Journal of Cancer Therapy
Vol.3 No.5(2012), Article ID:23447,7 pages DOI:10.4236/jct.2012.35070
Combined 18F-FDG PET/CT with Enhanced CT Perform One-Stop Shop Imaging for Assessing Pancreatic Carcinoma
SHAPE \* MERGEFORMAT ![]()
Department of Nuclear Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Email: *lb10363@rjh.com.cn, laurazm_cn@sina.com
Received August 8th, 2012; revised September 7th, 2012; accepted September 19th, 2012
Keywords: Pancreas; Carcinoma; Computed Tomography; Positron Emission Tomography; Enhanced CT
ABSTRACT
Objective: To evaluate the role of PET/CT with contrast enhanced CT in diagnosing and staging for pancreatic diseases and optimize the use of enhanced PET/CT as one-stop imaging modality. Methods: Fifty-six patients who presented with suspected pancreatic carcinoma underwent whole-body 18F-FDG PET/CT and enhanced CT imaging. Images were interpreted and compared with the histopathology findings. The sensitivity, specificity and accuracy of enhanced CT, PET/CT and combined PET/CT with contrast enhanced CT diagnosis were analyzed. The vascular invasion and distant metastases of pancreatic lesions on different imaging modality were analyzed and compared. Results: Among the fifty-six patients evaluated for primary tumor, thirty-nine patients had malignant diseases and seventeen had benign lesions. Sensitivity, specificity and accuracy of enhanced CT were 87.5%, 75% and 83.9%, those of non-enhanced PET/CT were 89.7%, 88.2% and 89.2%, of PET/CT with enhanced CT were 100%, 94.1% and 98.2%. Combined PET/CT with enhanced CT had highest sensitivity, specificity and accuracy in diagnosing pancreatic carcinoma. Average SUVmax of malignant lesions was 6.72 ± 3.84, compared with 2.56 ± 1.22 for patients with benign disease (P < 0.01). Combined PET/CT with enhanced CT can help to make accurate staging especially in assessing metastases and vascular invasion. Seven patients deemed surgical candidates were changed to non-surgical treatment. SUVmax didn’t correlate with distant metastases and survival time (Pearson = −0.243, P = 0.136). Distant metastases correlate with survival time (Pearson = −0.447, P = 0.004). Conclusion: PET/CT with contrast enhanced CT is of greater value in the diagnosis of pancreatic lesions as well as preoperative staging especially in assessing vascular invasion and distant metastasis. It is feasible to perform one-stop shop imaging by combining PET/CT with enhanced CT, supplying more accurate assessment before operation and help to select optimal therapeutic plan.
1. Introduction
Pancreatic carcinoma is one of leading causes of cancer death and increasing in incidence. Despite advances in diagnosing modalities, most patients with pancreatic cancer are still unresectable at the time of diagnosis. Surgery remains the only potential for long-term survival, with a resectability rate of around 15% - 20% in the latest review [1]. Even in patients with resectable disease, the 5-year survival rate is still around 20% [1-3]. Clearly, an effort has to be made for diagnosing early stage cancer, also by determining its clinical stage and by accurately predicting the prognosis.
However, diagnosis, clinical staging and treatment of pancreatic carcinoma remain difficult. Suspicions of pancreatic cancer are often raised by sonography or CT findings, including the presence of a low-attenuation pancreatic mass and dilatation of the pancreatic duct or biliary tree (to both). CT is the most common diagnostic imaging modality used in the preoperative diagnosis of pancreatic cancer. This technique can also access vascular involvement and invasion of adjacent organs. Unfortunately, interpretation of CT is sometimes difficult in mass-forming pancreatitis or in questionable findings.
The advantages of positron emission tomography using 18F-fluorodeoxyglucose (18F-FDG) on diagnosing pancreatic cancer, especially small lesions less than 2 cm in size, over the conventional modalities, including computed tomography (CT), have been reported [2]. Furthermore, 18F-FDG PET has also been reported to possibly play a role in predicting the prognosis of pancreatic cancer [2,4-6].
However, a limiting factor for the sensitivity of PET is the limited resolution of the method and the specificity is the difficulty to differ malignant diseases from pancreatitis. By the use of fusion systems combining PET and CT, diagnose may be improved, even though assessment of vascular invasion is still difficult. In this article, we compare the use of non-enhanced PET/CT and enhanced PET/CT on pancreatic tumor and suggested combining use of PET and enhanced CT to improve diagnosing accuracy.
2. Materials and Methods
2.1. Patient Population
Among seventeen patients with benign diseases, five patients underwent surgery and twelve patients underwent conservative therapy. Seven patients deemed surgical candidates were changed to non-surgical treatment because enhanced PET/CT exams found bone, lung, or liver distant metastases and organ infiltration. Bone, liver distant metastases and organ infiltration were confirmed by pathology, and lung metastases were confirmed by clinical follow up. All patients performed 6-mo follow-up period after examination.
2.2. PET/CT Imaging
18F-FDG PET/CT exams were performed with a GE Discovery STE16 PET/CT (General Electric Medical systems). Patients were required to fast for at least 4 h before PET scanning and serum glucose level was controlled under the level of 7.4 mmol/L. Approximately 60 min after the intravenous administration of 18F-FDG 0.12 - 0.15 mCi/kg, PET/CT scan was acquired.
Initially, CT scan was acquired starting from the level of the head to the thigh using the following parameters: 120 - 180 mAs, 140 kV, a gantry rotation, a slice thickness of 3.75 mm. The CT scan was acquired during breath holding in the normal expiratory position. The low dose CT data were used for attenuation correction and lesion localization, and the images were reconstructed using a standard iterative algorithm. Immediately after the CT acquisition, a PET scan was acquired.
Six to seven bed positions that included the head to the thigh were imaged. Emission images were acquired for 3 min per bed position. The axial field of view of this system is 15.6 cm. Integrated PET and CT images were obtained automatically on Xeleris (GE Healthcare) or Advantage workstations (GE Healthcare).
The acquired images were reviewed with software providing multi-planar reformatted images of PET alone, CT alone, and fused non-enhanced PET/CT and enhanced CT images with linked cursors using a Xeleris workstation (GE Healthcare).
Clinical information was used in addition to CT scans for localization of the pancreatic head to differentiate between abnormal uptake in the head of the pancreas and physiologic uptake in an adjacent loop of bowel. Visual analysis was performed using background liver uptake as a reference (uptake greater than liver background corresponding to the head of the pancreas on CT was considered malignant). The attenuation-corrected images were analyzed semi-quantitatively, using the SUVs by the physician who performed the visual interpretation. The SUV was calculated as: SUV = (activity in region of interest in mCi/mL)/(injected dose in mCi/weight in kg).
2.3. Enhanced CT Imaging
Afterward, enhanced CT scans of the abdomen were obtained with 5-mm collimation and a table speed of 5 mm/s (pitch 1 or 2) and 20 - 25 s after intravenous contrast administration (150 mL, Ultravist, at a rate of 3 mL/s) and then again 60 - 70 s during the portal phase. The images were reconstructed with 3.75 - 5 mm thicknesses.
Forty-two patients presented with PET/CT and enhanced CT of the abdomen performed at our hospital at the same time. Fourteen patients performed enhanced CT at outside institutions and these studies were not repeated because intravenous contrast was identified in the superior mesenteric vein, and pancreatic parenchyma enhancement was present. The time duration between the PET/CT and enhanced CT was no more than one week.
2.4. Image Interpretation
The PET/CT images were analyzed by 2 dual-boardcertified nuclear radiology physicians. Images were evaluated by consensus. First, the reader interpreted the PET images alone; in a second step, the reader interpreted the non-enhanced PET/CT images; and in a third step, the reader interpreted the enhanced PET/CT images. The PET images were analyzed for the presence and nature of lesions with focally increased 18F-FDG uptake. For all patients, the attenuation-corrected PET images were used for analysis. Lesions were interpreted as metastases if the uptake was higher than the uptake of the surrounding background tissue so that a focal lesion was clearly depicted. The enhanced-CT part was analyzed using the established criteria for the assessment of the primary tumor: vessel involvement, organ infiltration, and distant metastases.
2.5. Statistical Analysis
The chi-square test was employed for a statistical comparison of the sensitivity of 18F-FDG PET and CT. The Student’s test was used to compare the values of the SUVmax between the two groups. Correlations between the SUVmax with the maximum diameter of the lesion, distant metastases, and survival time were examined by the Pearson’s correlation test. Correlation between distant metastases with survival time was tested by the Pearson’s correlation test. Survival functions of two groups were analyzed by using Log-Rank test. All statistical analyses were performed using the SPSS software program (SPSS 13.0). A P value < 0.05 was considered statistically significant.
3. Results
3.1. Sensitivity for Diagnosing Pancreatic Disease
A total of 56 patients underwent both 18F-FDG PET/CT imaging and CT enhancement scanning for the diagnosis of suspected primary pancreatic adenocarcinoma during the study period. The distribution of patients is summarized in Table 1. Among these 56 patients, 39 malignant diseases and 17 benign lesions were identified. Among the 39 patients with pancreatic malignant diseases, maximum tumor diameter was measured by CT. Tumor size ranged from 0.5 cm to 5.6 cm.
Sensitivity, specificity and accuracy of enhanced CT were 87.5%, 75.0% and 83.9%, those of non-enhanced PET/CT were 89.7%, 88.2% and 89.2%, of PET/CT with enhanced CT were 100%, 94.1% and 98.2%. Combined PET/CT with enhanced CT had highest sensitivity, specificity and accuracy in diagnosing pancreatic carcinoma (Table 2).
Four false negative and two false positive PET/CT (SUVmax 2.5 as standard) scans were found. There were two reasons to select SUVmax 2.5 as standard. First reason to select SUVmax 2.5 is according to references [3-7]. Second we compared diagnosis accuracy of PET (SUVmax 2.0, 2.5, 3.0, respectively) and diagnosis accuracy with SUVmax 2.5 was highest. So, we select SUVmax 2.5 as standard. And there were four falsepositive and five false-negative in enhanced CT scans, which was correctly diagnosed by PET/CT. Combined PET/CT with enhanced CT, hybrid approach correctly diagnosed 55 cases finally. Accuracy of hybrid diagnose means is 98.2% higher than other single means.
3.2. Distribution of SUVmax
The distribution of SUVmax in benign and malignant pancreatic lesion is shown in Figure 1. The SUVmax of the patients with pancreatic adenocarcinoma ranged from 1.7 to 18.9 and that of benign disease ranged from 1.2 to 5.0. Overall mean SUVmax for patients with malignant disease was 6.72 ± 3.84, compared with 2.56 ± 1.22 for patients with benign disease. This difference was statistically significant (P < 0.05).
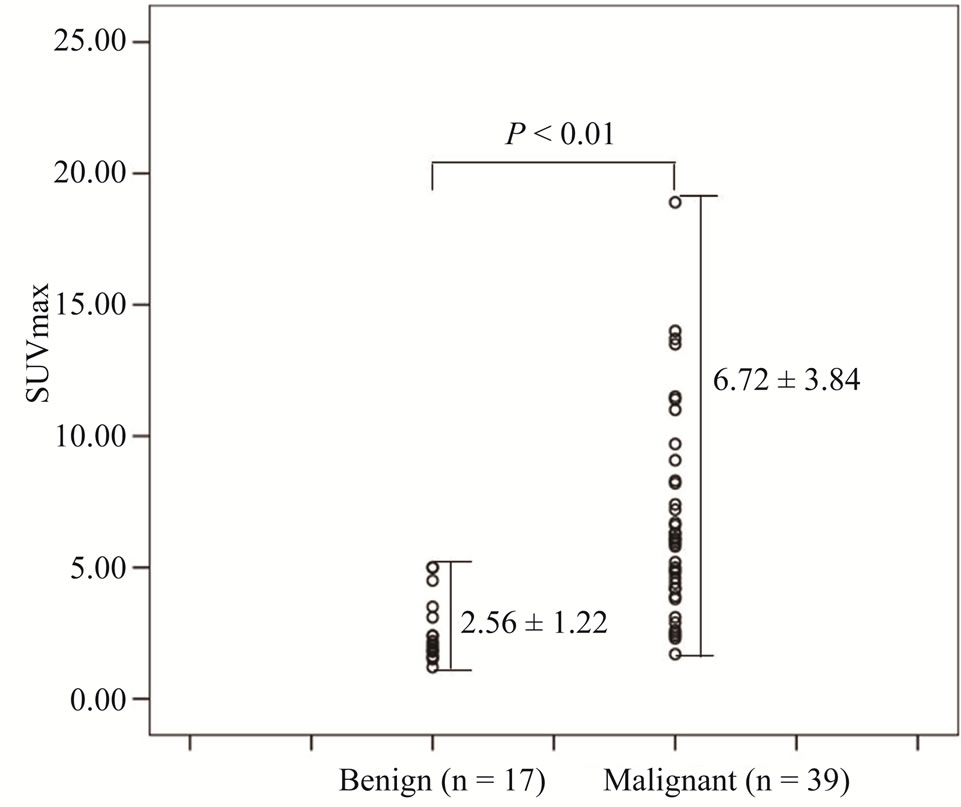
Figure 1. Distribution of standardized uptake values (SUVmax) for benign and malignant pancreatic lesions.
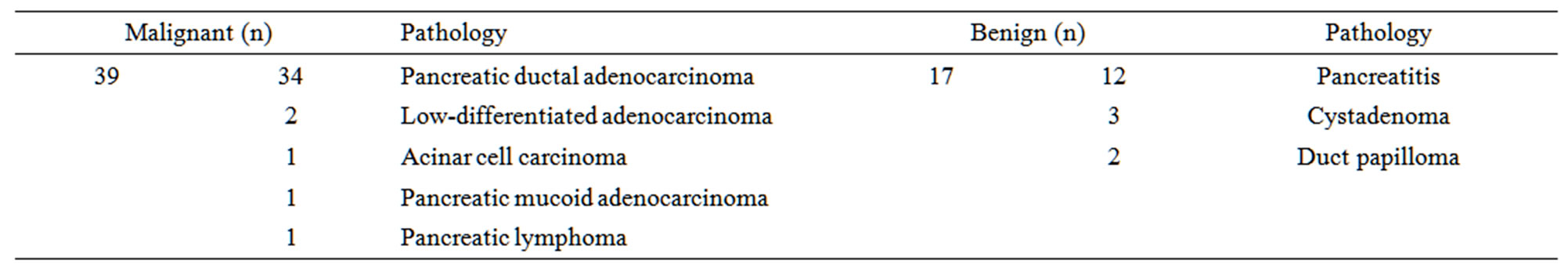
Table 1. Distribution of patient population (n = 56).
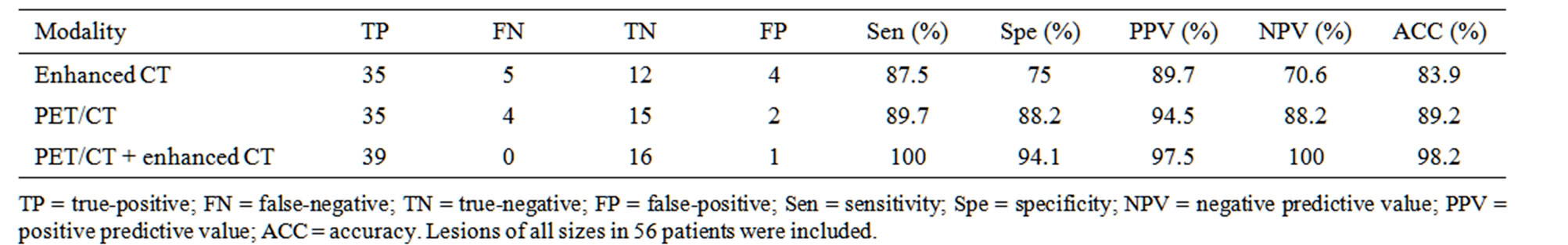
Table 2. Value of enhanced CT, PET/CT, PET/CT+ enhanced CT.
Among pancreatic malignant diseases, four failed to highly uptake 18F-FDG with SUVmax of 1.7, 2.1, 2.3, and 2.4. Five patients with pancreatitis showed high 18F-FDG uptake with SUV of 3.1, 3.5, 3.7, 4.5 and 5.0. An example of false negative PET/CT imaging for pancreatic cancer is presented in Figure 2.
Some benign inflammatory lesions accumulated 18FFDG and result in false-positive interpretations on PET images as Figure 3 showed.
3.3. Relationship between SUV and Lesion Size
Figure 4 showed correlation between the SUVmax and the maximum diameter of the primary lesion measured on CT by using Pearson’s correlation test (Pearson = 0.416, P < 0.01). Figure 4 also showed there were two patients had malignant lesions with diameter smaller than 2 cm among the three false-negative PET/CT examinations. Small lesions that suffer from partial volume averaging can lead to false-negative interpretation.
3.4. Value of Enhanced CT, PET/CT, Combined PET/CT with Enhanced CT in Assessing the Staging of Pancreatic Cancer
The sensitivity and specificity of PET, PET/CT, com-
 (a)
(a)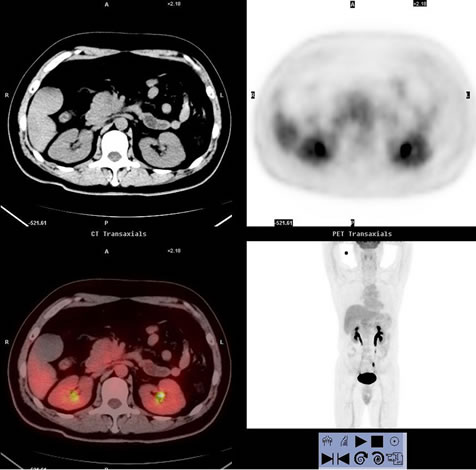 (b)
(b)
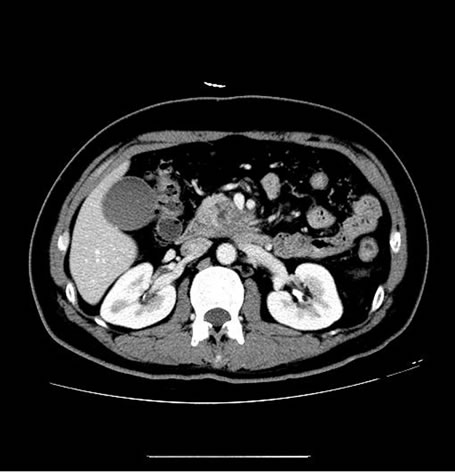
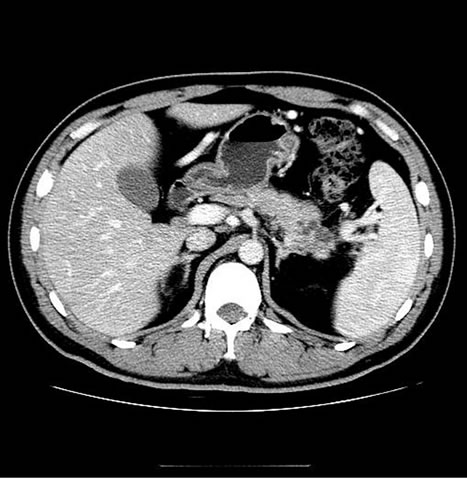
Figure 2. Images of a 43-year-old man, who presented with right upper quadrant pain for 5 days and proved to be pancreatic ductal adenocarcinoma (stage II) by histological examination. (a) showed PET/CT scan failed to reveal high uptake lesion in the head of pancreas (SUVmax 2.3); (b) Enhanced CT showed a low-attenuation mass within the head pancreas (1.6 cm in diameter), which indicate the lesion to be malignant.
bined PET/CT with enhanced CT in assessing vascular invasion, lymph node, liver, lung or bone metastasis showed in Table 3.
 (a)
(a)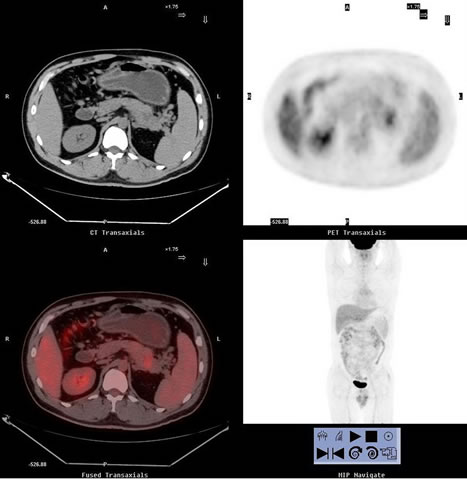 (b)
(b)
Figure 3. One false-positive case images of a 41-year-old man, who presented with left upper quadrant pain and vomiting for 5 years. (a) showed PET/CT scan revealed high uptake lesion in the tail of pancreas (SUVmax 3.1) which indicate the lesion to be malignant; (b) Enhanced CT imaging revealed multiple round low density lesions at the tail of pancreas, which indicated the lesion to be chronic pancreatitis and proved by histological examination.

Figure 4. Relationship between the standardized uptake values (SUVmax) and maximum diameter of malignant pancreatic lesions (P < 0.01). Horizontal line represent size and vertical line represent maximum SUV.
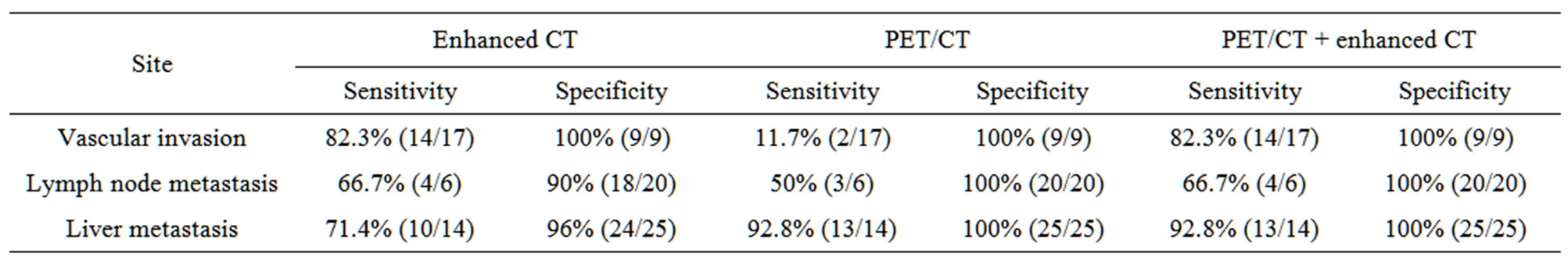
Table 3. Sensitivity and specificity of PET, PET/CT, combined PET/CT with enhanced CT in assessing vascular invasion and metastasis.
3.4.1. Vascular Invasion
Seventeen patients were confirmed to have vascular invasion of the superior mesenteric artery or celiac trunk. Non-enhanced PET/CT found only two cases with serious vascular invasion. Combined PET/CT with enhanced CT detected 14 cases with vascular invasion (one case showed as Figure 5), having higher sensitivity than non-enhanced CT.
3.4.2. Lymph Node Metastasis
Sensitivity of non-enhanced PET/CT was 50% and that of enhanced PET/CT combing enhanced CT increased to 66.7%.
3.4.3. Lung Metastases
Two patients had lung metastases, both of which were detected by enhanced CT, non-enhanced and enhanced PET/CT.
3.4.4. Liver Metastases
Fourteen patients had liver metastases. Enhanced CT detected 10 cases. Enhanced PET/CT detected thirteen cases, having higher sensitivity than enhanced CT.
3.4.5. Bone Metastases
Three patients had bone metastases. Enhanced CT failed to find bone metastases because of local scan mode, PET/CT and enhanced PET/CT detected all cases with bone metastases.
3.5. Correlation between SUVmax, Distant Metastases and Survival Time
SUVmax didn’t correlate with distant metastases by using Pearson’s correlation test (Pearson = 0.133, P = 0.418) and didn’t correlate with survival time (Pearson = −0.243, P = 0.136). Distant metastases correlate with survival time (Pearson = −0.447, P = 0.004). As Figure 6 showed Median survival time of group 0 (patients with out distant metastases) was 14 ± 2.94 months, which of
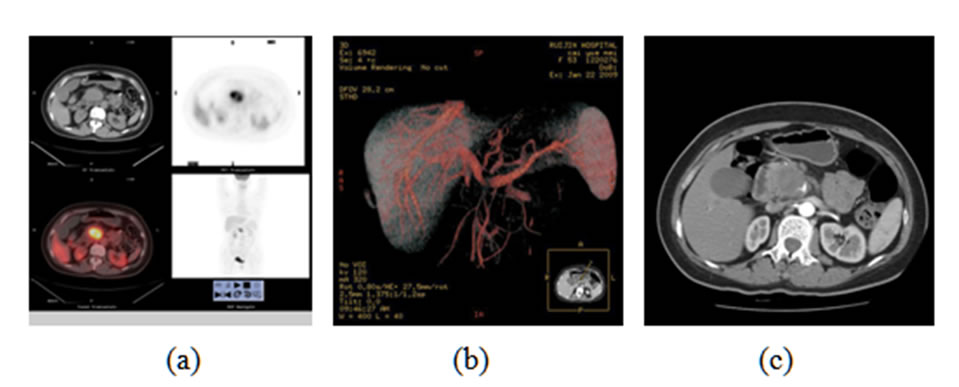
Figure 5. (a) showed PET/CT scan revealed high uptake lesion in the head of pancreas (SUVmax 13.7) which indicate the lesion to be malignant. But non-enhanced PET/CT failed to show vascular invasion; (b) Axial enhanced CT and (c) 3D VR images revealed infiltration of the superior mesenteric artery.
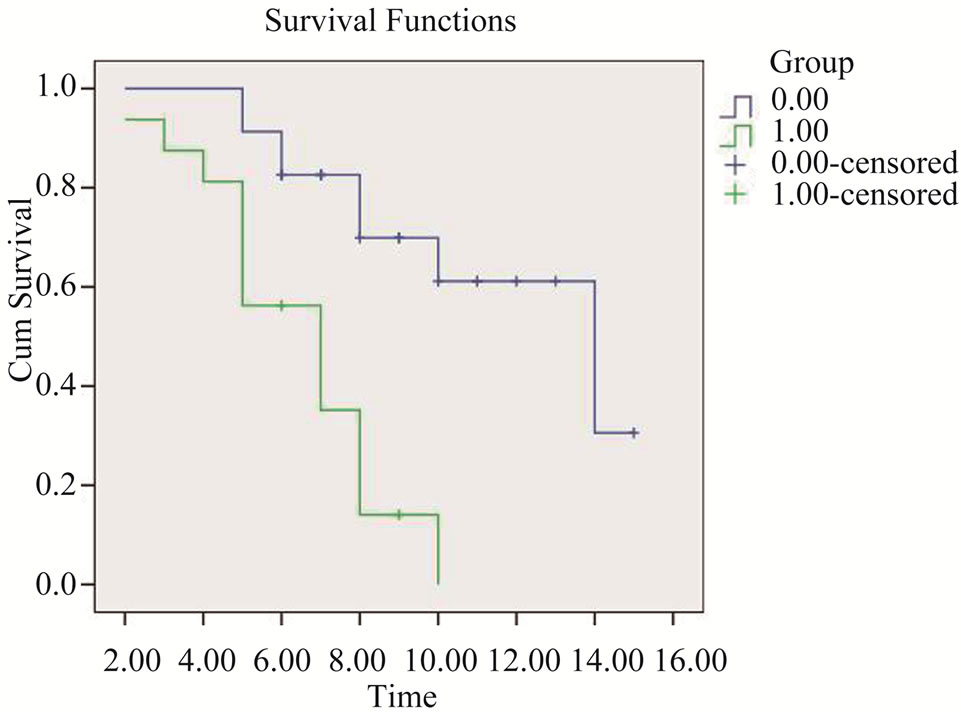
Figure 6. Survival functions of group 0 (patients without distant metastases) and group 1 (patients with distant metastases).
group 1 (patients with distant metastases) was 7 ± 1.17 months. Survival functions of two groups were difference statistically by using Log-Rank test (X2 = 14.5, P = 0.000).
4. Discussion
Pancreatic cancer is one of the most lethal human cancers and it continues to be a major unsolved health problem worldwide. Only patients with localized disease and a tumor size less than 2 cm with no lymph node metastases can expect long-term survival after surgery [1,2,7]. Therefore, increased efforts should be focusing on diagnosing the early stage disease, staging and predicting prognosis accurately before surgery. The preoperative differentiation between malignant and benign lesions in patients with suspected pancreatic carcinoma remains a diagnostic challenge. In contrast to the inherent limitations of anatomic imaging modality as CT, 18F-FDG PET/CT imaging, which integrate functional imaging and anatomic imaging, appears to represent a significant advance in the detection and staging of pancreatic malignancy. However, non-enhanced PET/CT also has false-positive (negative) interpretations and was difficult in assessing vascular invasion, which can be improved by combining enhanced CT. combining PET/CT with enhanced CT is promising to increase diagnosis accuracy and staging, performing one-stop shop imaging for diagnosing and staging of pancreatic carcinoma [3].
The role of combined PET/CT with enhanced CT in diagnosis pancreatic diseases.
In this study, we compared the clinical value of non-enhanced PET/CT, enhanced PET/CT in diagnosing pancreatic diseases, which showed that combined PET/ CT with enhanced CT is extremely useful in the detection and diagnosis of pancreatic carcinoma. PET/CT had higher sensitivity than enhanced CT, which is in the same range as those reported by other investigators who reported sensitivity of 85% - 100%, specificity of 67% - 99% and accuracy of 85% - 93% [7-13]. Combined PET/CT with enhanced CT raised sensitivity and specificity. So, it is of great value to combine enhanced CT in diagnosing pancreas diseases.
Benign inflammatory lesions, including chronic active pancreatitis with abscess formation, can accumulate 18F-FDG and result in false-positive interpretations on PET images [14,15]. In this setting, five inflammatory lesions accumulated 18F-FDG and result in false-positive interpretations on PET images which caused lower specificity (Figure 3 showed). Non-enhanced CT correctly differentiated three inflammatory lesions but failed in the other two, which was correct diagnosed by enhanced CT.
PET has limited spatial resolution compared with CT. Small lesions that suffer from partial volume averaging can lead to false-negative interpretation [16]. There were 2 false-negative cases smaller than 2 cm in the setting as Figure 4 showed. There was correlation between the SUVmax and the maximum diameter of the primary lesion. So we should pay more attention to small lesions with diameter less than 2 cm for the higher false-negative rate in PET diagnosis. PET/CT combined by enhanced CT is useful.
The role of 18F-FDG PET in staging of the disease.
Accurate staging is imperative to select optimal therapeutic plan and improve survival after curative resection. This study showed enhanced PET/CT being of great superiority in preoperative pancreatic cancer staging.
Firstly, enhanced PET/CT showed advantages in vascular invasion assessment. Infiltration of the superior mesenteric artery or celiac trunk is not suitable for surgery. Non-enhanced PET/CT can only show few serious vascular invasions, two of seventeen in this study. Combined with enhanced CT, most vascular invasions can be detected, which can apply accurate information for better therapy decisions. Vessels can be delineated clearly in enhanced CT and three dimensional imagines, such as volume rendered images, multiple planar reconstructions imagines, can help to assess artery infiltration accurately.
Second, it is hard for both 18F-FDG PET and CT in diagnosing lymph nodes metastases. Sensitivity of non-enhanced PET/CT in detecting lymph metastases is 50% and enhanced PET/CT is 66.7%. Previous papers reported that one possible reason for the apparent low sensitivity of 18F-FDG PET was the close proximity of the peripancreatic and paraaortic lymph node to the primary tumor, which can obscure their detection [2]. Combined PET/CT with enhanced CT, sensitivity improved a little to 66.7% because enhanced CT can supply higher density resolution, which help to distinguish lymph node metastases from other peripheral tissue.
The important impact of 18F-FDG PET and PET/CT on staging has been in its ability to identify distant metastases [3,17]. According to the previous reports, the sensitivity of 18F-FDG PET for detecting hepatic metastases is about 70% [18]. In this study, fourteen patients were confirmed to have metastases in liver and enhanced PET/CT detected thirty. Liver was the most frequently affected organ and enhanced PET/CT had high sensitiveity.
PET alone is difficult to find lung metastases especially smaller than 1 cm. CT provided more in find small lung metastases, and PET/CT increased sensitivity into 100%. Enhanced PET/CT did not supply more in finding lung metastases than non-enhanced PET/CT. Sensitivity in detecting bone metastases is high for each imaging modality.
Accurately staging and predicting the prognosis of pancreatic cancer play an important role in helping to select proper treatment plan. Enhanced PET/CT can help to make correct staging and proper therapy plan as our study and previous articles reported [3]. SUV may represent tumor growth and also resemble the tumor’s biological behavior. In this study, we also analyzed the correlation between SUV and distant metastases, survival time, which seldom reported by previous articles [3-7]. The results of our study were that the SUVmax did not correlate with distant metastases and survival time, while distant metastases correlate with survival time. Median survival time of patients without distant metastases was longer than which of patients with distant metastases (X2 = 14.5, P = 0.000). The important impact of enhanced PET/CT on staging has been in its ability to identify distant metastases, thus we can accurately predict survival status.
5. Conclusion
In conclusion, enhanced PET/CT is of greater value to the diagnosis of pancreatic lesions than other methods as non-enhanced PET/CT, as well as preoperative staging especially in assessing vascular invasion and distant metastasis. It is feasible to perform one-stop shop imaging by combining PET/CT with enhanced CT, supplying more accurate assessment before operation and help to select optimal therapeutic plan.
6. Acknowledgements
This work was supported by Shanghai Leading Academic Discipline Project (Project Number: S30203), China,the China Ministry of Health Sector Funds (201002020), and Chinese Nature Science Foundation Project (NSFC 30900375).
REFERENCES
- D. Li, K. Xie, R. Wolff, et al., “Pancreatic Cancer,” The Lancet, Vol. 363, No. 9414, 2004, pp. 1049-1057. doi:10.1016/S0140-6736(04)15841-8
- F. Pakzad, A. M. Groves and P. J. Ell, “The Role of Positron Emission Tomography in the Management of Pancreatic Cancer,” Seminars in Nuclear Medicine, Vol. 36, No. 3, 2006, pp. 248-256. doi:10.1053/j.semnuclmed.2006.03.005
- K. Strobel, S. Heinrich, U. Bhure, J. Soyka, P. Veit-Haibach, B. C. Pestalozzi, P. A. Clavien and T. F. Hany, “Contrast-Enhanced 18F-FDG PET/CT: 1-Stop-Shop Imaging for Assessing the Resectability of Pancreatic Cancer,” Journal of Nuclear Medicine, Vol. 49, No. 9, 2008, pp. 1408-1413. doi:10.2967/jnumed.108.051466
- O. W. Hamer and S. Feuerbach, “How Useful Is Integrated PET and CT for the Management of Pancreatic Cancer?” Nature Reviews Gastroenterology & Hepatology, Vol. 3, No. 2, 2006, pp. 74-75.
- C. M. Parsons, J. L. Sutcliffe and R. J. Bold, “Preoperative Evaluation of Pancreatic Adenocarcinoma,” Journal of Hepato-Biliary-Pancreatic Surgery, Vol. 15, No. 4, 2008, pp. 429-435. doi:10.1007/s00534-007-1240-7
- C. Sperti, C. Pasquali, F. Chierichetti, et al., “18-Fluorodeoxyglucose Positron Emission Tomography in Predicting Survival of Patients with Pancreatic Carcinoma,” Journal of Gastrointestinal Surgery, Vol. 7, No. 8, 2003, pp.539-959. doi:10.1016/j.gassur.2003.09.002
- V. Casneuf, L. Delrue, A. Kelles, N. Van Damme, J. Van Huysse, F. Berrevoet, M. De Vos, P. Duyck and M. Peeters, “Is Combined 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Superior to Positron Emission Tomography or Computed Tomography Alone for Diagnosis, Staging and Restaging of Pancreatic Lesions?” Acta Gastro-Enterologica Belgica, Vol. 70, No. 4, 2007, pp. 331-338.
- J. C. Slollfuss, G. Glatting, H. T. Friess, et al., “F-(Fluorine-fluoro-2-deoxy-d-glucose PET in Detection of Pancreatic Cancer: Value Quantitative Image Interpretation,” Radiology, Vol. 175, No. 2, 1995, pp. 339-344.
- H. Saisho and T. Yamaguchi, “Diagnostic Imaging for Pancreatic Cancer: Computed Tomography, Magnetic Resonance Imaging, and Positron Emission Tomography,” Pancreas, Vol. 28, No. 3, 2004, pp. 273-278. doi:10.1097/00006676-200404000-00011
- C. L. Ho, F. Dehdashti, L. K. Griffeth, et al., “F-18 FDGPET Evaluation of Indeterminate Pancreatic Masses,” Journal of Computer Assisted Tomography, Vol. 20, No. 3, 1996, pp. 363-369. doi:10.1097/00004728-199605000-00006
- C. Sperti, S. Bissoli, C. Pasquali, L. Frison, G. Liessi, F. Chierichetti and S. Pedrazzoli, “18-Fluorodeoxyglucose Positron Emission Tomography Enhances Computed Tomography Diagnosis of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas,” Annals of Surgery, Vol. 246, No. 6, 2007, pp. 932-939. doi:10.1097/SLA.0b013e31815c2a29
- J. M. Farma, A. A. Santillan, M. Melis, J. Walters, D. Belinc, D. T. Chen, E. A. Eikman and M. Malafa, “PET/ CT Fusion Scan Enhances CT Staging in Patients with Pancreatic Neoplasms,” Annals of Surgical Oncology, Vol. 15, No. 9, 2008, pp. 2465-2471. doi:10.1245/s10434-008-9992-0
- H. Minn, K. R. Zasadny, L. E. Quint and R. L. Wahl, “Lung Cancer: Reproducibility of Quantitative Measurements for Evaluating 2-[F-18]-Fluoro-2-deoxy-d-glucose Uptake at PET,” Radiology, Vol. 196, No. 1, 1995, pp. 167-173.
- T. Higashi, T. Saga, Y. Nakamoto, I T. shimori, K. Fujimoto, R. Doi, M. Imamura and J. Konishi, “Diagnosis of Pancreatic Cancer Using Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography (FDG PET)—Usefulness and Limitations in Clinical Reality,” Annals of Nuclear Medicine, Vol. 17, No. 4, 2003, pp. 261-279. doi:10.1007/BF02988521
- M. Zimny, U. Buell, C. G. Diedrichs, et al., “False-positive FDG-PET in Patients with Pancreatic Masses: An Issue of Proper Patient Selection?” European Journal of Nuclear Medicine and Molecular, Vol. 25, No. 1325, 1998, p. 1352.
- H. E. J. offman, S. C. Huang, M. E. Phelps, et al., “Quantitation in Positron Emission Computed Tomography: Effect of Object Size,” Journal of Computer Assisted Tomography, Vol. 3, No. 3, 1979, pp. 299-308.
- S. Bang, H. W. Chung, S. W. Park, et al., “The Clinical Usefulness of 18-Fluorodeoxyglucose Positron Emission Tomography in the Differential Diagnosis, Staging, and Response Evaluation after Concurrent Chemoradiotherapy for Pancreatic Cancer,” Journal of Clinical Gastroenterology, Vol. 40, No. 10, 2006, pp. 923-929. doi:10.1097/01.mcg.0000225672.68852.05
- C. G. Diederichs, L. Staib, J. Vogel, et al., “Values and Limitations of 18F-Fluorodeoxyglucose-Positron-Emission Tomography with Preoperative Evaluation of Patients with Pancreatic Masses,” Pancreas, Vol. 20, No. 2, 2000, pp. 109-116. doi:10.1097/00006676-200003000-00001
NOTES
*Corresponding author.

