlina, (1998) Palladium sorption on vinylpyridine ion exchang- ers from chloride solutions obtained from spent catalysts. Hydrometallurgy, 48, 65-72. [23] O. N. Kononova, N. G. Goryaeva, N. B. Dostovalova, S. V. Kachin, and A. G. Kholmogorov, (2007) Recovery of palladium from acidic solutions using carbon adsorbents. Khimiya Tverdogo Topliva. 4, 71-75. [24] O. N. Kononova, A. N. Lukianov, M. V. Derevyashkin, A. G. Kholmogorov, S. V. Kachin, and N. G. Goryaeva, (2008) Sorption of palladium on carbon adsorbents from nitric acid solutions. J. Porous Mater. 15, 61-66. [25] O. N. Kononova, K. A. Shatnykh, K. V. Prikhodiko, D. M. Kashirin, and A. G. Kholmogorov, (2009) Sorption of gold (I) and silver (I) from thiosulfate solutions on anion  O. N. Kononova et al. / Natural Science 1 (2009) 166-175 SciRes Copyright © 2009 http://www.scirp.org/journal/NS/ 175 exchangers. Tsvetnye Metally, 1, 40-43. [36] R. A. Shoytsov, (1972) Spectrophotometrical investiga- tion of palladium (2+) state in aqueous solutions. State University, Moscow, Moscow, PhD-thesis. [26] F. E. Beamish, (1966) The analytical chemistry of the noble metals. Pergamon Press, Oxford, London, Edin- burgh, New York, Toronto, Paris, Frankfurt, 41-45. [37] V. S. Shmidt, N. A. Shorokhov, A. A. Vashman, and V. E. Samsonov, (1982) Study of complex 2223 )(). J. Inorg. Chem., Moscow, 27(5), 1254-1256. (OHNOPd [27] S. I. Ginzburg, N. A. Ezerskaya, I. V. Prokofieva, N. V. Fedorenko, Y. I. Shlenskaya, and N. K. Belskiy, (1972) Analytical chemistry of platinum group metals. Nauka, Moscow, 165-166. [38] P. I. Antyukhin, (1993) About correlation between solu- bility constants of metal hydroxides and formation con- stants of their neutral hydroxocomplexes. Sibirskiy Khimicheskiy Zhurnal, 1, 21-25. [28] F. Umland, A. Janssen, D. Thierig, and G. Wünsch, (1971) Theorie und praktische Anwendung von Komplexbild- nern. Akademische Verlagsgesellschaft, Frankfurt am Main, 364-365. [39] L. Bellami, (1963) Infrared spectra of complex molecules. Inostrannaya Literatura, Moscow, 383-590. [29] F. Helfferich, (1962) Ion exchange. McGraw-Hill, New York, 335-360. [40] V. A. Uglyanskaya, G. A. Chikin, V. F. Selemenev, and G. A. Zavialova, (1989) Infrared spectroscopy of ion ex- change materials. Izdatelstvo VGU, Voronezh, 150-207. [30] F. Helfferich and J. A. Marinsky, (1966) Ion exchange kinetics. (ed.) Ion exchange. A series of advances. McGraw-Hill, Buffalo, New York, 281-331. [41] K. Nakamoto, (1986) Infrared and raman spectra of in- organic and coordination compounds. Wiley, New York, 360-536. [31] D. C. Harris, (1996) Quantitative chemical analysis. Freeman and Company, New York, 374-376. [42] F. A. Cotton and G. Wilkinson, (1969) Advanced inor- ganic chemistry. A Comprehensive Text, Mir, Moscow, 460-469. [32] J. H. Pollard, (1977) A handbook of numerical and statis- tical techniques. Cambridge University Press, Cambridge, 342-345. [43] P. Kubelka, and F. Munk, (1931) Ein Beitrag Zur Optik Der Farbanstriche. Z. Techn. Phys., 12, 593 -601. [33] Y. A. Kokotov, (1990) Ion exchangers and ion exchange. Khimiya, Leningrad, 47-52. [44] G. D. Brykina, D. Y. Marchenko, and O. A. Shpigun, (1995) Solid-phase spectrophotometry. J. Anal. Chem., Moscow, 50(5), 484-491. [34] Y. A. Zolotov, G. M. Varshal, and V. M. Ivanov, (2003) Analytical chemistry of platinum group metals. Editorial URSS, Moscow, 16-88. [45] V. V. Barchenkov, (1982) Sorption technology of recov- ery of gold and silver from ores. Nauka, Moscow, 155-286. [35] S. E. Livingstone, (1967) The chemistry of ruthenium, rhodium, palladium, osmium, iridium and platinum. Per- gamon Press, Oxford, New York, Toronto, Sydney, Paris, Braunschweig, 178-211. Openly accessible at
|