Chinese Medicine
Vol. 3 No. 1 (2012) , Article ID: 18123 , 4 pages DOI:10.4236/cm.2012.31010
Antimicrobial Activity of Cannabis sativa L.
1Department of Biochemistry, Nutrition and Toxicology, Central Veterinary Research Laboratories, Khartoum, Sudan
2Department of Microbiology and Parasitology, Medicinal and Aromatic Plants Research Institute, National Centre for Research, Khartoum, Sudan
Email: aalmagboul@hotmail.com
Received July 27, 2011; revised December 1, 2011; accepted December 15, 2011
Keywords: Antimicrobial; Cannabis sativa Seeds; Whole Plant; Methanol Extract; Petroleum Ether Extract
ABSTRACT
The oil of the seeds, petroleum ether and methanol extracts of the whole plant of Cannabis sativa belonging to the family Cannabinaceae were screened for their antimicrobial activity against two Gram positive organisms (Bacillus subtilis, Staphylococcus aureus), two Gram negative organisms (Escherichia coli, Pseudomonas aeruginosa) and two fungi namely Aspergillus niger and Candida albicans using the cup plate agar diffusion method. The oil of the seeds of Cannabis sativa exerted pronounced antibacterial activity (21 - 28 mm) against Bacillus subtilis and Staphylococcus aureus, moderate activity (15 mm) against Escherichia coli and high activity (16 mm) against Pseudomonas aeruginosa and inactive against the two fungi tested. The petroleum ether extract of the whole plant exhibited pronounced antibacterial activity (23 - 28 mm) against both Bacillus subtilis and Staphylococcus aureus organisms, high activity (16 mm) against Escherichia coli and inactive against Pseudomonas aeruginosa and both fungi. The methanol extract of the whole plant showed also pronounced antibacterial activity (29 mm) against Bacillus subtilis, low activity (12 mm) against Staphylococcus aureus and high activity (16 - 18 mm) against both Gram negative organisms, inactive against Aspergillus niger and low activity (13 mm) against Candida albicans. The minimum inhibitory concentrations of Cannabis sativa methanol extracts of the seeds and the whole plant against the standard organisms were determined using the agar plate dilution method. The standard organisms were tested against reference antibacterial and antifungal drugs and the results were compared with the activity of the extracts.
1. Introduction
Cannabis sativa is a member of the family Cannabinaceae. Cannabis sativa preparation is known by various names worldwide. It is called Marijuana in America; Bhang, Ganja and Charas in India; Kif in North Africa; Dogga in South Africa; Krori in Tunisia, Habak in Turkey; Hashish in Middle East; Djomba or Liamba in Central Africa and Brazil; Sodom, Tampl, Gum, Gauge and stuff in Kinshasa, Swala and Whiskt in Ghana; Grifa in Mexico and Macohna in some parts of South America [1].
In Sudan, the most famous names of Cannabis preparations are Bango and Hashish. The name Bango in Sudan may be derived from the Indian name Bhang.
The chemical definition encompasses a variety of distinct chemical classes. The classical cannabinoids are structurally related to Tetrahydrocannabinol commonly abbreviated as THC, the nonclassical cannabinoids, the aminoalkylindoles, the eicosanoids related to the endocannabinoids, 1,5-diarylpyrazoles, quinolines and arylsulphonamides and additional compounds that do not fall into these standard classes but bind to cannabinoid receptors. The term cannabinoids also refers to a unique group of secondary metabolites found in the cannabis plant, which are responsible for the plant’s peculiar pharmacological effects. Currently, there are three general types of cannabinoids; phytocannabinoids occur uniquely in the cannabis plant; endogenous cannabinoids are produced in the bodies of humans and animals and synthetic cannabinoids which are similar compounds produced in the laboratory [2].
Cannabis was used in the twentieth century B.C in Egypt to treat sore eyes. In India, prior to the tenth century B.C., bhang was used as an anesthetic and antiphlegmatic [1]. It is also used in both the Hindu and Moslem systems of drugs as spasmolytic, hypnotic, analgesic in mental conditions and to increase resistance to severe physical stress [3]. Cannabis is administered to patients suffering from rabies, cholera, rheumatism, epilepsy and tetanus. Also observation on the analgesic, anticonvulsive and muscle relaxant. In northeastern India, some of the plants species including Cannabis sativa have been used for the treatment of specific human ailments such as allergies, burns, cuts and wounds, inflamemation, leprosy, leucoderma, scabies, smallpox and sexually transmitted diseases [4].
The plant was used as an anesthetic in surgery in ancient China. Cannabis in Europe and America became the drug of choice in the prevention of migraine attacks. Cases of uterine hemorrhage after birth, asthma, postpartum psychoses, various neuralgias and epilepsy were also successfully treated with Cannabis [3].
Kabelik et al., [5] reported that Cannabis extract was used for irrigation in diseases of the anus, applied to sore toe nails and the seed pulp was a favorite dish. Edible, industrial and medicinal oils were obtained from the seeds.
Cannabis has an ancient tradition of usage as a medicine in obstetrics and gynecology. Cannabis extracts may represent an efficacious and safe alternative for treatment of a wide range of conditions in women including dysmenorrheal, dysuria, hyperemesis gravidarum and menopausal symptoms [6].
Cannabis is grown and processed for many uses. Many plant parts are used as medicine for humans and livestock, whole seeds and seed oil are eaten by human, seeds and leaves are fed to animals, seeds oil and stalks are burned for fuel. Whole plants, leaves and wood have environmental uses, bark, fiber and seeds are also of ritual importance [7].
The antimicrobial activity from ethanol and petroleum ether extracts against multiple microorganisms was reported [8]. Essential oils extracted from five fiber varieties of C. sativa had antimicrobial activity with the degree of antimicrobial activity varying between cultivars [9]. C. sativa has been reported to have pharmacological and also antimicrobial effects [10-14]. Cannabidiol has been identified as a component of hemp oil effective against Gram positive bacteria and yeast [15]. The C. sativa chemotypes grown in northern latitudes are reported to have a higher ratio of cannabidiol to tetrahydrocannabinol resulting in stronger antimicrobial activity [15]. Cannabis sativa grown in Pakistan has strong antimicrobial activity against all strains of bacteria and fungi [8].
The objective of this work is to evaluate the antimicrobial activity of the extracts of Cannabis sativa grown in Sudan against standard bacteria and the fungi.
2. Materials and Methods
2.1. Plant Material
The seeds and the whole plant of Cannabis sativa were obtained from Niala, South Darfur, Sudan in 2008, cleaned off from the impurities and dried. It was identified by Dr.Mohammed Abd Elraheem Jah Alnabi (Institute for Forensic Sciences).
2.2. Preparation of the Crude Extract
50 grams of the air-dried and coarsely powdered plant material of Cannabis sativa was exhaustively extracted for 20 hours with petroleum ether (40˚C - 60˚C) in a Soxhlet apparatus. The petroleum ether extract was filtered and evaporated under reduced pressure. The extracted plant material was then air dried and repacked in the Soxhlet and exhaustively extracted with methanol. The methanol extract was filtered and evaporated under reduced pressure. Each residue was weighed and the percentage yield was determined.
2.3. Test Organisms
The extracts were tested against two Gram-positive organisms, Bacillus subtilis (NCTC8236) and Staphylococcus aureus (ATCC 25923), two Gram negative bacteria, Escherichia coli (ATCC25922), and Pseudomonas aeruginosa (27853) and two fungi Aspergillus niger (ATCC9763) and Candida albicans (ATCC7596). The bacterial cultures were maintained on nutrient agar slopes. They were grown in nutrient agar plate and incubated at 37˚C for 18 hrs before being used for the tests. All microorganisms were obtained from the stock cultures of the Department of Microbiology, Medicinal and Aromatic Plants Research Institute, National Centre for Research, Khartoum, Sudan.
2.4. Testing for Antibacterial Activity
The cup-plate agar diffusion method [16] was adopted to assess the antibacterial activity of prepared extracts.
One ml of the standardized bacterial stock suspension (108 - 109) C.F.U/ ml were thoroughly mixed with 100 ml of molten sterile nutrient agar which was maintained at 45˚C. 20ml aliquots of the inoculated nutrient agar were distributed into sterile Petridishes.
The agars were left to set and in each of these plates 4 cups (10 mm in diameter) were cut using a sterile cork borer (No.4) and agar discs were removed. Alternate cups were filled with 0.1 ml samples of each of the extracts using automatic microlitre pipette, and allowed to diffuse at room temperature for two hours. The plates were then incubated in the upright position at 37˚C for 18 hours.
Two replicates were carried out for each extract against each of the test organisms. Simultaneously positive controls involving the addition of the respective solvents instead of the extracts were carried out separately. After incubation the diameters of the resultant growth inhibition zones were measured, averaged and the mean values were tabulated.
2.5. Testing for Antifungal Activity
The same method as for bacteria was adopted instead of nutrient agar, Sabouraud dextrose agar was used. The inoculated medium was incubated at 25˚C for one day for the yeast and three to five days for Aspergillus niger.
The minimum inhibitory concentrations (MIC) of the methanol extracts against the standard organisms were determined using the agar dilution method [17].
3. Results and Discussion
The oil of the seeds, petroleum ether and methanol extracts of the whole plant of Cannabis sativa belonging to the family Cannabinaceae were screened for their antimicrobial activity against two Gram positive organisms (Bacillus subtilis, Staphylococcus aureus), two Gram negative organisms (Escherichia coli, Pseudomonas aeruginosa) and two fungi namely Aspergillus niger and Candida albicans (Table 1).
The oil of the seeds of Cannabis sativa exerted pronounced antibacterial activity (21 - 28 mm) against Bacillus subtilis and Staphylococcus aureus, moderate activity (15 mm) against Escherichia coli and high activity (16 mm) against Pseudomonas aeruginosa and inactive against the two fungi tested. This result is similar to that reported by Novak et al. [9] who found that the oil extracted from C.sativa had antimicrobial activity and this could be due to the presence of sesquiterpenes or to the presence of cannabidiol [15]). The petroleum ether extract of the whole plant exhibited pronounced antibacterial activity (23 - 28 mm) against both Gram positive organisms, high activity (16 mm) against Escherichia coli and inactive against Pseudomonas aeruginosa and both fungi. The methanol extract of the whole plant showed also pronounced antibacterial activity (29 mm) against Bacillus subtilis, low activity (12 mm) against Staphylococcus aureus and high activity (16 - 18 mm) against both Gram negative organisms, inactive against Aspergillus niger and low activity (13 mm) against Candida albicans. This result is different from that produced by Borchardt et al. [18] who found that the leaves and stems extract was only active against Staphylococcus aureus and also different from that reported by Wasim et al. [8] who reported that C.sativa grown in Pakistan has strong
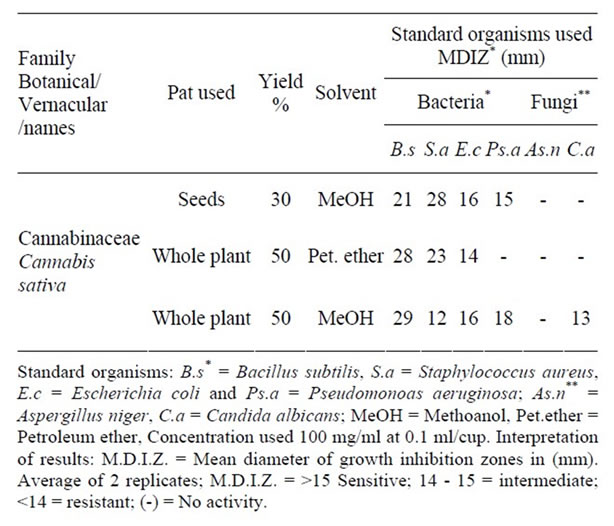
Table 1. Antimicrobial activity of Cannabis sativa extracts against the standard organisms.
antimicrobial activity against all strains of bacteria and fungi. Also our result is similar to that produced by Wasim et al. [8] who reported antimicrobial activity from ethanol and petroleum ether extracts against multiple microorganisms. This shows in our results that the Gram positive organisms are more active than the Gram negative organisms and the fungi (Table 1).
The oil of Cannabis sativa seeds exerted high antibacterial activity against Bacillus subtilis which is similar to that produced by 20 µg/ml Gentamicin. It inhibited Staphylococcus aureus which is similar to 10 µg/ml Benzyl penicillin and 40 µg/ml Cloxacillin. It also inhibited Escherichia coli similar to 10 µg/ml Gentamicin and inhibited Pseudomonas aeruginosa similar to 20 µg/m Gentamicin (Table 2).
The petroleum ether extract of the whole plant of Cannabis sativa inhibited Bacillus subtilis which is almost similar to 40 µg/ml Gentamicin and inhibited Staphylococcus aureus almost similar to 5 µg/ml Benzyl penicillin and 10 µg/ml Cloxacillin. It inhibited Escherichia coli almost similar to 10 ug/ml Gentamicin and inhibited Pseudomonas aeruginosa similar to 20 µg/ml Gentamicin (Table 2).
The methanol extract of the whole plant Cannabis sativa inhibited Bacillus subtilis and Pseudomonas aeruginosa almost similar to 40 µg/ml Gentamiin, Staphylococcus aureus similar to 5 µg/ml Gentamicin and inhibited Escherichia coli similar to 10 µg/ml Gentamicin (Table 2).
The activity of the methanol extract is very low even when compared with Nystatin (Table 3).
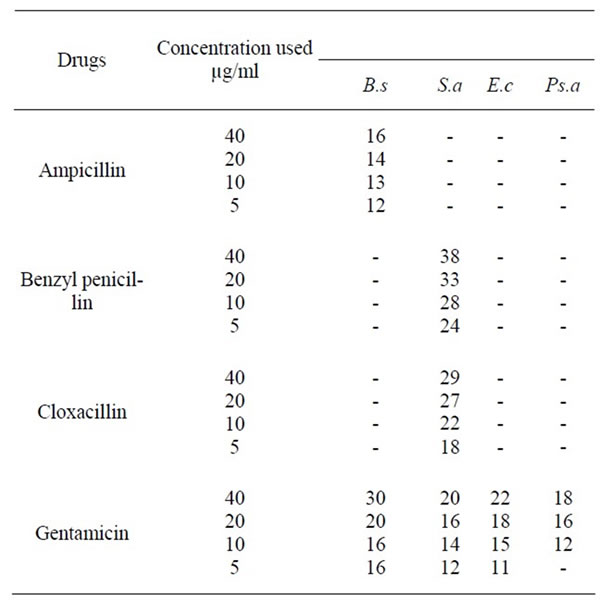
Table 2. Antibacterial activity of reference drugs against standard organisms.
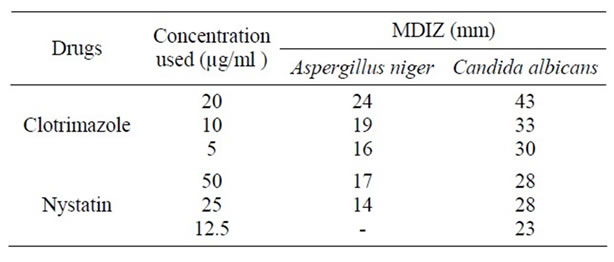
Table 3. Antifungal activity of reference drugs against the standard organisms.
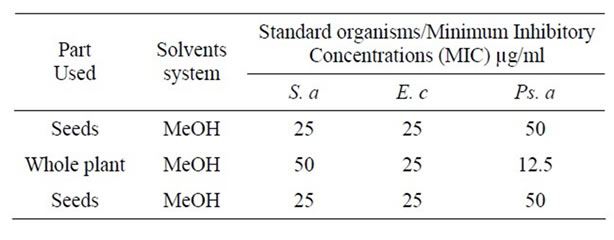
Table 4. Minimum Inhibitory Concentrations (MIC) of Cannabis sativa extracts against the standard organisms.
The minimum inhibitory concentrations of Cannabis sativa methanol extracts of the seeds and the whole plant against the standard organisms showed that the methanol extract of the seeds against Staphylococcus aureus and Escherichia coli is lower than that of Pseudomonas aeruginos i.e. it is active against S.aureus and E.coli and the (MIC) of the whole plant showed that both Gram negative organisms exhibited lower (MIC) than the Gram positive organism (Table 4).
REFERENCES
- N. Sachindra and A. Pradhan, “Marijuana Drug Abuse Clinical and Basic Aspects,” The C.V. Mosby Company, Saint Louis, 1977, pp. 148-173.
- D. M. Lambert and C. J. Fowler, “The End Cannabinoids System Drug Targets Lead Compounds and Potential Therapeutic Application,” Journal of Medicinal Chemistry, Vol. 48, No. 16, 2005, pp. 59-87.
- R. Mechoulam and N. Lander, “Cannabis a Possible Source of New Drugs,” Journal Pharmacy International, Vol. 1, 1980, pp. 19-21.
- B. Dilara and S. C. Nath, “Ethno Botanical Review of Medicinal Plants Used for Skin Diseases and Related Problems in Northeastern India,” Journal of Herbal Spices and Medicinal Plants, Vol. 7, No. 3, 2000, pp. 55- 93. doi:10.1300/J044v07n03_07
- K. J. Kabelik, Z. Krejei and F. Santavy, “Cannabis as a Medicament,” Bulletin on Narcotic, Vol. 12, No. 3, 1960, pp. 5-23.
- E. Russo, “Cannabis Treatments in Obstetrics and Gynecology: Historical Review,” Journal of Cannabis Therapeutics, Vol. 2, No. 3-4, 2002, pp. 5-35.
- R. C. Clarke, “Filed Interview Schedule and Questionnaire for Investigating Cannabis Use, Rockville National Institute on Drug Abuse,” Journal Indian Hemp, Vol. 7, No. 1, 2002, pp. 83-88. doi:10.1300/J237v07n01_07
- K. Wasim, I. U. Haq and M. Ashraf, “Antimicrobial Studies of the Leaf of Cannabis sativa L.,” Pakistan Journal of Pharmaceutical Sciences, Vol. 8, 1995, pp. 22- 38.
- J. Novak, K. Zitterl-Eglseer, S. G. Deans and C. M. Franz, “Essential Oils of Different Cultivars of Cannabis sativa L. and their Antimicrobial Activity,” Flavour Fragrance Journal, Vol.16, No. 4, 2001, pp. 259-262. doi:10.1002/ffj.993
- O. Polunin, “Flowers of Europe: A Field Guide,” Oxford University Press, London, 1969, pp. 56-57.
- D. Baker, G. Pryce, G. Giovannoni and A. J. Thompson, “The Therapeutic Potential of Cannabis,” Lancet Neurology, Vol. 2, No. 5, 2003, pp. 291-298. doi:10.1016/S1474-4422(03)00381-8
- J. L. Hartwell, “Plants Used against Cancer: A Survey 1967-1971,” Lloydia, 1971, pp. 30-34.
- S. Foster, “Herbs for Your Health,” Interwave Press, Loveland, 1996, pp. 56-57.
- C. Leizer, D. Ribnicky, A. Poulev, S. Dushenkov and I. Raskin, “The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition,” Journal of Nutraceuticals, Functional & Medical Foods, Vol. 2, No. 4, 2000, pp. 35-53. doi:10.1300/J133v02n04_04
- C. R. Langezaal, A. Chandra and J. J. C. Scheffer, “Antimicrobial Screening of Essential Oils and Extracts of Some Humulus lupulus L. Cultivars,” Pharmacy World & Science, Vol. 14, No. 6, 1992, pp. 353-356. doi:10.1007/BF01970171
- F. Kavanagh, “Analytical Microbiology,” Academic Press, New York and London, Vol. II, 1972.
- J. E. Blair, E. H. Lenette and I. P. Truant, “Manual of Clinical Microbiology. American Society for Microbiology,” Baltimore, Maryland and Wilkins Company, Washington DC, 1970.
- J. R. Borchardt, D. L. Wyse, C. C. Sheaffer, K. L. Kauppi, R. G. Fulcher, N. J. Ehlke, D. D. Biesboer and R. F. Bey, “Antimicrobial Activity of Native and Naturalized Plants of Minnesota and Wisconsin,” Journal of Medicinal Plants Research, Vol. 2, No. 5, 2008, pp. 98-110.

