Journal of Cancer Therapy
Vol.2 No.5(2011), Article ID:16612,9 pages DOI:10.4236/jct.2011.25089
D-Aminoacid Oxidase-Fe2O3 Nanoparticle Complex Mediated Antitumor Activity in Swiss Albino Mice
![]()
1Amala Cancer Research Centre, Amala Nagar, Thrissur, India; 2Department of Materials Science, Tmfy-MSE, Royal Institute of Technology, Stockholm, Sweden; 3Pushpagiri Institute of Medical Sciences & Research Centre, Thiruvalla, India.
E-mail: *ckknair@yahoo.com
Received October 20th, 2011; revised November 21st, 2011; accepted December 5th, 2011.
Keywords: D-Aminoacid oxidase, oxidation therapy, Fe2O3, antitumor, nanoparticles, comet Assay, apoptosis
ABSTRACT
D-Aminoacid oxidase (DAO) was isolated from fresh porcine kidney; its cytotoxic potential was studied under in vitro and in vivo conditions. The isolated DAO was complexed with Fe2O3 nanoparticles and its potential as an oxidation therapeutic agent was analysed. The ability of the complex in eliciting H2O2 mediated cytotoxicity was studied on Dalton’s lymphoma ascites cells (DLA). The induction of apoptosis in DLA cells by Fe2O3-DAO complex was studied by morphological examination and alkaline single cell gel electrophoresis (comet assay). The antitumor activity of the complex was investigated by oral administration of the complex and the substrate D-alanine to tumor bearing Swiss albino mice and by targeting the complex to the tumor site, using an externally applied magnetic field. Fe2O3-DAO along with D-alanine showed remarkable cytotoxicity in a substrate concentration-dependent manner. Both morphological examination and comet assay revealed that Fe2O3-DAO/D-alanine induced apoptosis. Oral administration of Fe2O3-DAO and D-alanine along with magnetic targeting significantly suppressed tumor growth in mice. The present report provides the first evidence for the promising application of enzyme bound nanoparticles for targeted oxidation therapy.
1. Introduction
Reactive oxygen species (ROS) e.g., superoxide anion (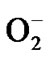 ), hydrogen peroxide (H2O2), and hydroxyl radical (
), hydrogen peroxide (H2O2), and hydroxyl radical (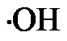 ) are generated in all aerobic organisms and are indispensable for signal transduction pathways that regulate cell growth [1] and reduction-oxidation (redox) status. However, overproduction of these highly reactive metabolites can initiate lethal chain reactions that involve oxidation of various bio molecules and damage cellular integrity and survival. If they can be selectively delivered into tumors, ROS may exert remarkable antitumor potential due to their reaction with vital cellular targets. This strategy of cancer treatment by generation of ROS selectively in tumor cells has been named “oxidation therapy” [2,3]. In oxidation therapy, the principle is to generate excess ROS selectively in tumor tissue causing maximum tumor killing without affecting the normal tissues.
) are generated in all aerobic organisms and are indispensable for signal transduction pathways that regulate cell growth [1] and reduction-oxidation (redox) status. However, overproduction of these highly reactive metabolites can initiate lethal chain reactions that involve oxidation of various bio molecules and damage cellular integrity and survival. If they can be selectively delivered into tumors, ROS may exert remarkable antitumor potential due to their reaction with vital cellular targets. This strategy of cancer treatment by generation of ROS selectively in tumor cells has been named “oxidation therapy” [2,3]. In oxidation therapy, the principle is to generate excess ROS selectively in tumor tissue causing maximum tumor killing without affecting the normal tissues.
Among the ROS, H2O2 readily crosses cellular membranes and causes oxidative damage to DNA [1] proteins[4] and lipids by direct oxidation [5]. It was also reported that H2O2 induces apoptosis in many tumor cells in vitro [6,7] via activation of the caspase cascade. H2O2 is relatively unstable and is a small water-soluble molecule. Those characteristics hamper the utility of H2O2 as an antitumor agent in vivo for selective delivery to tumor site. H2O2 used alone was ineffective when injected into tumor or into the circulation [8,9] because of its rapid clearance and decomposition by catalase in erythrocytes. Thus the use of H2O2-generating enzymes has been proposed as an alternative approach to an H2O2 dependent oxidation therapy. In our study we isolated, D-aminoacid oxidase (DAO) from porcine kidney and exploited its potential as an H2O2-generating enzyme. D-Aminoacid oxidase (DAO) (D-aminoacid: oxygen oxidoreductase (deaminating); EC 1.4.3.3) catalyzes the oxidative deamination of D-amino acids, producing the corresponding α-keto acid and ammonia with concomitant reduction of molecular oxygen to hydrogen peroxide (H2O2). The generation of H2O2 can be easily modulated by varied infusion of its substrates (D-aminoacids) as shown by the in vitro data. Since the D-amino acids are scarce in mammalian organisms, it is to be introduced as the substrate for effective oxidation therapy [10].
DAO have a short in vivo half-life. The molecular size of DAO (Mr 39,000) is slightly smaller than the renal excretion threshold (Mr 50,000), so it would be excreted gradually as observed previously for other small proteins or polymer drugs smaller than Mr 40,000 [11]. To overcome this drawback, DAO was conjugated with a Fe2O3 (coated with Poly Vinyl Pyrolidone (PVP)) nanoparticles. Earlier works showed that chemical modification results in an increased in vivo half-life, a reduced antigenicity of the native protein, inhibition of proteolytic degradation and also helps in the effective targeting [10,12]. The complexing of the enzyme with nanoparticles improved the stability of the enzyme (unpublished data). Earlier reports from our lab and several others showed that Fe3O4- doxorubicin complexes can be targeted directly to the site of the tumor by using external magnetic field [13].
Earlier studies revealed that biocompatible macromolecules accumulate and remain in solid tumor because of the unique characteristics of the tumor vasculature and the impaired lymphatic clearance system. This phenomenon was named the ‘EPR effect’ of macromolecules and lipids in solid tumor. This EPR effect was validated in many experimental solid tumors [14,15]. In the present work we are trying to exploit the potential of magnetic nanopartcle—enzyme complex to be targeted using an external magnetic field and the EPR effect by which the complex once entered the tumor may get trapped within the tumor vasculature. In this paper, we describe the isolation and purification of DAO, synthesis of Fe2O3 nanoparticles and preparation of Fe2O3 nanoparticles— DAO complex. The prepared complex was further analysed for its anti tumor potential under in vitro conditions. The in vivo antitumor activity was analysed in tumor bearing swiss albino mice by targeting the nanoparticleDAO complexes to the tumor site using an externally applied magnetic field, after oral administration of the complexes and D-alanine (substrate).
2. Materials and Methods
2.1. Animals
Female Swiss albino mice of 8 - 10 weeks old, weighing 22 - 25 g were obtained from the Small Animal Breeding Section (SABS), Mannuthy, Thrissur, Kerala. They were kept under standard conditions of temperature and humidity in the Centre’s Animal House Facility. The animals were provided with standard mouse chow (Sai Durga Feeds and Foods, Bangalore, India) and water adlibitum. All animal experiments in this study were carried out with the prior approval of the Institutional Animal Ethics Committee (IAEC) and were conducted strictly adhering to the guidelines of Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by the Animal Welfare Division of Government of India.
2.2. Chemicals
Sodium pyrophosphate, D-alanine, 4-amino-3-hydrazino -5-mercapto-1,2,4-triazole (purpald reagent) and Poly Vinyl Pyrolidone (PVP) were from Sigma chemical company Inc., St.louis, MO, USA. Sodium benzoate and ammonium sulphate ((NH4)2SO4) were from Himedia Laboratories Pvt. Ltd. Mumbai. All other reagents were all of analytical grade and purchased from reputed Indian manufacturers.
2.3. Preparation of Fe2O3 Nanoparticles Coated with PVP
Fe2O3 nanoparticles were prepared by co-precipitation technique in an inert atmosphere. A typical synthesis process is discussed below. In brief, stoichiometric quantities of metal chlorides (ferric chloride and ferrous chloride 2:1 molar ratio) were dissolved in milli-Q water. Ammonia solution (28%) was added drop wise into the metal chloride solution with vigorous stirring until the pH became 11 - 12, and subsequently heating the slurry at 80˚C for 1 h. PVP (K-15, M.W = 15,000) was added to the above slurry and further heated for 1 h and cooled to room temperature and the salt solution was decanted and the precipitate was repeatedly washed to remove any impurity ions. The resulting Fe3O4 nanoparticles were heated at 180˚C - 210˚C to yield Fe2O3 nanoparticles [13, 16].
2.4. Isolation of Enzyme
DAO was isolated from fresh porcine kidney using ammonium sulphate ((NH4)2SO4) precipitation technique [17]. Fresh porcine kidneys were obtained from Veterinary College, Mannuthy, Thrissur, Kerala, India. It was homogenized in 0.016 M pyrophosphate buffer (pH 8.3). To the solution, Sodium benzoate (1 g/L) was added and heated at 40˚C for 30 minutes and then was cooled at 25˚C. After cooling, pH was adjusted to 5.2 with 2N acetic acid and centrifuged at 10,000 rpm for 10 minutes. After centrifugation the supernatant was taken and 250 g/L ammonium sulphate was added. Again centrifugation was carried out at 10,000 rpm to obtain the precipitate which was dissolved in 0.016 M pyrophosphate buffer (pH 8.3). Heating was repeated at 52˚C, and centrifugation and reprecipitation with ammonium sulphate was performed thrice. The final precipitate was dissolved in pyrophosphate buffer and dialyzed against the same buffer. The final enzyme was stored at –20˚C.
2.5. Enzyme Activity of DAO
Enzyme activity was determined colorimetrically using, 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (purpald reagent) as a colouring reagent [18] and color developed was measured at 550 nm. D-Alanine was used as the substrate with an initial concentration of 0.1 M. The enzyme reaction was carried out at 37˚C in 0.133 M pyrophosphate buffer (pH 8.3), where 1 unit of DAO activity is defined as the rate of formation of 1 µmol of H2O2 per min.
2.6. Preparation of Fe2O3-DAO Complex
Fe2O3 (300 mg) was mixed with 0.3 ml of isolated DAO (Enzyme activity—12.6 U/ml, protein concentration—1 mg/ml) in 0.133 M pyrophosphate buffer (pH 8.3), incubated overnight at 4˚C in refrigerator and was sonicated using an ultrasonicator for ten minutes. The enzyme nanoparticle complex was centrifuged at 15,000 rpm for 15 minutes. The supernatant was collected and the pellet was suspended in 0.5 ml of 0.133 M pyrophosphate buffer (pH 8.3) and kept in refrigerator. Enzyme activity of both the pellet and supernatant were measured. As the enzyme activity was detected only in pellets, it is further used for the experimental studies.
2.7. Cytotoxic effect of Fe2O3-DAO in Presence of Different Concentrations of D-Alanine, in Vitro
The ability of Fe2O3-DAO complex to produce H2O2 in presence of different concentrations of D-alanine was determined. In vitro cytotoxicity of the complex towards DLA (Dalton’s lymphoma ascites) cells was also analysed. The DLA cells (1 × 106 cells/ml) were incubated with Fe2O3-DAO complex in presence of different concentrations of D-alanine. After the incubation, the cell viability was estimated by means of Trypan blue dye exclusion method. The percentage mortality of cells was calculated and a graph was plotted with percentage mortality of cells against time of incubation (in min).
2.8. Induction of Apoptosis in Vitro
DLA cells were aspirated from ascitic mice. The cells were washed three times using phosphate buffered saline (PBS). The cells (1 × 106 cells/ml) were incubated in DMEM medium containing 10% foetal calf serum (FCS) in presence or absence of the Fe2O3-DAO complex with 0.2 M D-alanine.
2.9. Morphological Analysis
To detect the morphological changes during apoptosis, cell suspension was smeared on a clean glass slide. The smear was stained with May-Grunwald-Giemsa staining. The slides were observed under bright field microscope for apoptotic cells (having condensed or fragmented nuclei and cytoplasmic blebbing). Apoptotic index was calculated using the equation.
Apoptotic index
= (Number of apoptotic cells/Total number of cells) × 100
2.10. Comet Assay
In order to detect apoptosis induction by Fe2O3-DAO complex, on DLA cells, comet assay was performed [19] with minor modifications. Microscopic slides were coated with 200 μl of 0.8% low melting point agarose containing 50 μl of DLA cell suspensions and the slides were kept at 4˚C. After solidification, the slides were immersed in prechilled lysing solution containing 2.5 M NaCl, 100 mM Na2 EDTA, 10 mM Tris-HCl, pH 10, 1% DMSO, 1% Triton X and kept for 1 hour at 4˚C. After lysis, slides were drained properly and placed in a horizontal electrophoretic apparatus filled with freshly prepared electrophoresis buffer containing 300 mM NaOH, 1 mM EDTA, 0.2% DMSO, pH ≥ 13. The slides were equilibrated in buffer for 20 minutes and electrophoresis was carried out for 30 minutes at 20 V, 300 mA. After electrophoresis the slides were washed gently with 0.4 mM Tris-HCl buffer, pH-7.4 to remove alkali. The slides were again washed with distilled water, kept at 37˚C for 2 hours to dry the gel and silver staining was carried out [20-23].
2.11. In Vivo Antitumor Activity of Fe2O3-DAO
Solid Tumor in Animals-Solid tumor was grown in hind limbs of animals by transplanting DLA cells (1 × 106 cells/animal) simultaneously in to the right hind legs of thirty female Swiss albino mice (6 - 8 weeks old, body weight 20 - 25 g). When the tumor was grown to a diameter 0.8 - 10 mm (10th day after tumor transplantation), the animals were divided into six groups of five animals each. The animals were orally administered with the Fe2O3 nanoparticles complexed with the enzyme DAO, as well as nanoparticles and enzyme alone or water. Thirty minutes later, the animals were orally administered with Dalanine (0.5 mmoles in 0.2 ml/mouse) and treated as detailed below:
Group I: 0.1 ml Distilled water;
Group II: 0.1 ml Fe2O3 (30 mg/ml);
Group III: 0.1 ml Fe2O3-DAO (30 mg Fe2O3/ml, 0.1 mg protein/ml);
Group IV: 0.1 ml DAO (0.1 mg protein/ml) + D-alanine (0.5 mmol/mouse);
Group V: 0.1 ml Fe2O3-DAO (30 mg Fe2O3/ml, 0.1 mg protein/ml) + D-alanine (0.5 mmol/mouse);
Group VI: 0.1 ml Fe2O3-DAO (30 mg Fe2O3/ml, 0.1 mg protein/ml) + D-alanine (0.5 mmol/mouse) + magnetic treatment for 15 minutes.
The animals in the groups II, III and VI were given a magnetic treatment for 15 minutes on the tumor bearing limb with a horse shoe magnet (1 G field) each day, 20 minutes after the D-alanine administration. The treatments were continued for five consecutive days. The hind leg thicknesses were measured using a vernier calliper once in two days from 10th day of tumor induction. The tumor volume was calculated as follows:
Tumor radius
= Radius of tumor induced leg − Radius of normal leg Tumor volume = 4/3π r3 where r is the tumor radius.
2.12. Statistical Analysis
The results are presented as mean ± SD of the studied groups. Statistical analyses of the results were performed using ANOVA with Tukey-Kramer multiple comparisons test.
3. Results
3.1. Enzyme Purification
Enzyme activity and specific activity was determined at every stages of isolation. The yield and activity of the enzyme at various stages of the purification is summarized in Table 1.
The recovery of the enzyme was 11.72%. The final specific activity of the DAO at 37˚C is 0.2538 U/mg protein.
3.2. Characterization of Fe2O3 and Fe2O3-DAO Complexes
The FTIR spectra of magnetic Fe2O3 and Fe2O3-DAO are shown in the Figures 1 and 2. A weak peak at 2061.88 cm−1 appeared in the spectra of Fe2O3-DAO could be assigned to the C-O bonds in the enzyme molecule. This peak was absent in the spectra of Fe2O3 indicating enzyme attachment onto the particles. The results of the XRD analysis of the nanoparticle and enzyme complexes are presented in Figures 3 and 4.
3.3. In Vitro Cytotoxicity of Fe2O3-DAO
Cytotoxicity of Fe2O3-DAO was first examined via an in vitro system using Dalton’s lymphoma ascites (DLA) cells. Different concentrations of D-alanine was used as the substrate because of its high turnover rate. DAO alone and D-alanine alone showed no marked cytotoxicity against DLA cells. In contrast, Fe2O3-DAO along with D-alanine showed remarkable cytotoxicity in a substrate concentration-dependent manner (Figure 5). This is attributed to the H2O2 generation by the enzyme-substrate reaction system. Maximum percentage mortality (99%) was obtained when 50 mM D-alanine was used.
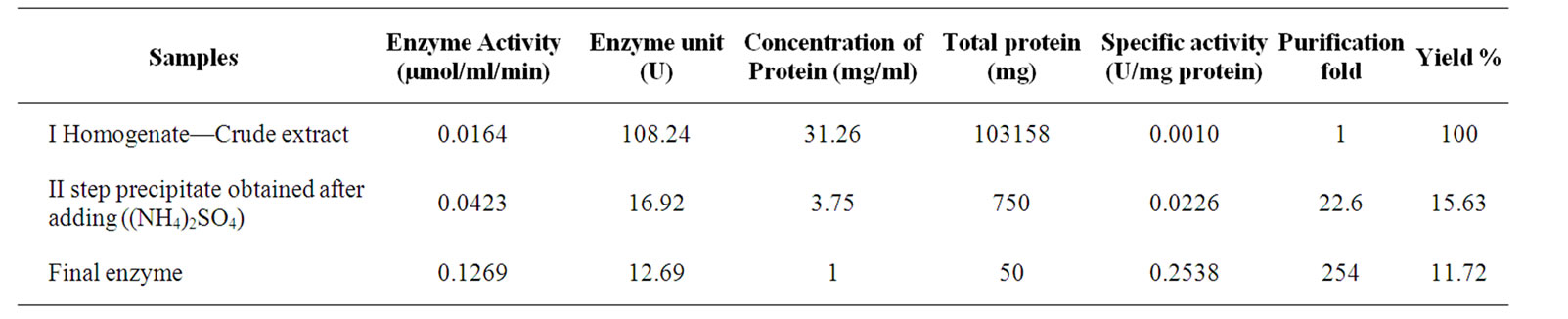
Table 1. Characterization of DAO at various steps of isolation.

Figure 1. FTIR spectra of Fe2O3 magnetic nanoparticles.
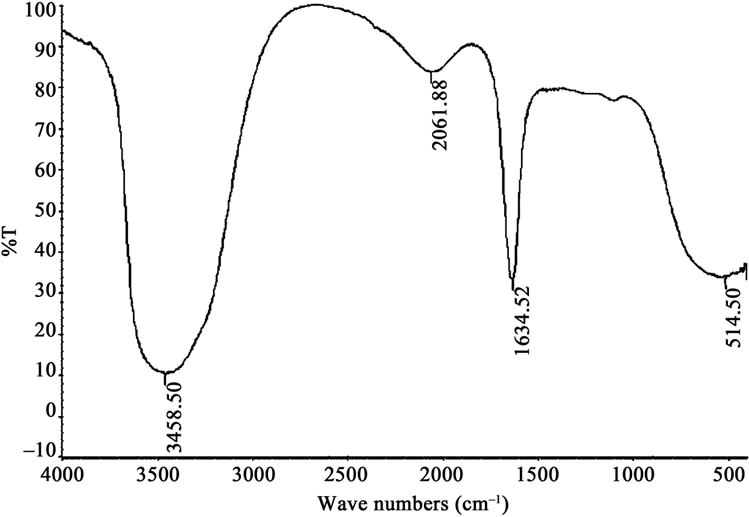
Figure 2. FTIR spectra of Fe2O3-DAO complex.
3.4. Induction of Apoptosis in DLA Cells, in Vitro
Induction of apoptosis on DLA cells was studied by analyzing the morphological pattern of DLA cells. The apoptotic cells were visualized as having cytoplasmic blebbing and nuclear shrinkage (Figure 6). The results showed that D-alanine along with Fe2O3-DAO showed maximum apoptotic index (95.5%). The DAO (0.36 U) along with substrate (0.2 M) also gave an apoptotic index of about 85.2 ± 5.55. The cells incubated with D-alanine or Fe2O3-DAO alone showed less apoptosis. The results are shown in Table 2.
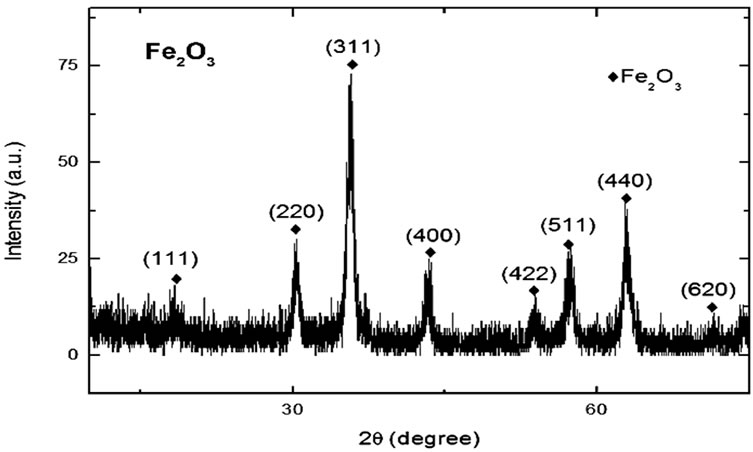
Figure 3. XRD pattern of Fe2O3 nanoparticles coated with PVP.

Figure 4. XRD pattern of Fe2O3-DAO complex.
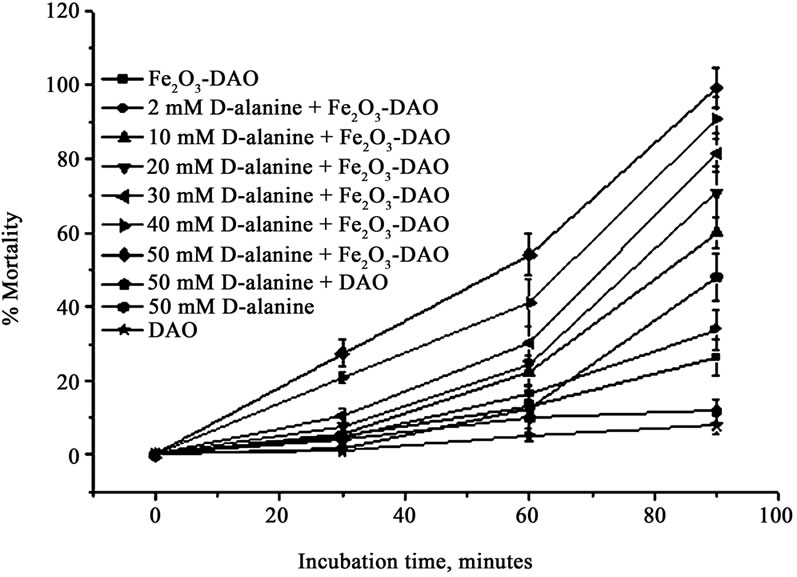
Figure 5. Cytotoxic effect of Fe2O3-DAO in presence of different concentrations of D-alanine on DLA cells in vitro.
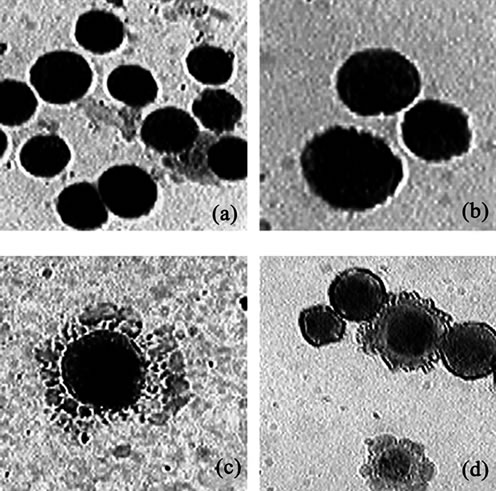
Figure 6. Representative figures of DLA cells showing apoptosis after May-Grunwald-Giemsa staining. (a) Untreated cells; (b) Cells treated with Fe2O3-DAO; (c) Cells treated with Fe2O3-DAO and D-alanine showing cytoplasmic blebbing; (d) Cells treated with DAO and D-alanine showing apoptotic and non-apoptotic cells.
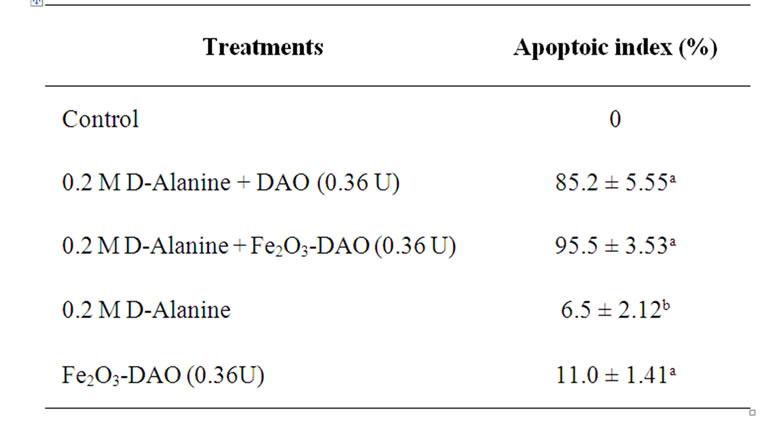
Table 2. Apoptoic index in DLA cells treated with Fe2O3- DAO in presence of D-alanine. (“a” represents, p < 0.001 compared to respective control, “b” represents, p < 0.05 compared to respective control).
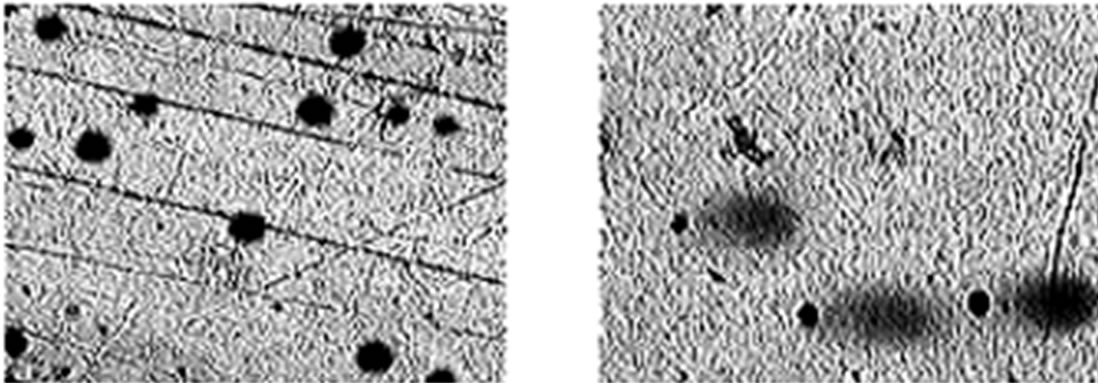
To further study the induction of apoptosis, DLA cells incubated with Fe2O3-DAO and D-alanine were subjected to comet assay (Figure 7). Cellular DNA damage was observed when DLA cells were treated with Fe2O3- DAO along with substrate D-alanine. The comets posses a fan like tail indicative of apoptosis (Figure 7(b)). The extent of DNA damage produced was nearly equal when the cells treated with DAO with its substrate D-alanine (Figure 7(c)). The cells treated with Fe2O3-DAO alone (Figure 7(d)) and D-alanine alone (Figure 7(e)) did not exhibit any DNA damage which may be due to the lack of formation of H2O2 at the reaction site. Thus the result provided the extent of cellular DNA damage exerted through the induced oxidative stress by H2O2 generated in situ through enzyme action.
 (a)
(a)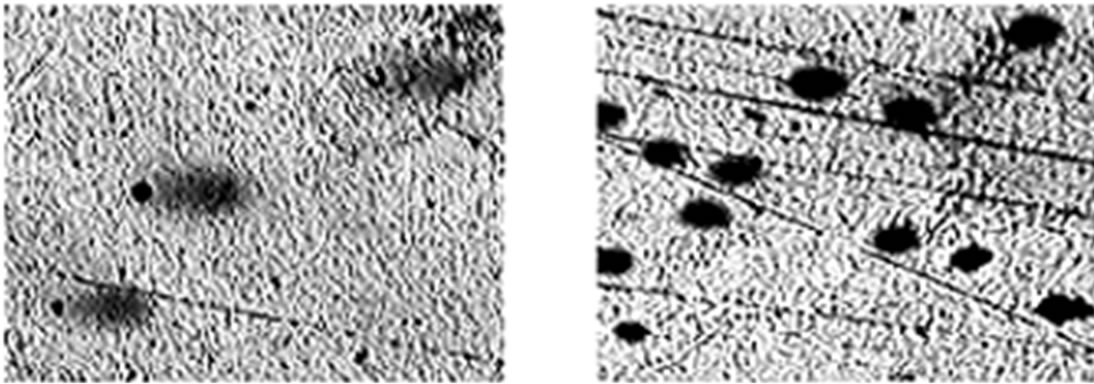 (b)
(b)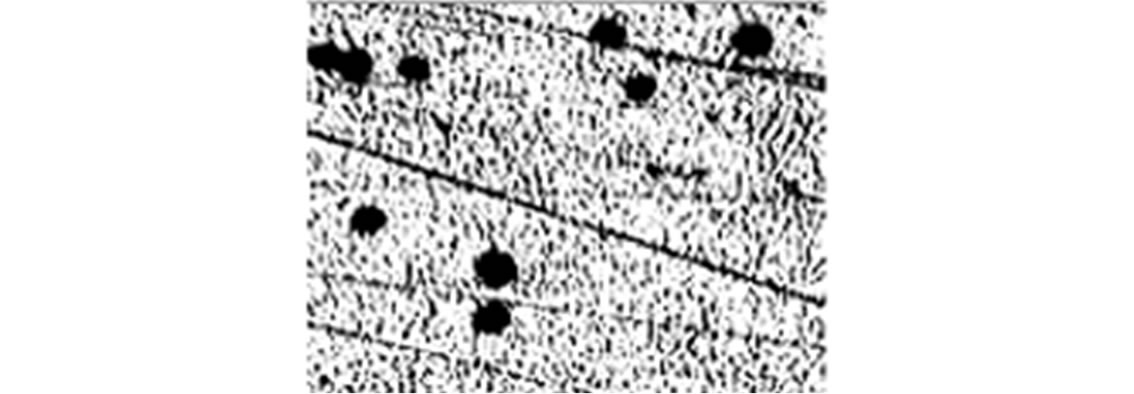 (c)
(c)
Figure 7. Representative images of DLA cells after comet assay. (a) The untreated DLA cells; (b) DLA cells treated with Fe2O3-DAO and D-alanine showing Fan-like comets indicative of apoptosis; (c) DLA cells treated with enzyme (DAO) and D-alanine showing DNA damaged and apoptotic comets; and (d) DLA cells treated with Fe2O3-DAO; (e) DLA cells treated with D-alanine.
3.5. In Vivo Antitumor Activity of Fe2O3-DAO
Fe2O3-DAO was administrated orally and after an adequate lag time to allow the accumulation of Fe2O3-DAO in the tumor by the application of an external magnetic field (Figures 8 and 9), the substrate D-alanine was administered. This treatment procedure allows generation of cytotoxic H2O2, predominantly at the tumor site.
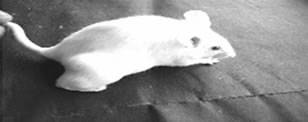
After oral administration of DAO-Fe2O3 nanoparticles and D-alanine, the tumor sites of tumor bearing animals were allowed to place under the influence of an external magnetic field “m” by keeping a magnet at the tumor site for 15 minutes every day for 5 consecutive days. The results are presented in Figures 8 and 9. Figure 9 gives representative photographs of the animals and their tumor bearing limbs and Figure 8 presents the data on the tumor volume, following the treatments.
As shown in Figure 9(f1), tumor growth was signifycantly suppressed in mice administered Fe2O3-DAO and D-alanine along with magnetic treatment in the fourth day. In contrast, no significant antitumor effect was observed in mice treated with native Fe2O3-DAO plus Dalanine without magnetic treatment (Figures 9(e1) and (e2)). Fe2O3-DAO and D-alanine alone treatment did not show any antitumor activity (Figures 9(c1), (c2), (d1), and (d2)).
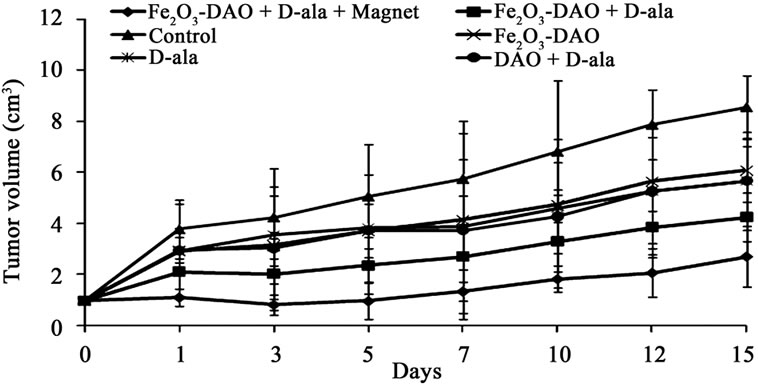
Figure 8. Effect of administration of Fe2O3 nanoparticles coated with PVP and complexed with DAO and magnetic targeting on growth of DLA solid tumor on hind limb of mice. After transplanting the DLA cells (1 × 106 cells), on 10th day the tumor grows to a size of 1 cm3 and different treatments are started as detailed in the text.
 (a)
(a) (b1) (b2)
(b1) (b2) (c1) (c2)
(c1) (c2) (d1) (d2)
(d1) (d2) (e1) (e2)
(e1) (e2) (f1) (f2)
(f1) (f2)
Figure 9. Effect of administration of Fe2O3 nanoparticles coated with PVP and complexed with DAO and magnetic targeting on DLA solid tumor growth on hind limb of mice. (a) represent animals on the initial day of commencement of the experiment; (b1) & (b2) represents untreated control animals; (c1) & (c2) represents animals treated with Fe2O3-DAO; (d1) & (d2) represents animals treated with D-alanine; (e1) & (e2) represents animals treated with Fe2O3-DAO and Dalanine without magnetic treatment; (f1) & (f2) represents animals treated with Fe2O3-DAO and D-alanine with magnetic treatment. The suffix “1”indicates 4th day of treatment and “2” indicates 15th day of treatment.
4. Discussion
Reactive oxygen species (ROS), including hydroxyl radical (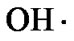 ), superoxide anion (
), superoxide anion ( ), peroxynitrite (ONOO–) and hydrogen peroxide (H2O2), are highly reactive and potentially hazardous by-products of cellular metabolism. Overproduction of ROS, e.g. H2O2, causes oxidative damage to the DNA, proteins and lipids leading to cell death. ROS are highly cytotoxic and may exert remarkable antitumor potential if they can be selectively produced in cells of tumors for “oxidation therapy” [10]. Nanoparticles can be used to target drugs to generate ROS at the tumor site. In our study we tried to exploit the potential of ferric nano complex of D-amino acid oxidase (Fe2O3-DAO) to generate H2O2 at tumor site when its substrate D-amino acids (D-alanine) are supplied exogenously. As D-amino acids are not usually present in mammalian organisms in significant levels it is to be administered exogenously.
), peroxynitrite (ONOO–) and hydrogen peroxide (H2O2), are highly reactive and potentially hazardous by-products of cellular metabolism. Overproduction of ROS, e.g. H2O2, causes oxidative damage to the DNA, proteins and lipids leading to cell death. ROS are highly cytotoxic and may exert remarkable antitumor potential if they can be selectively produced in cells of tumors for “oxidation therapy” [10]. Nanoparticles can be used to target drugs to generate ROS at the tumor site. In our study we tried to exploit the potential of ferric nano complex of D-amino acid oxidase (Fe2O3-DAO) to generate H2O2 at tumor site when its substrate D-amino acids (D-alanine) are supplied exogenously. As D-amino acids are not usually present in mammalian organisms in significant levels it is to be administered exogenously.
Under in vitro conditions, the killing of DLA cells were enhanced in presence of the Fe2O3-DAO and its substrate D-alanine. The result suggests that the cytotoxic mechanism of Fe2O3-DAO and its substrate is through inducing apoptosis, which was assessed by alkaline single cell gel electrophoresis or comet assay (figure 7). As shown in in vitro studies, DAO alone, D-alanine alone or Fe2O3- DAO alone did not have any cytotoxicity. The cytotoxicity became apparent only when DAO and D-alanine were accessible simultaneously (Figure 5). The cellular DNA damage induced by the ferric-enzyme-substrate complex on DLA cells is due to the induction of apoptosis in cells. The induction of apoptosis by ferric enzyme nanocomplex with D-alanine results in extensive damage to cellular DNA as revealed in comet assay. Here, a greater portion of DNA has fragmented and migrated sufficiently to make the long tail. The intense damage to cellular DNA might have caused the cells to go for apoptosis as revealed by morphological pattern of the DLA cells with cytoplasmic blebbing and nuclear shrinkage. Treatment of the cells with D-alanine along with Fe2O3-DAO showed an apoptotic index of 95.5% [24,25].
The new type of cancer therapy, described here, depends on targeting the H2O2-generating enzyme (DAO) to the tumor site using magnetic nanoparticles and the enzyme converting a pharmacologically inert substance (D-alanine) to a highly cytotoxic metabolite, H2O2. Recent studies have demonstrated that intravenous administration of DAO conjugated with poly ethylene glycol (PEG) along with intra peritoneal administration Dproline exhibited marked antitumor activity under in vivo condition by increasing ROS formation at the tumor site [10,12]. Tumor targeting by PEG-xanthine oxidase (PEGXO) complex along with the substrate hypoxanthine also has been reported to enhance the tumor-targeting efficacy and the antitumor activity of xanthine oxidase (XO) [26]. The present study, demonstrated the remarkable antitumor activity of two nontoxic components, Fe2O3-DAO and D-alanine on mouse solid tumor model. Tumor targeted delivery of Fe2O3-DAO by applying an external magnetic field was possible and subsequent administration of D-alanine selectively generated potent cytotoxic agent, H2O2 at the tumor site. Consequently, effective antitumor activity by H2O2 could be accomplished, minimising toxicity to normal tissues and organs. Key findings of the present study are: 1) tumor-targeted delivery of an H2O2-generating enzyme (DAO) can be accomplished; and 2) tumor regression due to generation of H2O2 by exogenous administration of the substrate, D-alanine.
The present study strongly suggested the feasibility of effectively controlling tumor growth by targeting orally administered nanoparticle bound enzyme with the help of an external magnetic field to enhance the efficacy of the treatment, suggesting the rationale of development of a new anticancer strategy.
5. Acknowledgements
SCAD thanks CSIR, Government of India for the award of JRF. Studies at Stockholm have been funded by The EU Erasmus program (KMS) and the Swedish Agency VINNOVA. KMS was on deputation from3 Department of Physics, Amrita Viswa Vidyapeetham University, Amritapuri Campus, Kollam 690 525, Kerala, India.
REFERENCES
- K. B. Bechman and B. N. Ames, “Oxidative Decay of DNA,” The Journal of Biological Chemistry, Vol. 272, No. 32, 1997, pp. 633-636.
- O. Ben-Yoseph and B. D. Ross, “Oxidation Therapy: The Use of a Reactive Oxygen Species-Generating Enzyme System for Tumor Treatment,” British Journal of Cancer, Vol. 70, 1994, pp. 1131-1135. doi:10.1038/bjc.1994.460
- L. D. Stegman, H. Zheng, E. R. Neal, O. Ben-Yoseph, L. Pollegioni, M. Pilone and B. D. Ross, “Induction of Cytotoxic Oxidative Stress by D-Alanine in Brain Tumor Cells Expressing Rhodotorula gracilis: A Cancer Gene Therapy Strategy,” Human Gene Therapy, Vol. 9, No. 2, 1998, pp. 185-193. doi:10.1089/hum.1998.9.2-185
- B. S. Berlett and E. R. Stadtman, “Protein Oxidation in Aging, Disease, and Oxidative Stress,” The Journal of Biological Chemistry, Vol. 272, No. 33, 1997, pp. 20313- 20316. doi:10.1074/jbc.272.33.20313
- B. Halliwell and J. M. Gutteridge, “Free Radicals, Lipid Peroxidation, and Cell Damage,” The Lancet, Vol. 324, No. 8411, 1984, p. 1095. doi:10.1016/S0140-6736(84)91530-7
- T. Matsura, M. Kai, Y. Fujii and K. Yamada, “Hydrogen Peroxide-Induced Apoptosis in HL-60 Cells Requires Caspase-3 Activation,” Free Radical Research, Vol. 30, No. 1, 1999, pp. 73-83. doi:10.1080/10715769900300081
- H. Yamakawa, Y. Ito, T. Naganawa, Y. Banno, S. Nakashima, S. Yoshimura, M. Sawada, Y. Nishimura, Y. Nozawa and N. Sakai, “Activation of Caspase 9 and 3 during H2O2-Induced Apoptosis of PC12 Cells Independent of Ceramide Formation,” Neurological Research, Vol. 22, No. 6, 2000, pp. 556-564.
- H. N. Green and J. W. Westrop, “Hydrogen Peroxide and Tumor Therapy,” Nature, Vol. 181, 1958, pp. 128-129. doi:10.1038/181128a0
- K. Sugiura, “Effect of Hydrogen Peroxide on Transplanted Rat and Mouse Tumors,” Nature, Vol. 182, 1958, pp. 1310-1311. doi:10.1038/1821310a0
- J. Fang, T. Sawa, T. Akaike and H. Maeda, “Tumor-Targeted Delivery of Polyethylene Glycol-Conjugated for Antitumor Therapy via Enzymatic Generation of Hydrogen Peroxide,” Cancer Research, Vol. 62, No. 11, 2002, pp. 3138-3143.
- H. Maeda, L.W. Seymour and Y. Miyamoto, “Conjugates of Anticancer Agents and Polymers: Advantages of MacRomolecular Therapeutics in Vivo,” Bioconjugate Chemisry, Vol. 3, No. 5, 1992, pp. 351-362.
- J. Fang, D. Deng, H. Nakamura, T. Akuta, H. Qin, A. K. Iyer, K. Greish and H. Maeda, “Oxystress Inducing Antitumor Therapeutics via Tumor-Targeted Delivery of PEGConjugated D-Amino Acid Oxidase,” International Journal of Cancer, Vol. 122, No. 5, 2008, pp. 1135-1144. doi:10.1002/ijc.22982
- O. D. Jayakumar, R. Ganguly, A. K. Tyagi, D.K. Chandrasekharan and C. K. K. Nair, “Water Dispersible Fe3O4 Nanoparticles Carrying Doxorubicin for Cancer Therapy,” Journal of Nanoscience and Nanotechnology, Vol. 9, 2009, pp. 6344-6348. doi:10.1166/jnn.2009.1369
- L. W. Seymour, Y. Miyamoto, H. Maeda, M. Brereton, J. Strohalm, K. Ulbrich and R. Duncan, “Influence of MoLecular Weight on Passive Tumor Accumulation of a Soluble Macromolecular Drug Carrier,” European Journal of Cancer, Vol. 31A, No. 5, 1995, pp. 766-770. doi:10.1016/0959-8049(94)00514-6
- L. W. Seymour, S. P. Olliff, C. J. Poole, P. G. De Takats, R, Orme, D. R. Ferry, H. Maeda, T. Konno and D. J. Kerr, “A Novel Dosage Approach for Evaluation of SMANCS [Poly-(strene-co-maleyl-half-n-butylate)-neocarzinostatin] in the Treatment of Primary Hepatocellular Carcinoma,” International Journal of Oncology, Vol. 12, No. 6, 1998, pp. 1217-1223.
- J. Grabis, G. Heidemane and D. Rasmane, “Preparation of Fe3O4 and γ-Fe2O3 Nanoparticles by Liquid and Gas Phase Processes,” Materials Science (Medziagotyra), Vol. 14, 2008, pp. 292-295.
- J. D. Choi, “The Nature of N-5 Reactivity in Flavins and Inhibitor Complexes,” 1978. http://etd.lib.ttu.edu/theses/available/etd-04102009-1295015070716/unrestricted/31295015070716.pdf
- T. Watanabe, Y. Motomura and T. Suga, “A New ColoriMetric Determination of and Urate Oxidase Activity,” Analytical Biochemistry, Vol. 86, No. 1, 1978, pp. 310- 315. doi:10.1016/0003-2697(78)90347-0
- D. K. Chandrasekharan, V. T. Kagiya and C. K. K. Nair, “Radiation Protection by 6-Palmitoyl Ascorbic Acid-2- Glucoside: Studies on DNA Damage in Vitro, ex Vivo, in Vivo and Oxidative Stress in Vivo,” Journal of Radiation Research, Vol. 50, No. 3, 2009, pp. 203-212. doi:10.1269/jrr.08090
- H. Cerda, H. Delincee, H. Haine and H. Rupp, “The DNA ‘Comet Assay’ as a Rapid Screening Technique to Control Irradiated Food,” Mutation Research, Vol. 375, No. 2, 1997, p. 167. doi:10.1016/S0027-5107(97)00012-2
- K. Konca, A. Lankoff, A. Banasik, H. Lisowka, T. Kuszewski, S. Gozdz, Z. Koza and A. Wojcik, “Across Platform Public Domain PC Image Analysis Programme for the Comet Assay,” Mutation Research, Vol. 534, No. 1-2, 2003, pp. 15-20.
- D. Sandeep and C. K. K. Nair, “Protection of DNA and Membrane from γ-Radiation Induced Damage by the Extract of Acorus calamus Linn.: An in Vitro Study,” Environmental Toxicology and Pharmacology, Vol. 29, No. 3, 2010, pp. 302-307. doi:10.1016/j.etap.2010.02.006
- G. G. Nair and C. K. K. Nair, “Amelioration of γ-Radiation Induced Genomic Insult and Oxidative Stress in Whole Body Irradiated Swiss Albino Mice by Sesamol,” International Journal of Low Radiation, Vol. 8, No. 1, 2011, pp. 20-34.
- S. Wada, T. V. Khoa, Y. Kobayashi, T. Funayama, K. Yamamoto, M. Natsuhor and N. Ito, “Detection of Radiation-Induced Apoptosis Using the Comet Assay,” Journal of Veterinary Medical Science, Vol. 65, No. 11, 2003, pp. 1161-1166. doi:10.1292/jvms.65.1161
- R. C. Wilkins, D. Wilkinson, H. P. Maharaj, P. V. Bellier, M. B. Cybulski and J. R. McLean, “Differential Apoptotic Response to Ionizing Radiation in Subpopulations of Human White Blood Cells,” Mutation Research, Vol. 513, No. 1-2, 2002, pp. 27-36.
- T. Sawa, J. Wu, T. Akaike and H. Maeda, “Tumor-Targeting Chemotherapy by a Xanthine Oxidase-Polymer Conjugate That Generates Oxygen-free Radicals in Tumor Tissue,” Cancer Research, Vol. 60, 2000, pp. 666- 671.

