Natural Science
Vol.5 No.7(2013), Article ID:34118,9 pages DOI:10.4236/ns.2013.57102
Food habits of Barbus luteus in main outfall drain, Iraq
![]()
Department of Biology, College of Science, Thi-Qar University, Thi-Qar, Iraq; Afrah.abid@yahoo.com
Copyright © 2013 Afrah A. Maktoof. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 24 April 2013; revised 25 May 2013; accepted 2 June 2013
Keywords: Main Outfall Drain; Food Habits; Barbus luteus
ABSTRACT
The alimentary canal contents of 360 Barbus luteus caught from MOD between January 2010 to December 2010 were examined. The phytoplankton, zooplanktons, detritus, diatoms, insects and unidentified digestive food were identified in the alimentary canal of fish. The results of diet analysis showed that the B. luteus was omnivorous. The species of fish tended to consume more plant origin food. The aquatic plants and tissues were the main part of thier food followed by chlorophyta and cyanophyta. The results revealed that the species obtained or fell their food item from the bed of rivers or near to it.
1. INTRODUCTION
Fish are ecology important and there are often intense commercial and recreational interests surrounding their study [1].
Feeding behaviors of fish is the major important factor affecting their nutrition and growth. Changing environmental conditions such as those resulting from eutrophication affect the availability of food types.
The study of the food and feeding habits of freshwater fish species is a subject of continuous research because it constitutes the basic for the development of a successful fisheries management program on fish capture and culture [2]. One of the key factors to successful fish culture is the understanding of some biological fundamentals especially food and feeding behavior.
Various works have been done on the diet of Barbus luteus and other fish species from varies river. [3], in his study, found two types of freshwater fish: B. luteus and Aspius vorax in Al-Hammer Marsh which are omnivores and benthic in feeding. Aspius vorex is regarded as carnivores and B. luteus depended basically on filament algae, higher aquatic plant, animals and humus. [4] stated that the common carp fed on zooplankton and phytoplankton matter, which indicated a mixed feeding [5] and it prefered animals rather than plants [6]. [7] studied the feeding interference of four types of fish from cyprindine pices in Al-Habbaniya lake. This study showed the nature of food for each of C. carpio, Carrassus carassus, B. luteus and Barbus xanthopterus. It also showed that Barbus xanthopterus depended on mixed feeding and tended to eat animals, whereas C. carpio, Carrassus carassus and B. luteus depended on mixed feeding. [8] studied the feeding interference for five types of Barbus genus in Himreen dam. These include Barbus grypes, Barbus xanthopterus, B. luteus, Barbus eosinus and Barbus barbulus. This study demonstrated that Barbus eosinus depended on animals in feeding as they fed on small fish, whereas Barbus grypes, Barbus xanthopterus and Barbus barbulus depended on mixed feeding and tended to feed on animals. B. luteus depended on mixed feeding, which fed on animals and plants approximately. Previous works showed a greatly varied diet in different environment, ranging from larvae Chironomds and oligochaetes to seeds and plants. Therefore, the purpose of this study was to investigate food composition and to determine monthly variations in the diet of the species in main outfall drain, south Iraq.
2. MATERIAL AND METHODS
2.1. Description of Study Area
Third river is a river use to discharge the effluents of agriculture activities from its both side. It is extended from Al-Shaklawiya near Baghdad north until Al-Basrah at the south with length about 565 km [9]. It is dividing into three sectors (North, Mid and South), the south sector (study area) extended from the end of the mid sector until Shatt Al-Basarah in the south, with length about 165 km. The discharge of water is must be mention the range or monthly or annually mean 220 m3/sec in this sector [10]. New branch was constructed this sector with length 7 km, feed to the marshes south Al-Nassiriya city.
Three stations were selected in the south sector of this river to implement, the present study, these are station 1(St.1) was near Al-Holandee bridge and the general caragge in the center of Al-Nassiriya city, St.2 was 20 km far from the first station, while St.3 was in the beginning of the new branch (Figure 1).
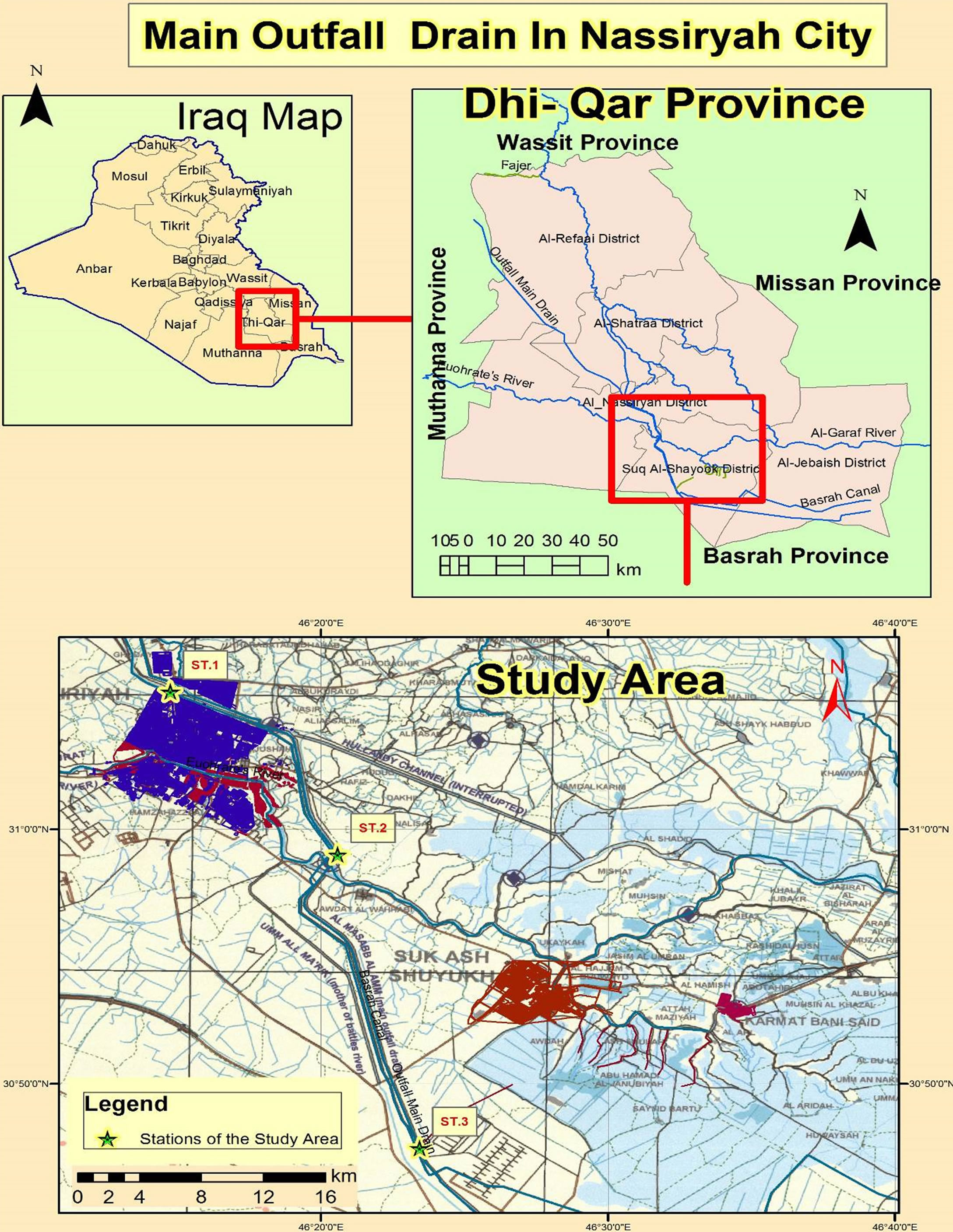
Figure 1. Map of the study area showed the study stations.
2.2. Measurement of Some Physical Properties
2.2.1. Air temperature
The air temperature was measured by digital portable multimeter; model 340 i/Set, which made in Germany.
2.2.2. Water Temperature
The water temperature was measured directly in the field by digital portable multimeter; model 340 i/Set, which were made in Germany.
2.2.3. Sample Collection
Fish samples were obtained from the fishermen caches using gill nets. A total of three hundred and sixty (360) fish as a sample. The sample were identified and collected between December 2010 and November 2011. The specimens were put in freezers soon after capturing them. Then the sample was sent to specific laboratory. In the laboratory, the sample were serially numbered and the weight and length of each specimen were taken recorded to the nearest gram and centimeters. The total length was taken from the tip of the head to the end of the tail. The gut length of each fish was taken. Since, there is no real stomach in cyprinidae species, the foregut (the anterior part of the alimentary cannal) was cut and put in 5% formalin to be used for the next test. The gut was dissected and washed well in a pteri dish in 10 ml of water. The gut was examined under microscope (Olympus type). The examination of the food specimens under microscope was done according to [11-14].
2.3. Method of Food Analysis
Various methods were used to analysis food of fish and emphasized that the method that used for any fish of the 360 must suitable for its diet. Instead of counting alimentary canal content, frequency of occurrence method [15] and point’s method [16] were used. This was calculated the proportions of organisms found in total food, which is based on the following formula:
F%: (n/N)*100 F%: frequency of occurrence of the species n: number of existence of the species N = number of the examined fish.
Ranking index [17] was calculated using the fallowing equation Ranking Index (RI%) = Points% × Occurrence% Because of the food exposure to grinding that resulted by digestion. The percentage of Ranking Index for each food content was calculated and used to compare it with percentage of points Feeding Intensity Fullness index of feeding fish according to [18] is as follows:
Feeding Intensity = No. of points/No. of feeding fish.
Feeding activity It represents the percentage of feeding fish of the examined fish [19].
Feeding activity = No. of Feeding fish/No. of examined.
3. RESELTS
3.1. Air and Water Temperature Measure
The air temperature during the study period for each location in Main outfall drain ranged between 16.5˚C - 39.31˚C. The lowest value measured was 16.5˚C at station 1 in January, whereas the highest value was 39.31˚C at station 3 in August. The water temperature of the area of study ranged between 12.6˚C - 32.1˚C. The lowest value was in station 1 in January, while the highest was in station 3 in August. These results are shown in Figures 2 and 3.
From the Statistical analysis results, it was found that the air temperature was differed significantly (P < 0.001), from water temperature values through the months of study. The positive correlation that was recorded between the air and water temperature was (P < 0.0001, r = 0.69).
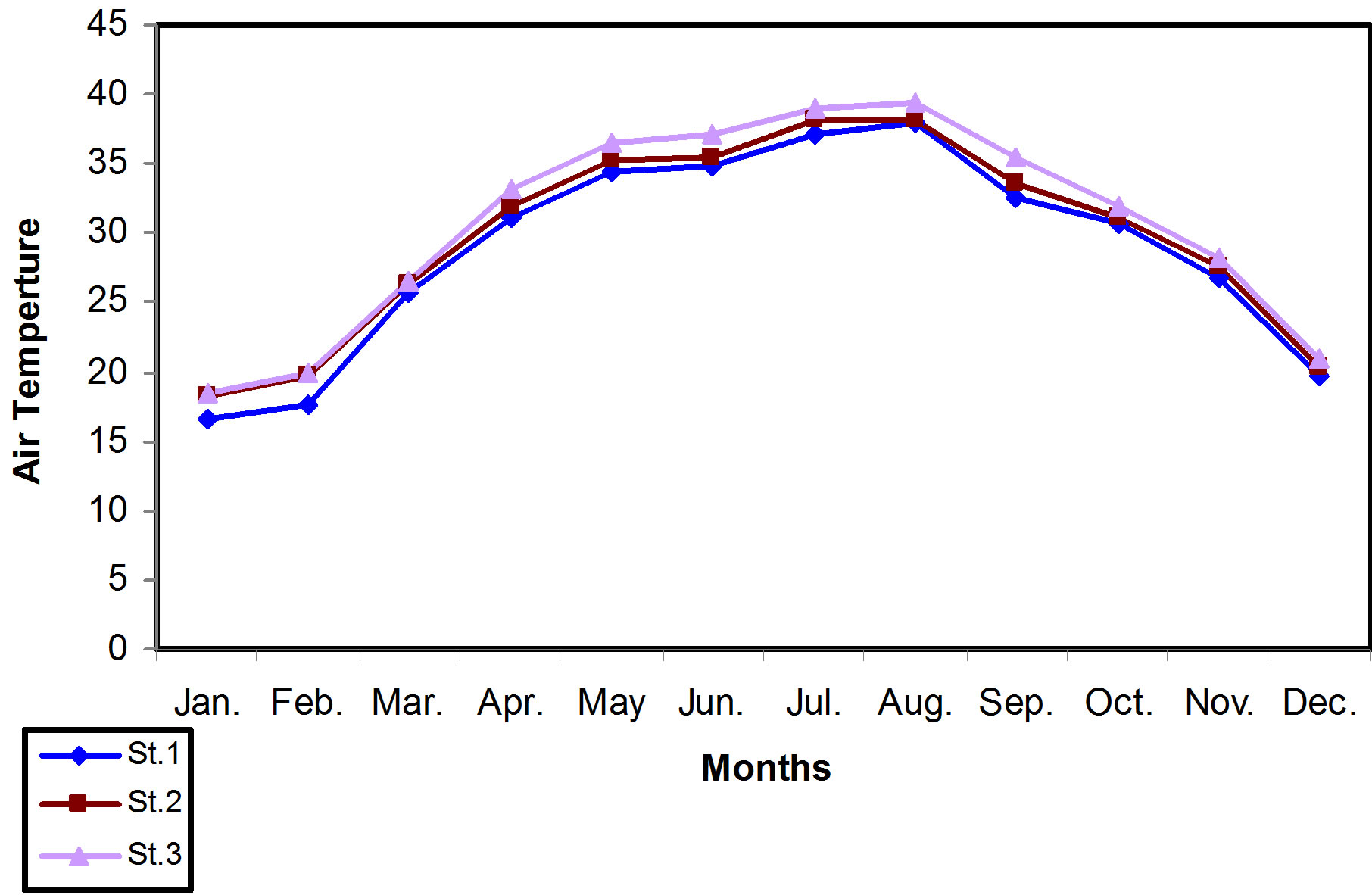
Figure 2. Monthly mean of air temperature variations for all locations of the study.
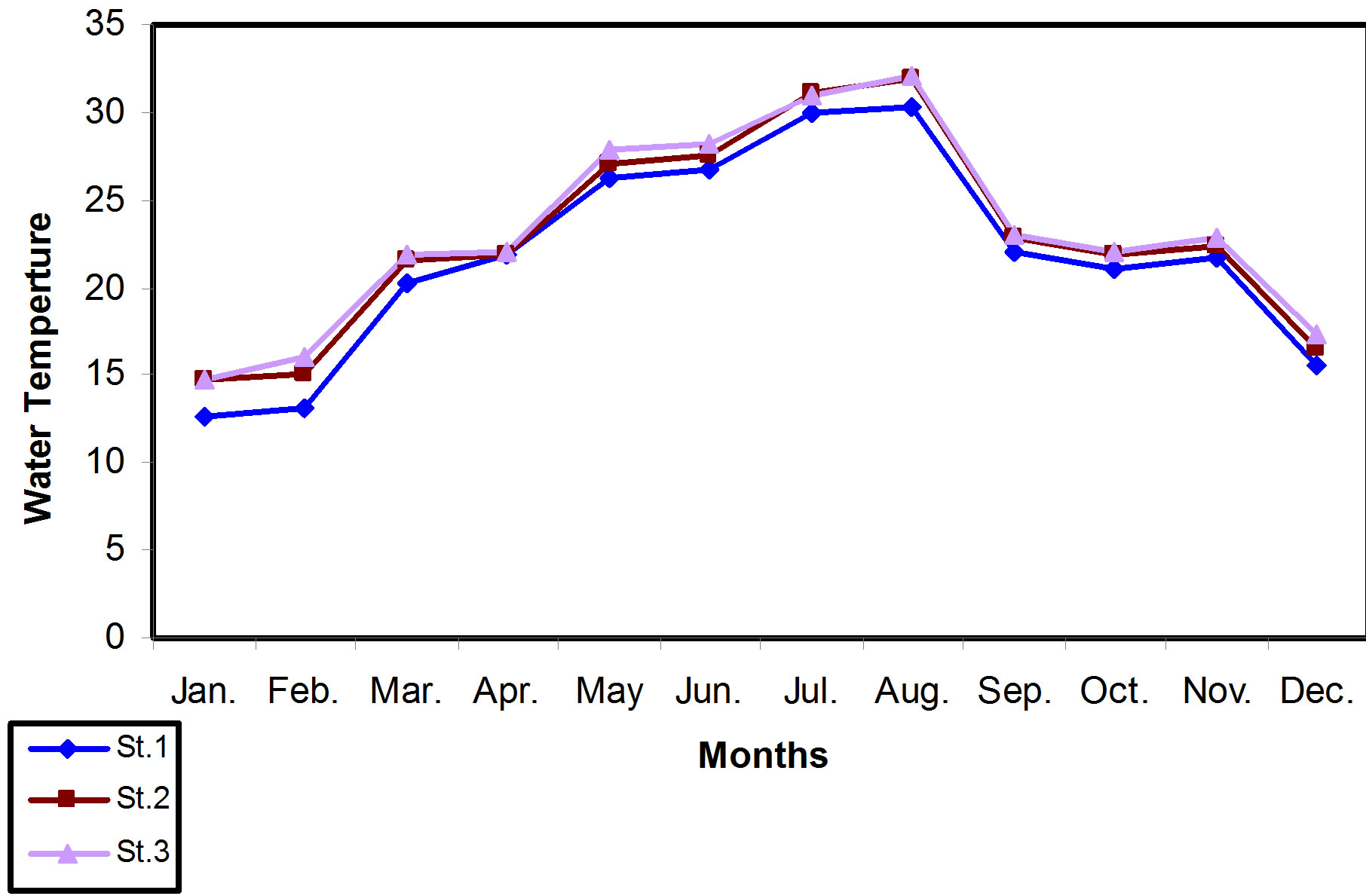
Figure 3. Monthly mean of water temperature variations for all locations of the study.
3.2. Food and Feeding Habits of B. luteus
3.2.1. Feeding Intensity and Activity for B. luteus
It has been recognized that the intensity of the feeding out of the points given for each digestive canal by its repletion where values ranged from (10.83 point/fish) during February and (23.23 point/fish) in July (Figure 4). It reached its peak during the hot months (July and August), as it reached its maximum value (23.23 point/fish and 21.60%), respectively. On the contrary the severity of feeding has decreased during the cold months (December, January and February), where it reached its lowest value (10.83 point/fish) during February (Figure 4). The feeding activity has also differed in the value according to the different months of the year where it reached 100% in the months of January, April, June, July and August. On the other hand, the intensity of feeding during the cold weather has reached the lowest value specially the month of December (76.47 point/fish) (Figure 5). Generally, hot months have recorded the highest values in the intensity and activity feeding.
From statistical analysis which relates temperature of water and air with feeding intensity and activity, it has been found that feeding intensity and activity have a relationship with those temperatures. There were signifi-
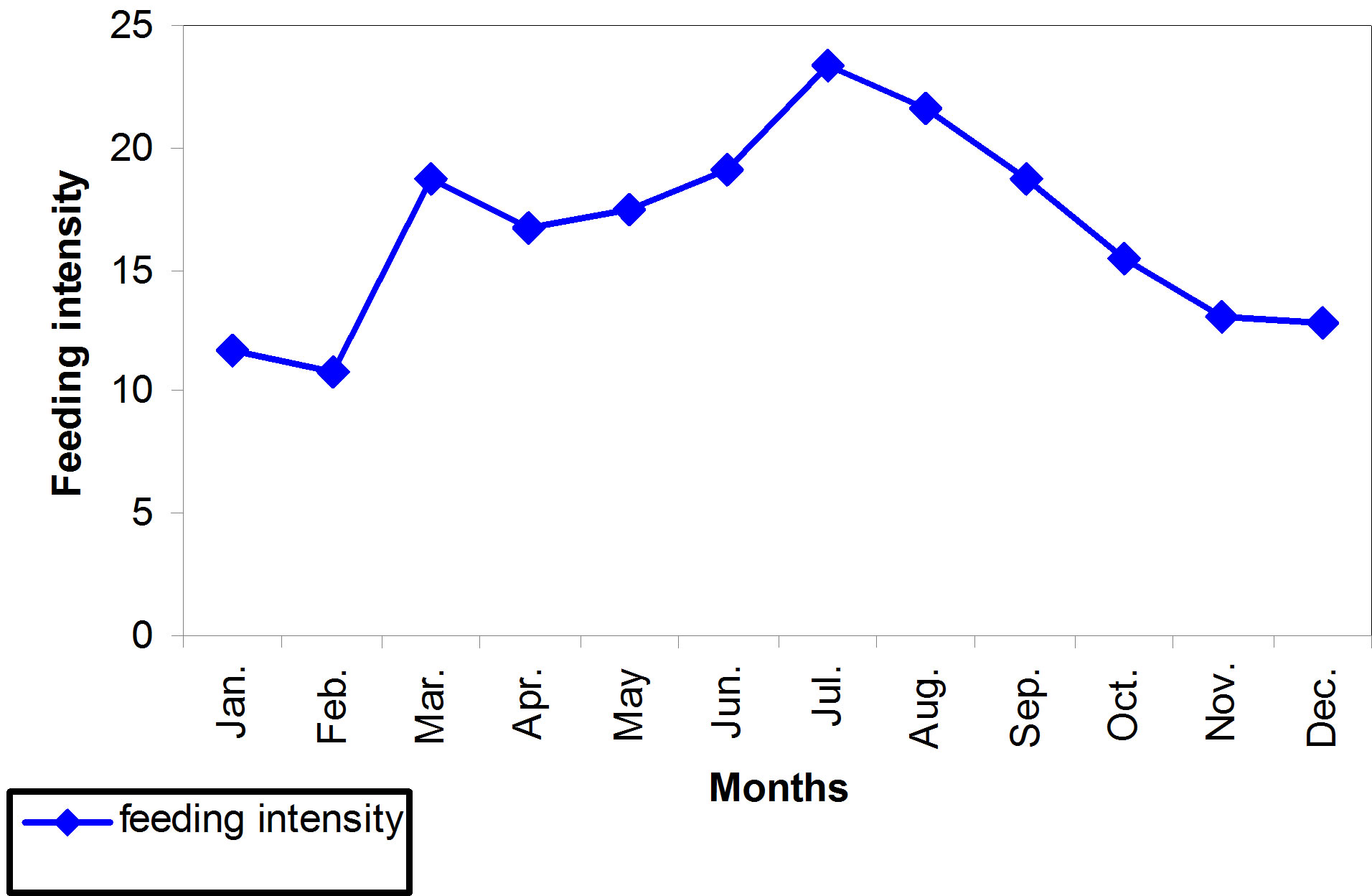
Figure 4. Feeding intensity during different Months for B.luteus.
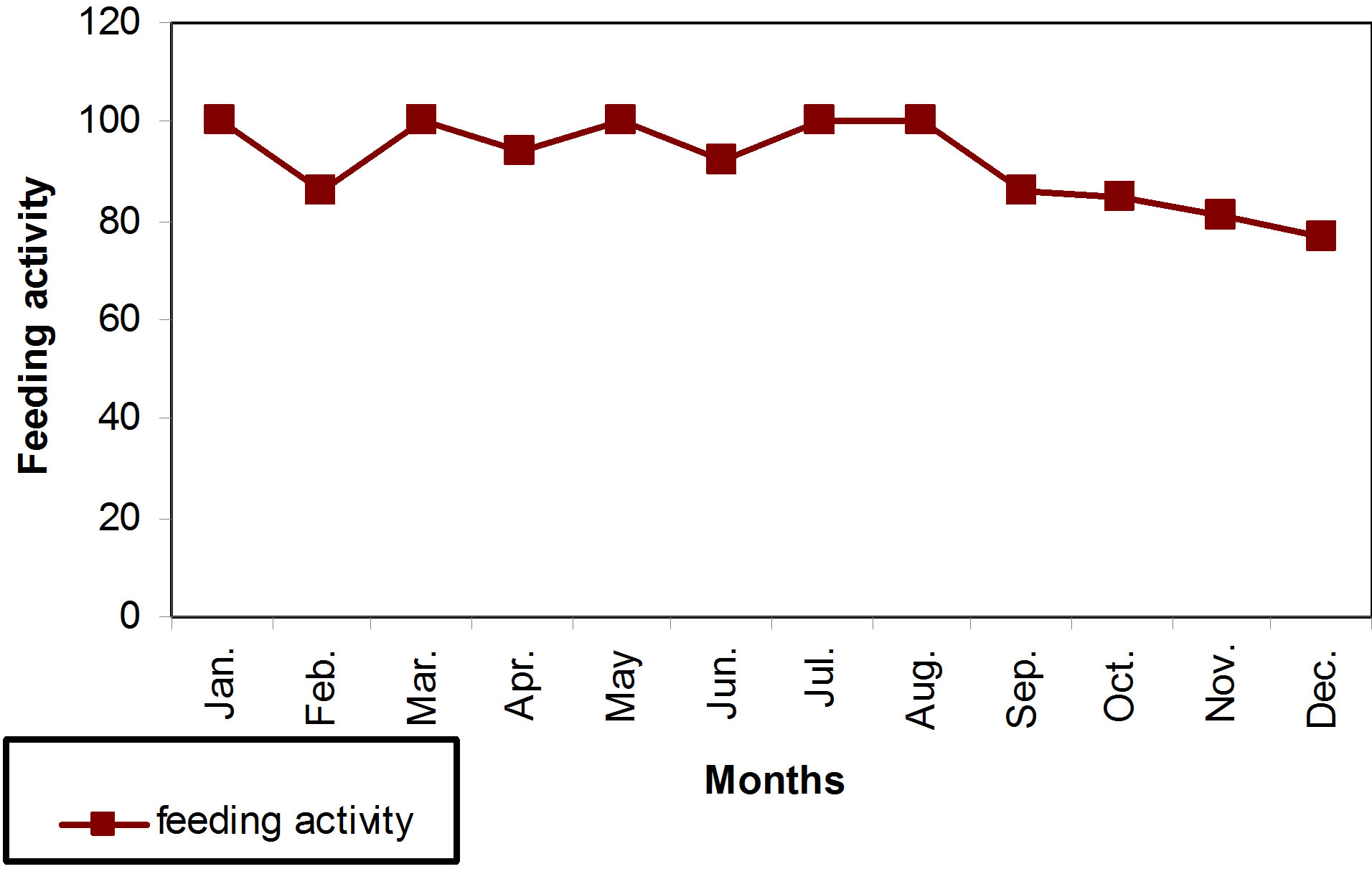
Figure 5. Feeding activity during different months for B.luteus.
cant differences between feeding activity and water temperature (P < 0.05, r = 0.77) and air temperature (P < 0.05, r = 0.72) among the month, whereas the feeding intensity has a significant correlation with water temperature and air temperature.
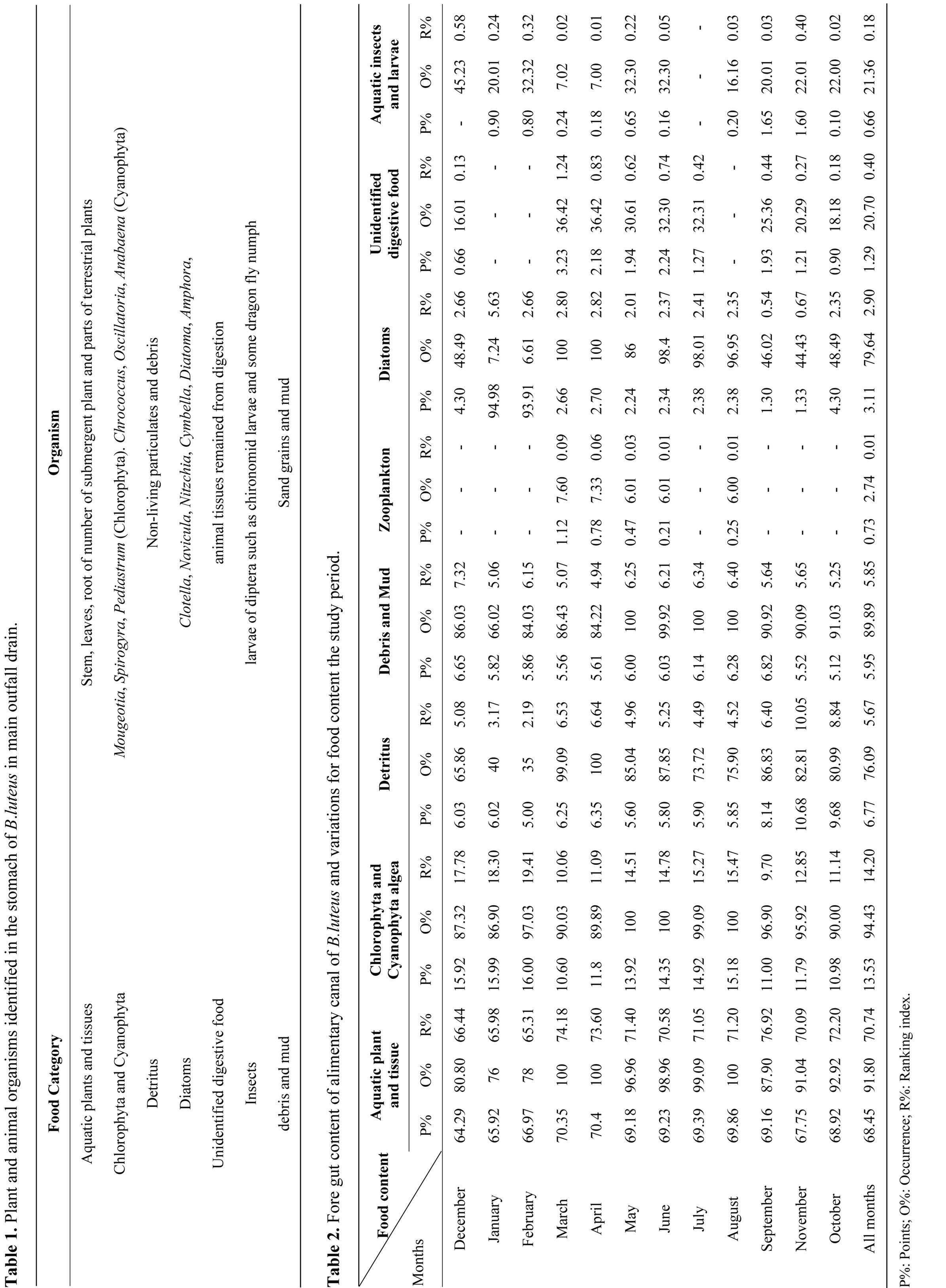
3.2.2. Food Content for B. luteus
Fore gut content of 360 fish were classified into eight main categories based on organisms’ habitat in study area (Table 1). The food of B. luteus consisted mainly of aquatic plants and tissues in the proportions of 68.45% from food volume and 70.74% from ranking index. The recorded frequency value was 91.80%. The genera identified Mougeotia, Scendesmus, Spirogyria from Chlorophyta group and Chrococcus, Oscillatoria and Spirulina from Cyanophyta were found to from 13.53% and 14.20%. The highest frequency of chlorophyta and cyanophyta recorded was 94.43% rather than other food content in alimentary canal for B. luteus (Table 2). The percentages of detritus were 6.77% and 5.67% from food content in alimentary canal by frequency observed which was 76.09%.
Debris and Mud the debris and mud formed 5.95% and 5.85% from food content and the frequency was 89.89% (Table 2).
Cyclotella, Navicula, Nitzchia, Cymbella, Diatoma and Amphora consumed from diatoms group reached 3.11% and 2.90% respectively of the total food content by both analysis methods (Table 2).
Unidentified digestive food: It is of animal origin that is exposed to digestion and cannot be classified. These were found in low ratios throughout the year, except in March (3.23%) and May (2.24%) (Table 2).
Aquatic insects and larvae: The percentage of aquatic insects and larvae was low 0.66% and 0.18% by frequency 21.36% throughout the year.
Zooplankton: Its ratio observed in fore gut of B. luteus were (0.73% and 0.01%) and frequency (2.74%) (Table 2).
Chlorophyta and Cyanophyta (94.43%) aquatic plant and tissue (91.80%) showed higher frequency than other food content in alimentary canal. The ratio of plant food origin was more than the ratios of animal food of origin. Therefore fish were dependent on food of plant origin.
3.2.3. Monthly Food Content Variation B. luteus
The variations of food content ratio were described as the following:
Aquatic plants and tissues: The highest ratio of aquatic plants and tissues that was consumed in March was (70.35%) as indicated by points and values from ranking index (74.18%), while the lowest values were in December which reached (64.29% and 66.44%) respectively (Table 2).
Chlorophyta and Cyanophyta algae: It has been revealed that the ratios of Chlorophyta and Cyanophyta consumed in March were low (10.60% and 10.06%), whereas the highest value recorded in February (16.0% and 19.14%) by point method and Ranking index (Table 2).
Debris and Mud: It was found in the food of B. luteus during the study period, as a result of continuous search for food in the benthic sediment. The highest ratio was during September (6.82%) from point and (5.64%) from ranking index, the lowest ratio were (5.12%) out of total food points and (5.25%) from ranking index during November (Table 2).
Detritus it is the important food content for B. luteus throughout the months of the year. The highest during October was 10.05% from ranking index (Table 2).
Diatoms: The highest ratio observed in January was (5.79%, 7.24%), while the lowest ratio in September were (2.35%, 0.54%) (Table 2).
Unidentified ingested food: It exists in food content of B. luteus throughout different months of the year, except January, February and July. The highest ratio has been recorded in March (3.23% and 1.24%), whereas the lowest ratios were (4.30%, 2.66%) in December (Table 2).
Aquatic insects and larvae: The presence of these food items have seen in all months of the year, expect in July and December (Table 2).
Zooplankton: The ratios of the presence of zooplankton have been recorded only in March, April, May, June and August with the following ratios (1.12%, 0.78%, 0.47%, 0.21% and 0.25%) respectively out of total food point (Table 2).
4. DISCUSSION
4.1. Food and Feeding Habit of B. luteus
4.1.1. Intensity and Activity of Feeding in B. luteus
Feeding intensity through the analysis of gut fullness has been correlated with seasons [20-22]. In the present results of feeding intensity decreases during the winter months, due either the temperaturedependent regulation on food intake [23], or lesser abundance of prey and the lowered metabolism of the fish, which probably reduce predation during cold months. This revealed the monthly variations in gut fullness to include a rather low feeding intensity during period of low temperature and the high feeding intensity. These findings strengthen the evidence that in MOD River, feeding intensity in B. luteus is related with the seasonal climatic change such as temperature, rather than the reproductive cycle. This agree with many studies such as [4,24] and disagree with [3,25]. [3] showed feeding intensity decrease in B. luteus as temperature rises, i.e. negative non-significant correlation with temperature may be due to the use of gill nets which may stresses for length hours, in which much of the diet could be digested. The monthly trend in the feeding activity of B. luteus fish is related to water temperature. Temperature is an important factor, which regulates the biogeochemical activities in the aquatic environment as well as they represent one of the most influence environment factors affecting on river dynamics and both the metabolism and growth of fish [26,27]. All metabolic and physiological activities and life processes such as feeding, reproduction, movements and distribution of aquatic organisms and algal bloom are greatly influenced by water temperature [28]. Water temperature is related to solar radiation and air temperature in the present study. Water temperature follows changes in air temperature because the water depth is shallow and the surface area more in comparison with volume [29]. This situation emphasized by the positive significant correlation between air temperature and water temperature. This phenomenon was confirmed by many researchers in many lakes and water bodies [30-34]. There are differences in the temperature among the stations during the day which come from the different times of samples taking. These are agreed with [35-37]. In this study, the temperature hits its maximum value during summer season, whereas it hits its minimum value during winter. [38] showed that the decrease in water temperature lead to the decoration activity of most aquatic invertebrate hidden under rocks or within bottom sand that decrease their existence food for fish. Temperature might have direct effect on fish food. This study conducts gradually in feeding intensity and feeding activity values. These conclusions agree with many studies on different fish in Iraq River [4,24,39] and agree with feeding intensity and feeding activity for C. carpio capture with B. luteus in the same study area.
4.1.2. Food Content for B. luteus
Fish’s Food study represents a significant part of life study because feeding is one of important functions of organisms. Many researchers considered that food study is an essential step for successful breeding fish and development of this field [40,41]. Examining the diet of these species showed that there was a high percentage of mud and detritus in their gut. This is an indication that these species are bottom grazers. [42] showed that detritus is commonly mixed with sand which indicates that fish feed at the bottom of river. [3,8] reported that feeding of B.luteus consist of organic matter mixed with debris and mud indicate of bottom nature in feeding. [43,44] reported same conclusion. According to results, B.luteus population are typically feeding omnivorous, Generally, B. luteus to feeding is benthic omnivorous. Available nutrient mostly found in environment. Enforced of B. luteus to depend on those sources. Therefore it was found that plants constituent are a major source in comparison with animal sources.
4.1.3. Monthly Variations of Food Habits
Results revealed that the highest aquatic plant and tissues were recorded in spring with a peak in April in MOD, whereas the lowest value was in winter. [45] showed that the difference of food nature of any individuals correlates with availability, and with the type of the food and capability of the individuals obtain the food in different environments. This agreed with [46] that fish depend on zooplankton because of the availability through high water level in Naissryia-Fohood marshes. [47] showed that aquatic plant and tissues were essential food for Cyprinion kais in Hameren dam. These results are obtained from the high water levels of the dam in the neighboring agricultural area. Green-algae and blue-green algae had a second rank followed by the higher plant. [40] showed that algae is found in blooming time during spring and autumn in water column and settles in bottom during settling time (summer and winter). [48] showed that algae and diatoms reach the bottom after complicated vital processes to become fish intake to feed on. As fish feed on benthic, the green-algae and blue-green algae have increased in the gut of fish to come in the second place after higher aquatic plant and tissues. Fish are captured from north part of MOD. It has been found that they feed on phytoplankton from different algae, detritus which come in the second place [39]. This study agreed with [49] who recorded that the dominant preferable food were aquatic plant and algae. In this study, it was found that the digestive tract content of B. luteus was dominated by detritus. It is an indication of its importance in feeding of B. luteus which comes third in ranks after higher aquatic plant and algae. Therefore, it could be concluded that the difference in food content is related to the effect of environment in different months. The monthly variation of diatoms in fish gut reaches optimum peak in January. The increase of diatoms resulted from nourishment through autumn and spring. On contrary in winter and summer, in this present study, the unidentified digestive food in the gut recorded the lowest existence in (December) but nothing of this was found in January and February, a period coinciding with the winter months. On the other hand it recorded an increase in the existence from April to September, a period coincided with the spring/summer months. Consequently the fluctuations in these food items of B. luteus were observed in MOD River which may be attributed to seasonal changes. The effect of water temperature was clear on fish apetite which increases with temperature rise, in addition to its effects on the increase of digestive enzymes speed. [50] Showed that food consumes increases with increase of temperature until optimum limit. The little and invariant available zooplankton through the year shows low percentage in the fish gut food. This present study showed that insects have been found in a little ratio during this study period because of non-existence or unavailability of insects near the benthic. However, the variation in fish gut contents is depending on numerous factors such as fish size, availability of different food items, light intensity and water temperature. This study showed that B. luteus was benthic omnivorous, which was agreed with [3,8] and disagree with [51,52].
REFERENCES
- Alaş, A. and Ak, A. (2007) A study on the population structure of the tench (Tinca tinca L., 1758) inhabiting Beyşehir Lake (Konya-Turkey). Turkish Journal of Irrenunciable para Extramadura, Camara Oficial de Comercio e Industria de Caceres, Caceres, 111-115.
- Oronsaye, C.G. and Nakpodia, F.M. (2005) A comparative study of the food and feeding habits of Chryichthys nigrodigitatus (Lecepede) and Brycinus nurse in a Tropical River. Pakistan Journal of Scientific and Industrial Research, 48, 118-121.
- Al-Mukhtar, M.A. (1982) Biological study of two type freshwater fish, Barbus luteus and Aspius vorax in AlHammar marsh. M.S. Thesis, University of Basrah, Basrah, 203 pages.
- Dawood, A.H. (1986) Biology of common carp Cyprinus carpio (L.) in Al-Hammar marsh, southern Iraq. M.S. Thesis, University of Basrah, Basrah, 94 pages.
- FFWCC (Florida Fish and Wildlife Conservation Commission) (2005) Florida’s exotic fresh water fishes. http://Florida. Fisheriescom/Fishes Nonnativehtm/toscar
- Mebane, C.A., Maret, T.R. and Hughes, R.M. (2003) An index of biological integrity (IBI) for pacific northwest river. Transactions of the American Fisheries Society, 132, 239-261.
- Al-Mashhadany, A.J. and Al-Shammaa, A.A. (2002) Food interaction of four species of (cyprinidae: Pisces) in AlHabania lake. Iraq Journal of Fish Biology, 21, 1-7.
- Al-Shammaa, A.A., Mohanad, R., Hussein, A.F. and Abid, B.K. (2009) Food interaction of five species from genus Barbus in Hemeren Dam reservoir. Dialya-Iraq. The 3rd Scientific Conference 2011, College of Science, Baghdad University, Baghdad, 154-159.
- Al-Kubaysi, A.A. (1996) The ecological study of suddam river. Ph.D. Thesis, College of Science, University Baghdad, Baghdad, 143 pages.
- Mohammed, S.S. (2010) Evaluation the quality of water in third river in Thi-Qar Governorate. M.S. Thesis, ThiQar University, Nasiriyah, 106 pages.
- Prescott, G.W. (1961) Algae of the Western Great lakes area. Brown Composition Publication, Dubuque, 977 pages.
- Segers, H. (1995) The lecanidae (Monogononta). University of Gent, Belgium.
- Desmet, W. H. (1996) The prolidae (Monogonata). SPB Academic Publishing, Amsterdam, 102 pages.
- Sahin, Y. (1991) Turkiye chironomidae potamofaunas. TUBITAK Temel Birimler Arastirma Grubu, TABG-869, 88.
- Elliot, J.M. (1977) Some methods, for the statistical analysis of samples of benthic invertebrates. Freshwater Biology Association Science Publication, University of California, No. 25, 156 pages.
- Bagenal, T.B. (1978) Methods for assessment of fish production in freshwaters. IBP Handbook No. 3, Blackwell Scientific Publication, 3rd Edition, London, 300 pages.
- Hobson, E.S. (1974) Feeding relationships of Telostean fishes on coral reefs in Kona. Hawaii Fish Bulletin, 72, 915-1031.
- Dipper, F., Bredges, C. and Menz, A. (1977) Age, growth and feeding in the ballon wroune Leburs bergylta (Ascanius, 1767). Journal of Fish Biology, 11, 105-120.
- Gordonn, J.D. (1977) The fish population in shore water of the West Costal Scotland. The food and feeding of the whiting (Merlanguis merlanguis L.). Journal of Fish Biology, 11, 513-529.
- Bowman, R.E. and Bowman, E.W. (1980) Diurnal variation in the feeding intensity and catch ability of silver hake, Merluccius bilinearis. Canadian Journal of Fisheries and Aquatic Sciences, 37, 1565-1572.
- Cabral, H.N. and Murta, A.G. (2002) The diet of blue whiting, hake, horse mackerel and mackerel off Portugal. Journal of Applied Ichthyology, 18, 14-23.
- Santc, M., Jardas, I. and Pallaora A. ( 2005) Feeding habits of horse mackerel, Trahurus trachurus (Linnaeus, 1758), from the central Adriatic Sea. Journal of Applied Ichthyology, 21, 125-130.
- Temming, A. and Hermann, J.P. (2001) Gastric evacuation of horse mackerel: The effects of meal size, temperature and predator weight. Journal of Fish Biology, 58, 1230-1245.
- Al-Shawardi, A.O. (2006) Ecology and biology of Crucian carp Carassius carassius (L. 1758) and Khishini Liza abu (Heckel, 1843) in Tharthar Arm and Tigris. Ph.D. Thesis, College of Science, Al-Mustansiriya University, Baghdad, 142 pages.
- Dadzie, S., Abou-Seedo, F. and AlQattan, E. (2000) The food and feeding habits of the silver pomfret, Pampus arenteus (Euphrasen), in Kuwait waters. Journal of Applied Ichthyology, 16, 61-67.
- Herzing, A. and Winkler, A. (1986) The influence of temperature on the embryonic development of three cyprinoid fishes, Abramis brama, Chalcal burnus, Chalcoides mento and Vimba vimba. Journal of Fish Biology, 28, 171-181.
- Boyd, C.E. (1990) Water quality in ponds for aquaculture. Agriculture Experiment Station, Auburn University, Alabama, 482 pages.
- Weiner, E.R. (2000) Application of environmental chem.- istry. Lewis Publishers, London/New York. doi:10.1201/9781420032963
- Welch, P.S. (1952) Limnology. 2nd Edition, McGraw-Hill Book Co., New York.
- Munawar, M. (1970) Limnological studies on fish water pond of Hyderabad, India. Hydrobiologia, 31, 101-128.
- Antoine, S.E. (1977) Seasonal variations of the environmental characteristics and phytoplankton blooms of Tigris, Iraq. M.S. Thesis, Basrah University, Basrah, 134 pages.
- Al-Zubaidi, A.J.M. (1985) Ecological study on the algae (Phytoplankton) in some marshes near Qurna-Southern Iraq. M.S. Thesis, Basrah University, Basrah, 131 pages.
- Hussain, N.A. and Taher, M.A. (2007) Effect of daily variations, diurnal fluctuations and tidal stage on water parameters of East Hammar marshland, Southern Iraq. Marsh Bulletin, 2, 32-42.
- Al-Maliky, R.N.A. (2009) Biogeochemical assessment of trace metals in Al-Hammar marshes, Iraq. Ph.D. Thesis, Department of Biology, College of Science, University of Baghdad, Baghdad, 253 pages.
- Fahad, K.K. (2005) Study in the some physic-chemical features to the main drainage river at Nassiriya city. ThiQar Journal, 18, 65-72.
- Salman, J.M. (2006) Environmental study of possible pollutants in Euphrates River from Al-Hindiya Barrage to Al-Kufa-Iraq. Ph.D. Thesis, University of Babylon, Babylon.
- Al-Ghanemi, H.A.H. (2010) Use of aquatic plants as bioindicators of heavy metals in Euphrates River-Iraq. Master of Science Thesis, University of Babylon, Babylon.
- Pennak, R.W. (1953) Freshwater invertebrates of United States. The Ronald Press Company, New York.
- Al-Shamma΄a, A.A., Mahmmed, M.A. and Ahmed, J.H. (1996) The natural food of fish in Al-Qudisiya Dam reservoir 1-Cyprinus carpio. Journal of Essential Science Studies, 23, 143-150.
- Luther, G. (1962) The food habits of Liza macrolepis Smith and Mugil cephalus L. (Mugilidae). Indian Journal of Fish, 9, 604-626.
- Al-Shamma΄a, A.A., Mahmmed, M.A. and Al-Mashhadinay, A.J. (1999) The natural food of fish in Al-Qudisiya Dam reservoir. Barbus xanthopterus and another fish types from genus Barbus. Journal of Natural and Engineering studies science, 26, 137-149.
- Hickling, C.F. (1970) A contribution to the natural history of the English grey mullet (Pisces: mugilidae). Journal of the Marine Biological Association of the United Kingdom, 30, 609-633.
- Al-Nasiri, S.K. and Sirajul-Islam, A.K. (1978) Age and growth of Liza abu (Heckel) (Perciformes, Mugilidae). From southern Iraq. Bangladesh Journal of Zoology, 6, 85-89.
- Al-Shammaa, A.A. and Ali, A. (1993) Preliminary study of Barbus sharpeyi food in Al-Hammer marsh, Al-Fahoud. Basrah, Iraq. Mesopotamian Journal of Marine Science, 8, 881-892.
- Lagler, K.F. (1956) Freshwater fishery biology. William C. Brown, Dubugue.
- Al-Shamma’a, A.A. and Jasim, Z.M. (1993) The natural food of Liza abu during the flood in Al-Hammar Marsh, south Iraq-Zoology in the Middle East. Osteichthes, 9, 59-64.
- Al-Shawardi, A.O. (2000) Seasonal variations in food contents of macrostomus (Heckel, 1843) and Cyprinion kais (Heckel, 1843) in Hemeren Dam reservoir. Master of Science Thesis, University of Basrah, Basrah.
- Tampi, P.R. (1959) The ecological and fisheries characteristics of the salf water lagoon near man dap. The Marine Biological Association of India, 1, 113-130.
- Soaod, H.A. (2004) Food interaction of some species cyprinidae in karmat Ali, Southern Iraq. Basrah Journal of Science, 3, 2-17.
- Sarker, A.L. (1983) Feeding ecology of blue gill, Lempomis macrochirus (Rafinesque) in two heated reservoir of Texas 1. season of year and patterns of feeding. Bangladesh Journal of Zoology, 1, 23-48.
- Al-Kubaysi, A.A.L. (1990) Ecological study of phytoplankton and food content in alimentary canal of some fish in Iraq central. Master of Science Thesis, University of Baghdad, Baghdad.
- Al-Rekabi, J.W. (2010) Qualititive study for algae in some Iraqi fish gut in Al-Ghibayesh and Abu Zariq marshes. Master of Science Thesis, University of Baghdad, Baghdad.